Abstract
Bacterial biofilms impair the operation of many industrial processes. Deinococcus geothermalis is efficient primary biofilm former in paper machine water, functioning as an adhesion platform for secondary biofilm bacteria. It produces thick biofilms on various abiotic surfaces, but the mechanism of attachment is not known. High-resolution field-emission scanning electron microscopy and atomic force microscopy (AFM) showed peritrichous adhesion threads mediating the attachment of D. geothermalis E50051 to stainless steel and glass surfaces and cell-to-cell attachment, irrespective of the growth medium. Extensive slime matrix was absent from the D. geothermalis E50051 biofilms. AFM of the attached cells revealed regions on the cell surface with different topography, viscoelasticity, and adhesiveness, possibly representing different surface layers that were patchily exposed. We used oscillating probe techniques to keep the tip-biofilm interactions as small as possible. In spite of this, AