Abstract
The partition site, parS, promotes accurate segregation of the replicated P1 plasmid to daughter cells when the P1-encoded ParA and ParB proteins are supplied. The parS site was inserted into the Escherichia coli chromosome between the promoter and the structural gene for β-galactosidase, lacZ. There was little interference with lacZ expression when ParA and ParB were supplied in trans. However, when a mutant ParA protein, ParAM314I, was supplied along with ParB, expression of lacZ was shut down. ParAM314I, ParB, and parS appear to form a nucleoprotein complex that blocks transcription. Mutations in parA and parB that relieved the parAM314I-dependent block were found. In addition, new mutations which impose the block were selected. Five of the latter mapped to parA and one to parB; all had a propagation-defective phenotype (ParPD) similar to that of parAM314I. Thus, whereas a null par mutant P1 plasmid segregates its DNA randomly, these mutants prevent even random distribution of the plasmid. We propose that ParA protein normally interacts transiently with the ParB-parS complex for partition to proceed but that the mutations block ParA dissociation. This “permanent” ParA-ParB-parS complex acts as a transcription block. Consistent with this hypothesis, we found that three of the seven blocking mutations lie within regions of ParA and ParB that are known to interact with each other. When the transcription block is imposed, regional silencing of nearby genes occurs. However, the requirement for ParA and a mutant parA or parB allele distinguishes the transcription block from the regional ParB-dependent gene silencing previously described.
The plasmid prophage of bacteriophage P1 can be accurately maintainedin its Escherichia coli host at a copy number of only one or two per cell (21). Stable maintenance is dependent on the P1 par region, which promotes the active segregation of daughter plasmids prior to cell division (19). The essential components involved consist of the cis-acting partition site, parS, and the genes encoding the two P1 Par proteins, ParA and ParB (12). The ParB protein binds to specific sequences within parS (6). The central portion of the parS site consists of a specific binding sequence for integration host factor (IHF protein) that induces a severe bend in the DNA (6, 13, 14). IHF and ParB bind cooperatively to the parS site in vitro (6, 13, 14).
The ParA protein is an ATPase (8), which interacts specifically with the parS-ParB assembly (28) but only forms a stable complex with the other par components in vitro when continually charged with ATP (3, 5). Its ATPase activity, which is essential for partition, is stimulated by interaction with ParB and nonspecific DNA in vitro (7). ParA does not appear to contact parS directly (7). It was suggested that ParA is not a permanent part of a partition complex but interacts transiently with ParB during partition to promote some essential aspect of the process (8).
A mutant ParA protein, ParAM314I, acts to block plasmid maintenance (28). Unlike typical Par mutations, parAM314I completely prevents propagation of the plasmid under nonpermissive conditions. The mutant protein presumably acts by blocking replication or by preventing segregation of the plasmid even by random diffusion such that one daughter cell retains all the plasmid copies (28). It was suggested that this mutation stabilizes the ParA interaction with the ParB-parS complex such that ParAM314I becomes locked into the complex, preventing some essential dissociation of the plasmid copies from each other, or from some host attachment site during partition (28).
The partition sites of the P1 and F plasmids have been associated with a silencing activity (17, 24). Silencing inhibits the expression of genes linked to, but often some distance from, the partition site. It occurs when P1 ParB, or the equivalent F SopB protein, is supplied to its cognate partition site. Silencing did not require the ParA component. It may reflect the formation of an extended coating of the DNA by ParB that is nucleated by binding to the partition site (24). Alternatively, it may be due to the nonspecific binding of ParB to a region of DNA surrounding the partition site promoted by binding of parS to some host structure containing multiple ParB molecules (17). In either case, the ParB-parS interaction promotes spreading of ParB binding to an extended region adjacent to parS.
Plasmid partition sites can be regarded as analogs of centromeres of eukaryotic chromosomes. For budding yeast, Doheny et al. (10) followed the formation of a protein complex at the centromere by placing it between the promoter and the open reading frame for a reporter gene. The formation of a complex was inferred by a block to the transcription of the gene, and mutations which relieved this block identified genes contributing to the formation of the complex (10). Here, we employ a similar approach to probe the formation of a complex at P1 parS and to select mutants that impose or relieve a transcription block.
MATERIALS AND METHODS
Media and general procedures.
Bacteria were grown in L medium (25). When necessary, ampicillin (Ap), chloramphenicol (Cm), kanamycin (Km), or spectinomycin (Sp) were added at 100, 10, 20, or 30 μg/ml, respectively, unless noted otherwise. MacConkey lactose (mac-lac), plates contained 1% lactose (18).
Escherichia coli strains.
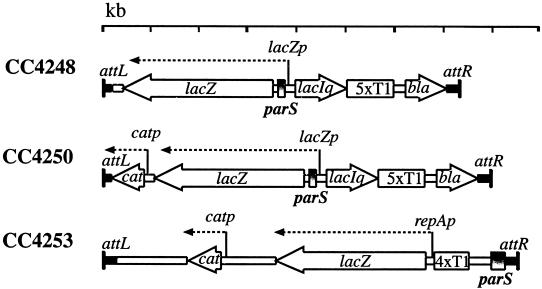
Strain BR6903, described as “construct I” by Rodionov et al. (24), has a parS site integrated in the chromosome approximately 1 kb upstream of the test locus consisting of a constitutive plasmid P1 repA promoter followed by the lacZ open reading frame (24). Approximately 1 kb downstream from lacZ is the cat (chloramphenicol acetyltransferase) gene with its own promoter. This strain was a gift from Michael Yarmolinsky. We transduced BR6903 to recA+ by using a linked marker, cysC3152::Tn10kan, to create CC4253 (Fig. 1). CC4248 contains the “transcription block” construct, and its construction is detailed below. CC4250 is CC4248 with the cat gene and its promoter inserted ∼200 bp downstream of lacZ in the λ phage sequences (Fig. 1). This strain was made using λ Red-mediated linear recombination by a modification to the protocols described by Yu et al. (30) to be described elsewhere. WJW26 and WJW45, gifts from Helen Wilson, are W3110 with Δ(lacI-Z)M445 and Δ(lacIZYA-argF)U169, respectively. ZH1142, a gift from Jian-guang Zhou, is W3110 Δ(lacIZYA-argF)U169 gal490 λ[N::lacZ immλ Δ(cro-bio)] rnc14. CC4249 is CC4248 with ihfA82::Tn10. LE30 is mutD5 strR aziR gal. CC2056 was used in the colony color partition assay as previously described (22).
FIG. 1.
Comparison of silencing and transcription block constructs at attλ in CC4248, CC4250, and CC4253. The diagram is to scale. The construct in CC4248 is similar to that found in CC4250 except the cat gene is not present (see Materials and Methods). The orientations of genes are indicated by the arrows. The parS site is oriented the same in all constructs and is such that the right border is the one adjacent to parB in the P1 plasmid. Strain CC4253 has an additional ∼170 bp of P1 DNA downstream of the parS site. catp, the promoter for the cat gene; lacZp, the lacUV5 promoter; repAp, the promoter for the P1 repA gene (23). 5xT1 and 4xT1, five and four copies, respectively, of the T1 transcriptional terminator from rrnB.
Construction of assay strain CC4248.
The transcription block construct found in CC4248 was first made on a plasmid, pALA2302. This plasmid contains the PlacUV5 promoter transcribing across the P1 parS site and into the lac operon as shown in Fig. 1. On the other side of the lac promoter are the lacIq gene, transcription terminators, and the bla (Ap resistance) gene, all transcribed away from parS. It was constructed as follows. Plasmid pALA2300 was made by introducing a synthetic linker containing BglII and BamHI sites into the StyI site of pALA161 (11). This placed the P1 parS site on a 134-bp BamHI fragment which was excised and introduced into the BamHI site of pALA1172 to create pALA2302. The orientation of parS was determined by restriction digestion and confirmed by DNA sequencing. It was such that its right border (Fig. 1) was the end adjacent to the parB gene in phage P1. Plasmid pALA1172 is pNK1475 (9) with the lacIq-containing EcoRI fragment from pNK627 cloned into the EcoRI site, oriented so that lacI is transcribed away from lacZ.
Phage λBDC531 (29) was used to pick up the transcription block construct from pALA2302 by using bla and lacZ homology. This phage stock was used to lysogenize strain WJW45 to make CC4243. P1 lysates were made on CC4243 and used to transduce the transcription block construct into ZH1142 by using the technique described by Yu and Court (29), selecting ApR (10 μg/ml) at 37°C to generate CC4245. The transcription block construct was then transduced into WJW26 (selecting ApR) to generate CC4248. The construct present at the phage lambda attachment site of this strain is shown in Fig. 1. Note that this construct contains only about 200 bp of phage λ DNA near attL and attR. The DNA sequence of the construct in CC4248, including the PlacUV5 promoter, parS and the start of lacZ, was determined and was found to be correct.
Vectors.
Plasmids pBR322 and pGB2 were as previously described (2). Plasmid pALA1858 consists of the PstI fragment of pSP102 (20), containing the Cm resistance gene, inserted into the PstI site of pBR322, thus inactivating the Ap resistance gene.
Plasmids.
The plasmids used in this study that contained the P1 par locus or modified versions of it are shown in Table 1. All par genes in this study were expressed using the constitutive promoter found in pALA1570 (8). In pALA1570, the constitutively expressed par operon is present on an EcoRI-BamHI fragment inserted between the same sites of pBR322. Likewise, mutant versions of par operons were inserted into pBR322 as HindIII-BamHI fragments. Cm resistance versions of these plasmids were made by cloning the PstI fragment of pSP102 into the PstI site of each plasmid.
TABLE 1.
Constitutively expressed par plasmids
| Mutation | Plasmid designations
|
|||
|---|---|---|---|---|
| pBR322Aprpar | pBR322Cmrpar (pALA1858) | pGB2 par (orientation 1)a | pGB2 par (orientation 2)a | |
| WT | pALA1570 | pALA2310 | pALA2306 | pALA1855 |
| parAM314I | pALA1835 | pALA2312 | pALA2308 | pALA1856 |
| parAA14V | pALA1863 | pALA1864 | pALA1862 | pALA1865 |
| parAD209Y | pALA1867 | pALA1868 | pALA1866 | pALA1869 |
| parAL329P | pALA1888 | pALA1887 | pALA1889 | pALA1890 |
| parAD209G | pALA1871 | pALA1872 | pALA1870 | pALA1873 |
| parAD152N | pALA1875 | pALA1876 | pALA1874 | pALA1877 |
| parBT12P | pALA1892 | pALA1894 | pALA1891 | pALA1893 |
| ΔparA | pALA1896 | pALA1895 | pALA2319 | |
| ΔparB | pALA1841 | pALA2314 | pALA2317 | |
| parAM314I ΔparB | pALA1842 | pALA2316 | pALA2318 | |
| ΔparA parBT12P | pALA1880 | |||
The par region is in orientation 1 when it is transcribed in the same direction as the Sp resistance gene and in orientation 2 when in the opposite orientation (see Materials and Methods).
Two series of plasmids were made with P1 par operons in a pGB2 vector. Inserting the HindIII-BamHI par-containing fragments into the same sites in pGB2 resulted in the par operon being transcribed in the same direction as the Sp resistance gene (orientation 1). Inserting the EcoRI-BamHI par-containing fragments into the same sites in pGB2 resulted in the par operon being transcribed in the opposite direction to the Sp resistance gene (orientation 2).
Plasmids that contained a large in-frame deletion of parA were made by cutting with XhoI and SacII and inserting an oligonucleotide consisting of the annealed single-stranded sequences, 5′TCGAGCTGCCGC and 5′GGCAGC. This deletion of parA maintains the parA stop codon and leaves the parB gene intact. To construct a large deletion of parB, the relevant plasmid was cut with BglII and XbaI and an oligonucleotide consisting of the annealed single-stranded sequences 5′GATCTAACTGATAT and 5′CTAGATATCAGTTA was inserted. This restored the 3′ end and stop codon of the parA gene, produced a complete deletion of the parB open reading frame, and introduced an EcoRV site.
Plasmids λ-P1:5RCm and λ-P1:5RKm are P1-λ hybrid constructs that can be grown lytically as λ phage or they can lysogenize E. coli as a low-copy-number plasmid under P1 replication control (26). They confer Cm and Km resistance, respectively. Unless noted otherwise, the plasmids were stably maintained, as they contained the par region.
The parS test plasmid was λ-P1:5RΔ1005::pALA1952, which contains the parS site but no par genes and can be monitored for segregation by the colony color assay (22). This plasmid also contains the cat gene.
Isolation of mutations.
Mutations were isolated as follows. Strain LE30 (mutD) was transformed with either pALA2306 or pALA2308. Plasmid DNA was extracted from the transformants by using Wizard Plus midi-preps (Promega, Madison, Wis.). These mutagenized plasmid preparations were used to transform CC4248 to Sp resistance on mac-lac-Sp plates.
Plasmid pALA2306 was mutagenized to isolate mutants that have a tighter transcription block to lacZ expression (white colonies on mac-lac plates). Cells transformed by mutagenized pALA2306 produced white colonies at a frequency of 2 × 10−4. Plasmid pALA2308 was mutagenized to generate mutations that relieve the parAM314I-dependent transcription block to lacZ expression (yielding red colonies on mac-lac plates). Red colonies from cells containing mutagenized pALA2308 were isolated at a frequency of 10−3. Candidate mutants were first struck on mac-lac-Sp plates to confirm their color. Mini-preps were then made and used to retransform CC4248 to confirm that the new phenotype was linked to the plasmid.
Estimating levels of par operon expression.
The levels of par operon expression from plasmids producing wild-type Par proteins were estimated by measuring the steady-state levels of ParA protein in cells grown in L broth.
Strain WJW26 was transformed with plasmid pALA2306, pALA1855, pALA2319, pALA1570, or pALA2310. A 50-ml culture was grown to an optical density at 600 nm (OD600) of 0.4 at 37°C in L broth supplemented with the appropriate antibiotic. A λ-P1:5RKm lysogen of WJW26, grown to an OD600 of 0.4 at 30°C in 50 ml of L broth supplemented with 20 μg of kanamycin/ml, was used as a reference sample. The cells were harvested by centrifugation at 5,000 × g for 15 min at 4°C. After the cells were washed with 50 ml of 25 mM Tris-HCl (pH 8.0), 1% (wt/vol) glucose, 0.1 mM EDTA, and 20 mM dithiothreitol (DTT), the pellets were resuspended in 2.5 ml of B-PER Bacterial Protein Extraction Reagent (Pierce, Rockford, Ill.) supplemented with 20 mM DTT.
The cells were lysed by incubating the resuspended pellets for 30 min at room temperature with 250 μg of lysozyme/ml and 10 U of Omnicleave Endonuclease (Epicentre Technologies, Madison, Wis.) per 1 OD600 of cell culture. The resulting lysate was cleared by centrifugation at 27,000 × g for 30 min at 4°C. The supernatant was desalted by chromatography on an 8.5-ml Sephadex G-25 column equilibrated with 200 mM ammonium sulfate, 40 mM HEPES (pH 7.5), 0.1 mM EDTA, 10 mM DTT, and 15% (vol/vol) glycerol. The lysates were stored at −70°C for further use.
The proteins were concentrated by precipitation with trichloroacetic acid and separated on a 12% (wt/vol) polyacrylamide mini gel. Western blottings were done as previously described (4). The blots were probed with rabbit polyclonal anti-ParA antibody and developed using the ECL Western blotting Analysis System according to the supplied protocol (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.). ParA protein of known concentration was used as a quantitative standard. By comparison of the ParA band intensity with that of a series of dilutions of the ParA protein standard, the weight of ParA protein per milliliter of culture was determined. Knowing the number of cells in the culture, the number of ParA proteins per cell was calculated.
Cat assays.
Chloramphenicol acetyltransferase assays were carried out using the Fast Cat Yellow kit from Molecular Probes (Eugene, Oreg.) by following the manufacturer's protocols. After thin-layer chromatography, the spots were quantitated using a Molecular Dynamics Typhoon 8600 analyzer by using its accompanying software.
RESULTS
Test strain for measuring transcription through parS site.
Strain CC4248 has the lacZ gene transposed to the phage λ attachment site. In addition, the P1 parS site was inserted between the promoter, PlacUV5, and the lacZ gene (Fig. 1). The strain formed red colonies on lactose indicator plates. Thus, the parS site itself does not block transcription from proceeding from the lac promoter into the lacZ gene. Assays of β-galactosidase (Table 2) showed that this modified lacZ operon was repressed by the LacIq repressor and was induced by isopropyl-β-d-thiogalactopyranoside (IPTG). The fully induced level (ca. 300 units) was below that normally achieved by the wild-type lacZ gene in its normal context (ca. 1,500 Miller units, data not shown). This may reflect the altered context of the lac promoter or the properties of the mutant LacIq repressor. However, part of the reduction appears to be due to IHF protein binding to the parS site, because introduction of an ihfA mutation into strain CC4248 caused lacZ expression to increase by some 50% (Table 2).
TABLE 2.
Levels of β-galactosidase in transcription block strain when Par proteins were provided
| Strain and/or plasmid | par operona | Miller units
|
|
|---|---|---|---|
| Uninduced | Induced | ||
| CC4248 | None | 1 | 303 |
| CC4248 ihfA82::Tn10b | None | 7 | 454 |
| CC4248 pBR322 | None | 1 | 323 |
| CC4248 pALA2310 | parA+parB+ | 2 | 222 |
| CC4248 pALA2312 | parAM314I parB+ | 1 | 18 |
| CC4248 pALA1895 | ΔparA parB+ | 2 | 189 |
| CC4248 pALA2314 | parA+ ΔparB | 6 | 375 |
| CC4248 pALA2316 | parAM314I ΔparB | 8 | 377 |
Each par operon has a constitutive promoter and is present on the Cm resistance version of pBR322 (pALA1858; see Materials and Methods). Values are averages of at least three experiments.
Strain CC4249 is CC4248 with an additional ihfA82::Tn10 mutation.
Wild-type P1 ParA and ParB impose only modest block to transcription through parS in transcription block reporter construct.
Plasmid pALA2310 produces P1 ParA and ParB from a constitutive promoter. The levels of Par proteins produced are suitable for supporting P1 plasmid partition (data not shown). When pALA2310 was introduced into the CC4248 reporter strain, the colonies remained red on lactose indicator plates and the induced level of β-galactosidase was only modestly reduced by the presence of the Par proteins (Table 2). Thus, any complex formed by ParA and ParB at the chromosomal parS site is unable to efficiently block transcription from the lac promoter into the lacZ gene.
parS site in CC4248 appears to be functional.
As supplying the wild-type Par proteins had little effect on lacZ transcription, the possibility existed that the parS site was nonfunctional or somehow inaccessible to binding proteins in this construct. To test this, we determined whether the parS site in the construct would exert incompatibility against an incoming plasmid containing the P1 Par system. Partition-mediated incompatibility prevents the stable establishment of replicon utilizing a par system when the cell already contains a copy of that system (1). This is due to the ability of the resident parS site to compete with the incoming replicon for binding of the par proteins or for binding to some cellular component required for partition (19).
Strain CC4248 was first lysogenized with the P1 mini-plasmid λ-P1:5RCm (Par+). The mini-P1 lysogens were then grown nonselectively for approximately 25 generations and retention of the plasmid was scored. In CC4248, 44% of the cells contained the λ-P1:5R plasmid after nonselective growth. In an isogenic control strain without the parS construct in the chromosome, 97% of the cells maintained the plasmid. We conclude the parS site in CC4248 is functional, at least as defined by this competition assay.
Mutant Par protein imposes efficient transcription block.
The transcription block tests were repeated using a mutant version of the constitutive par operon present on plasmid pALA2312. Plasmid pALA2312 has a point mutation in parA and produces the mutant ParA protein ParAM314I. With this plasmid, the reporter strain formed white colonies on lactose indicator medium, and the induced levels of β-galactosidase were reduced some 12-fold relative to the level achieved with the wild-type Par proteins (Table 2). The observed effect was dependent both on the parAM314I mutation and on the presence of the wild-type ParB protein: a derivative of pALA2312, pALA2316, which has a large in-frame deletion in parB did not impose the block (Table 2), and plasmid pALA1895, which contains an in-frame deletion of the parA gene removing the region containing the parAM314I mutation, largely relieved the transcription block, confirming that the mutant parA gene is required (Table 2). Thus, the mutant ParAM314I protein, in conjunction with wild-type ParB, can form a protein complex capable of blocking transcription from passing from the lac promoter into lacZ. The block was not due to overproduction of the proteins from the mutant plasmid, as all plasmids tested utilize the same constitutive promoter (28).
Block is dependent on level of Par protein synthesis.
Cells containing λ-P1:5RKm, a mini-P1 plasmid with a wild-type, autoregulated par operon, contained about 1,000 molecules of ParA per cell. We defined this as the 1× level of par operon expression (see Materials and Methods). Cells containing pALA2310, the pBR322-based plasmid with a constitutively expressed wild-type par operon, showed a 10× level of expression. Alternative constructs with the same constitutive promoter expressed the parA protein at 20× (pALA2306) and 0.5× (pALA1855) (see Materials and Methods). Table 3 shows that the 10× and 20× levels of par expression were sufficient to impose the transcription block with the parAM314I allele but that the 0.5× level was not. The equivalent plasmids carrying the wild-type par genes failed to exert a comparable transcription block (Table 3) even at the highest level of expression (20×).
TABLE 3.
Levels of Par proteins affect transcription block
| Plasmid | par operon | Level of par operon expressiona | Miller unitsb |
|---|---|---|---|
| pALA1855 | pcparA+parB+ | 0.5× | 340 |
| pALA2310 | pcparA+parB+ | 10× | 197 |
| pALA2306 | pcparA+parB+ | 20× | 183 |
| pALA1856 | pcparAM314I parB+ | 0.5×c | 353 |
| pALA2312 | pcparAM314I parB+ | 10×c | 15 |
| pALA2308 | pcparAM314I parB+ | 20×c | 14 |
Each par operon has a constitutive promoter. Plasmids are described in Materials and Methods. Plasmids pALA2306 and pALA2308 have the Par operon inserted in the vector pGB2 in orientation 1, such that the operon is transcribed in the same direction as Sp resistance (see Materials and Methods). Plasmids pALA1855 and pALA1856 also have the operon inserted into pGB2 but in the opposite orientation. Levels of operon expression were estimated by measuring the ParA content of the cells (see Materials and Methods). The 1 × level of expression is defined as the level of ParA expression in lysogens of λ-P1:5RKm.
The β-galactosidase assays were carried out after induction of the lacZ gene with 1 mM IPTG in strain CC4248 carrying the relevant plasmid (see Materials and Methods). Values are averages of at least three experiments.
The level of ParA protein was not determined for these cells but was inferred to be the same as the relevant wild-type parA+ constructs, as they have the same promoter.
Mutations which relieve transcription block imposed by ParAM314I.
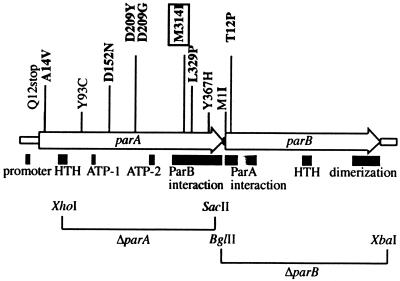
When plasmid pALA2308 (20× expression) was introduced into strain CC4248, the colonies were white on mac-lac indicator plates due to the transcription block imposed by the parAM314I mutant par operon. The plasmid was mutagenized (see Materials and Methods) and introduced into the indicator strain by transformation, selecting for spectinomycin-resistant colonies on mac-lac-Sp plates. As expected, most of the resulting colonies were white, but red colonies were present at a frequency of ∼1 × 10−3. Samples of these were isolated and purified, and the plasmid DNA was extracted from them. Four of these plasmid isolates were analyzed by DNA sequencing. Each of the four had the original parAM314I mutation plus an additional single mutation in the par operon. Three different mutations were found in parA and one was found in parB (Fig. 2). One parA mutation, parAQ12STOP, and the parBM1I start codon change are likely to be null mutations. This is consistent with the conclusion that both the mutant ParA protein and the wild-type ParB protein are required for a transcription block.
FIG. 2.
P1 par operon map. The mutations are shown above the map. Names correspond to the amino acid change and position. Thus, parAA14V has an alanine-to-valine change at the 14th ParA amino acid. Mutations in bold type are those that impose a transcription block in the test strain (Fig. 1). Those in regular type relieve the transcription block imposed by the previously isolated mutation, parAM314I (boxed). Features of the Par region are indicated as boxes below the map. HTH, helix-turn-helix motif probably involved in binding to the par operator sequence (ParA) or the parS site (ParB); ATP-1,2, first and second ATPase motifs. The regions implicated in the ParA-ParB interaction are also shown (23), as is the ParB dimerization domain (27).
New mutations that impose transcription block.
The plasmid carrying the wild-type parA and parB genes under constitutive promoter control, pALA2306 (20×), was subject to mutagenesis and introduced into the reporter strain, selecting for spectinomycin-resistant colonies on mac-lac-Sp plates. Most of the resulting colonies were red but occasional white colonies appeared (∼2 × 10−4). White colonies were purified and the plasmid DNA was extracted. The XhoI-SacII fragment, which includes the bulk of the parA gene (Fig. 2), was excised from each mutant candidate and inserted into a plasmid containing a par operon from which the XhoI-SacII fragment had been deleted (see Materials and Methods). The resulting plasmids from five of the candidate mutants were introduced into strain CC4248, and the colonies were white on mac-lac plates. Thus, the block to β-galactosidase activity was due to a mutation within the XhoI-SacII region of parA. This was confirmed by DNA sequencing. Each mutant proved to have a single base change from the wild-type sequence (Fig. 2). Two isolates failed to cause a block when the XhoI-SacII region was transferred, suggesting that they had a mutation outside this interval. Sequencing of the original mutant isolates showed that one had a single base change in parA, parAA14V. The other had a single base change in parB, parBT12P (Fig. 2).
Of the seven mutants that were sequenced, six different mutations were represented, five in parA and one in parB. The parA mutation, parAD209G, was isolated twice from different mutagenized plasmid preparations. The β-galactosidase levels of CC4248 containing these mutant par alleles are shown in Table 4.
TABLE 4.
Effect of mutations on transcription blocka
| Resident plasmid | par operon | Miller units |
|---|---|---|
| pGB2 vector | None | 220 |
| pALA2306 | pcparA+parB+ | 149 |
| pALA2319 | pcΔparA parB | 91 |
| pALA2308 | pcparAM314I parB+ | 14 |
| pALA1862 | pcparAA14V parB+ | 15 |
| pALA1866 | pcparAD209Y parB+ | 12 |
| pALA1889 | pcparAL329P parB+ | 49 |
| pALA1870 | pcparAD209G parB+ | 9 |
| pALA1874 | pcparAD152N parB+ | 8 |
| pALA1891 | pcparA+parBT12P | 11 |
| pALA1880 | pcΔparA parBT12P | 89 |
The β-galactosidase assays were carried out after induction of the lacZ gene with 1 mM IPTG. Transcription block assays were carried out in strain CC4248 carrying the relevant plasmid (see Materials and Methods). pc, constitutive par promoter. Values are averages of at least three experiments.
parBT12P mutation.
The parBT12P mutation was the only parB mutant isolated that imposes a block to transcription in the tester strain (Table 4). When parA was deleted from the plasmid carrying the parBT12P mutant operon, its ability to block lacZ transcription was greatly diminished (pALA1880; Table 4). Note that the parA deletion used was a large in-frame deletion of the parA open reading frame (Fig. 2) so parB expression could be maintained. Thus, the blocking effect of the parBT12P mutation is dependent on the presence of the ParA protein.
Mutant proteins are partition defective and yield propagation-defective phenotype.
The mutant par operons that impose a transcription block were excised from their original contexts and inserted into a pBR322 vector. The intact par operon under constitutive control was restored in these constructs with the relevant parA mutation in place (10× level of expression). Strains containing these plasmids were then used in the colony color partition assay (22). Cells carrying the control plasmid with a wild-type parAB operon were readily lysogenized with the parS test plasmid (λ-P1:5RΔ1005::pALA1952) by selecting for chloramphenicol resistance. Once established, the test plasmid was efficiently maintained in these cells without selection (Table 5). The test plasmid was also established readily in cells containing the pBR322 vector, but the parS test plasmid was rapidly lost without selection. Cells expressing the various mutant par operons, however, behaved differently from either of these cases. With the majority of the mutants, it was not possible to introduce the parS test plasmid even with selection into cells expressing the mutant operons (Table 5). In the two exceptional cases, small colonies were obtained under selection after 48 h of incubation. The parS plasmid was extremely unstable in these cells (Table 5). The very slow growth of the test colonies on medium selective for the parS plasmid presumably reflects this rapid plasmid loss. We conclude that the six new par mutant operons resemble those of the parAM314I mutant in not only being unable to support the partition of a parS-containing plasmid but preventing even the establishment of the plasmid within the cell (28). This ParPD (propagation-defective) phenotype may indicate that a complex at parS interferes with the plasmid DNA replication or that the plasmid copies are unable to dissociate from each other and always remain in one of the daughter cells at cell division (28).
TABLE 5.
Ability of transcription block mutations to support partition
| Resident plasmid | par operona | Cm-resistant colonies on overnight growthc | % Plasmid retention (25 generations) |
|---|---|---|---|
| pBR322 vector | None | ∼1 × 105 | 2 |
| pALA1570 | pcparA+parB+ | ∼1 × 105 | 92 |
| pALA1835 | pcparAM314I parB+ | <1 | NTd |
| pALA1863 | pcparAA14V parB+ | ∼5 × 101b | 18 |
| pALA1867 | pcparAD209Y parB+ | <1 | NT |
| pALA1888 | pcparAL329P parB+ | ∼5 × 101b | <1 |
| pALA1871 | pcparAD209G parB+ | <1 | NT |
| pALA1875 | pcparAD152N parB+ | <1 | NT |
| pALA1892 | pcparA+parBT12P | <1 | NT |
| pALA1841 | pcparA+ ΔparB | ∼1 × 105 | 1 |
Pc, Promoter constitutive.
No colonies were obtained after overnight growth, but small colonies were obtained after 48 hr; these were tested for plasmid retention. Values are averages of three experiments.
Number of lysogens of λ-P1:5RΔ1005::pALA1952 per assay. Each assay contained approximately 107 viable cells.
NT, no colonies were obtained for testing.
Most parPD mutants are recessive to wild type.
Partition tests were carried out in cells simultaneously carrying a mutant and a wild-type par operon under constitutive control (Table 6). The wild-type operon was present on pALA1570, a pBR322-based plasmid with a 10× level of par operon expression. The mutant operons were on compatible pGB2-based plasmids that resulted in a 20× level of par operon expression. Table 6 shows that the majority of the parA mutant alleles were recessive to the wild type under these conditions. The exceptions were M314I and D209G, which could not be classified in this test (Table 6). The parBT12P was recessive to the wild type (Table 6). The dominance of the wild-type locus over the recessive alleles had a limit. When the wild-type proteins were expressed at a sharply lower level than those of the mutants (0.5× versus 10× levels), all the mutants acted as if they were dominant (data not shown).
TABLE 6.
Dominance tests of mutant versus wild-type par operonsa
| Resident pGB2-based plasmid | par operon on pGB2-based plasmid | % parS plasmid retention (25 generations) |
|---|---|---|
| pGB2 vector | None | 94 |
| pALA2306 | pcparA+parB+ | No colonies |
| pALA2308 | pcparAM314I parB+ | <1 |
| pALA1862 | pcparAA14V parB+ | 90 |
| pALA1866 | pcparAD209Y parB+ | 89 |
| pALA1889 | pcparAL329P parB+ | 95 |
| pALA1870 | pcparAD209G parB+ | <1 |
| pALA1874 | pcparAD152N parB+ | 94 |
| pALA1891 | pcparA+parBT12P | 98 |
Maintenance of a mini-P1 plasmid carrying the P1parSsite (λ-P1:5RΔ1005::pALA1952) was determined by the colony color partition assay. All cells had a second resident plasmid pALA1570, a pBR322-based plasmid expressing the wild-type par operon from a constitutive promoter (10× level of expression). Note that the test is valid only when the mutant proves to be recessive. This is because, when both plasmids were expressing wild-type protein, the plasmid was unstable and no lysogens were formed. This is consistent with previous findings that overproduction of Par proteins gives P1 plasmid instability (15). Thus, mutants that appear to be dominant may indeed be dominant to the wild-type protein or may simply be mimicking the behavior of the wild-type protein.
Relationship of transcription block to gene silencing.
It has been shown that silencing of genes in a broad region surrounding a chromosome-integrated parS site can occur when Par proteins are supplied. The phenomenon was seen when both wild-type ParA and ParB proteins were supplied, but ParA was found to be unnecessary (24). By this operational definition, the parS region in the strain used in our transcription block assay was only marginally prone to silencing. This strain makes use of a parS site placed between the lacZ structural gene and its promoter, yet the ParB protein alone or ParB with wild-type ParA had very little effect on lacZ expression (Table 7). We conclude that the parS site in this context is unable to act as an efficient site for ParB to load and spread to the adjacent DNA. Thus, regional silencing by ParB alone is not a universal phenomenon. It likely depends on the context of the parS site. Control experiments using the strain of Rodionov et al. showed strong regional silencing in the presence of ParB alone, as originally shown (24) (Table 7).
TABLE 7.
Comparison of cat and lacZ gene activities
| Resident plasmid | par proteins supplied | Relative cat activitya
|
Induced relative β-galactosidase activityb
|
||
|---|---|---|---|---|---|
| Strain CC4250 | Strain CC4253 | Strain CC4250 | Strain CC4253 | ||
| pGB2 vector | None | 1 | 1 | 1 | 1 |
| pALA2319 | ParB | 0.55 | 0.16 | 0.65 | 0.02 |
| pALA2306 | ParA ParB | 0.39 | 0.16 | 0.62 | 0.07 |
| pALA2308 | ParAM314I ParB | 0.18 | 0.10 | 0.04 | 0.01 |
Strain CC4250 is a derivative of the transcription block strain CC4248 that has a cat gene inserted downstream of lacZ (Fig. 1). Strain CC4253 is a rec+ variant of the regional silencing strain of Rodionov et al. (24), BR6903. All values were normalized to the value obtained with the relevant strain (CC4250 or CC4253) when the pGB2 vector was present. Values are averages of three experiments. The cat assays were carried out as described in Materials and Methods. No IPTG was added so that the lacZ gene of strain CC4250 was uninduced.
The β-galactosidase values for CC4250 pGB2 and CC4253 pGB2 were 247 and 386 Miller units, respectively.
Does regional silencing occur at all in the transcription block strain? We measured the activity of a cat gene expressed from its own promoter placed some 3.5 kb from the parS site (strain CC4250; Fig. 1) and found that cat expression was not silenced significantly in the transcription block strain when ParB alone or when wild-type ParA and ParB were supplied. However, regional silencing was seen under conditions similar to those that generate the transcription block itself, i.e., when ParB was present with the mutant protein, ParAM314I (Table 7). Controls showed that the Radionov et al. strain (CC4253; Fig. 1) exhibited silencing of the cat gene with ParB alone as originally shown (24). The cat expression in this construct was not much affected by the presence of ParA or ParAM314I (Table 7). We conclude that regional silencing does occur in the transcription block strain, but like the transcription block itself, it requires both ParA and ParB and one of the Par proteins must contain a parPD mutation.
DISCUSSION
We have demonstrated that the ParAM314I mutant protein can block transcription through parS. This block was dependent on the presence of the parS binding protein, ParB. Thus, the mutant ParA and wild-type ParB proteins appear to form a specific complex at parS that is present through all or most of the cell cycle and is stable enough to prevent the frequent progression of RNA polymerase through the parS site. The finding that secondary mutations in either parA or parB can reverse this block is consistent with this conclusion. The parAQ12STOP chain-terminating mutation and the parBM1I start codon change are likely to be null mutations in parA and parB, respectively, reflecting a requirement both for the mutant ParA protein and the wild-type ParB protein for a transcription block.
The wild-type ParA-ParB-parS complex does not participate in an efficient block and effects lacZ expression only slightly when ParA and ParB are present at high levels. We have previously suggested that wild-type ParA participates in a complex at parS only transiently during the cell cycle and that the ParAM314I mutant protein is blocked at some stage in the partition process that locks it permanently into the complex (28). Our current observations are consistent with this and provide further evidence for the existence and nature of this “locked” complex. The complex clearly requires both ParA and ParB proteins to be present in a complex bound to parS. The requirement for ParA for the transcription block is most clearly shown in the case of the parBT12P mutant, where an in-frame deletion within ParA diminishes the transcription block.
In order for the ParA-ParB-parS complex to block transcription, one of the proteins needs to contain a parPD mutation. Three of the seven effective mutations lie within regions known to be involved in a ParA-ParB interaction. Two of these are in the carboxy-terminal region of ParA, and one is on the amino terminus of ParB (Fig. 2). We suggest that these changes stabilize the ParA-ParB interaction, preventing the proteins from dissociating from each other and from the parS site at a critical step in the partition process. We are presently investigating the binding properties of the mutant proteins to see if they promote formation of a stable ParA-ParB-parS complex in vitro.
Regional silencing occurs in the transcription block strain, but only under conditions similar to the transcription block itself: it requires ParA, ParB, and a parPD mutation. We suggest that the properties of the parS site can be affected by the surrounding DNA. In the Rodionov et al. configuration (Fig. 1), the parS site can act as a loading site for wild-type ParB in the absence of ParA. This presumably allows ParB to spread to the adjacent DNA to promote silencing. It is not clear whether a stable complex is formed on the parS sequence itself under these conditions. In contrast, ParB alone has very little effect on parS or the surrounding DNA in the transcription block configuration as constructed here. The parS site does not act as an efficient loading site for ParB alone, and only a modest effect is seen on lacZ or cat expression. However, in the presence of both ParA and ParB and when one of these proteins contains a parPD mutation, a stable complex that blocks transcription through the site is formed at parS. This stable complex can now act as a loading site for ParB to spread to the adjacent DNA so that partial silencing of distant genes can occur.
Two general principles are implied. First, ParA in conjunction with ParB is capable of forming a specific stable complex at parS when one of the proteins is distorted by mutation. These mutations likely enhance an interaction that wild-type ParA normally exhibits; i.e., they stabilize the ParA interaction with ParB and/or the partition site and thereby prevent transcription from traversing parS. Second, regional silencing by ParB alone or by wild-type ParA and ParB at physiological concentrations is not a universal phenomenon. Which context results in a parS site with properties that most resemble the active site in the actively partitioning plasmid? It is unclear. However, genes in the vicinity of the parS site cannot be constantly silenced in P1 or mini-P1 plasmids that are properly partitioned. Otherwise, we could not follow the markers they carry and they could not replicate due to the requirement for expression of the adjacent rep gene. Some plasmids containing parS do appear to be subject to silencing. In these cases, the site doesn't function for plasmid partition and the plasmid is rapidly lost when the Par proteins are present (16). Thus, the properties of our integrated parS site may be closer to that of the functional plasmid parS than that present in the Rodionov et al. (24) silencing strain.
Acknowledgments
This work was supported in part by a National Institutes of Health National Service Award Fellowship (No. 5 F32 GM16971) to J.A.S.
We thank Oleg Rodionov, Helen Wilson, Michael Yarmolinsky, and Jian-guang Zhou for providing strains and details of the silencing construct found in CC4253. We thank Don Court for critically reading the manuscript.
REFERENCES
- 1.Austin, S., F. Hart, A. Abeles, and N. Sternberg. 1982. Genetic and physical map of a P1 miniplasmid. J. Bacteriol. 152:63-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolivar, F., R. L. Rodriguez, P. J. Green, M. D. Betlach, H. W. Boyer, J. H. Crosa, and S. Falkow. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95-113. [PubMed] [Google Scholar]
- 3.Bouet, J. Y., and B. E. Funnell. 1999. P1 ParA interacts with the P1 partition complex at parS and an ATP-ADP switch controls ParA activities. EMBO J. 18:1415-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brendler, T., A. Abeles, and S. Austin. 1995. A protein that binds to the P1 origin core and the oriC 13mer region in a methylation-specific fashion is the product of the host seqA gene. EMBO J. 14:4083-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davey, M. J., and B. E. Funnell. 1994. The P1 plasmid partition protein ParA: a role for ATP in site-specific binding. J. Biol. Chem. 269:29908-29913. [PubMed] [Google Scholar]
- 6.Davis, M. A., and S. J. Austin. 1988. Recognition of the P1 plasmid centromere analog involves binding of the ParB protein and is modified by a specific host factor. EMBO J. 7:1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis, M. A., K. A. Martin, and S. J. Austin. 1992. Biochemical activities of the ParA partition protein of the P1 plasmid. Mol. Microbiol. 6:1141-1147. [DOI] [PubMed] [Google Scholar]
- 8.Davis, M. A., L. Radnedge, K. A. Martin, F. Hayes, B. Youngren, and S. J. Austin. 1996. The P1 ParA protein and its ATPase activity play a direct role in the segregation of plasmid copies to daughter cells. Mol. Microbiol. 21:1029-1036. [DOI] [PubMed] [Google Scholar]
- 9.Davis, M. A., R. W. Simons, and N. Kleckner. 1985. Tn10 protects itself at two levels from fortuitous activation by external promoters. Cell 43:379-387. [DOI] [PubMed] [Google Scholar]
- 10.Doheny, K. F., P. K. Sorger, A. A. Hyman, S. Tugendreich, F. Spencer, and P. Hieter. 1993. Identification of essential components of the S. cerevisiae kinetochore. Cell 73:761-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman, S., K. Martin, and S. Austin. 1986. The partition system of the P1 plasmid, p. 285-295. In S. Levy and R. Novick (ed.), Banbury report 24: antibotic resistance genes: ecology, transfer, and expression. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 12.Friedman, S. A., and S. J. Austin. 1988. The P1 plasmid-partition system synthesizes two essential proteins from an autoregulated operon. Plasmid 19:103-112. [DOI] [PubMed] [Google Scholar]
- 13.Funnell, B. E. 1988. Participation of Escherichia coli integration host factor in the P1 plasmid partition system. Proc. Natl. Acad. Sci. USA 85:6657-6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes, F., and S. Austin. 1994. Topological scanning of the P1 plasmid partition site. J. Mol. Biol. 243:190-198. [DOI] [PubMed] [Google Scholar]
- 15.Hayes, F., L. Radnedge, M. A. Davis, and S. J. Austin. 1994. The homologous operons for P1 and P7 plasmid partition are autoregulated from dissimilar operator sites. Mol. Microbiol. 11:249-260. [DOI] [PubMed] [Google Scholar]
- 16.Lobocka, M., and M. Yarmolinsky. 1996. P1 plasmid partition: a mutational analysis of ParB. J. Mol. Biol. 259:366-382. [DOI] [PubMed] [Google Scholar]
- 17.Lynch, A. S., and J. C. Wang. 1995. SopB protein-mediated silencing of genes linked to the sopC locus of Escherichia coli F plasmid. Proc. Natl. Acad. Sci. USA 92:1896-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, J. R. 1992. A short course in bacterial genetics: a laboratory manual for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Nordstrom, K., and S. J. Austin. 1989. Mechanisms that contribute to the stable segregation of plasmids. Annu. Rev. Genet. 23:37-69. [DOI] [PubMed] [Google Scholar]
- 20.Pal, S., R. J. Mason, and D. K. Chattoraj. 1986. P1 plasmid replication. Role of initiator titration in copy number control. J. Mol. Biol. 192:275-285. [DOI] [PubMed] [Google Scholar]
- 21.Prentki, P., M. Chandler, and L. Caro. 1977. Replication of the prophage P1 during the cell cycle of Escherichia coli. Mol. Gen. Genet. 152:71-76. [DOI] [PubMed] [Google Scholar]
- 22.Radnedge, L., M. A. Davis, and S. J. Austin. 1996. P1 and P7 plasmid partition: ParB protein bound to its partition site makes a separate discriminator contact with the DNA that determines species specificity. EMBO J. 15:1155-1162. [PMC free article] [PubMed] [Google Scholar]
- 23.Radnedge, L., B. Youngren, M. Davis, and S. Austin. 1998. Probing the structure of complex macromolecular interactions by homolog specificity scanning: the P1 and P7 plasmid partition systems. EMBO J. 17:6076-6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodionov, O., M. Lobocka, and M. Yarmolinsky. 1999. Silencing of genes flanking the P1 plasmid centromere. Science 283:546-549. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 26.Sternberg, N., and S. Austin. 1983. Isolation and characterization of P1 minireplicons, λ-P1:5R and λ-P1:5L. J. Bacteriol. 153:800-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surtees, J. A., and B. E. Funnell. 1999. P1 ParB domain structure includes two independent multimerization domains. J. Bacteriol. 181:5898-5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youngren, B., and S. Austin. 1997. Altered ParA partition proteins of plasmid P1 act via the partition site to block plasmid propagation. Mol. Microbiol. 25:1023-1030. [DOI] [PubMed] [Google Scholar]
- 29.Yu, D., and D. L. Court. 1998. A new system to place single copies of genes, sites, and lacZ fusions on the Escherichia coli chromosome. Gene 223:77-81. [DOI] [PubMed] [Google Scholar]
- 30.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]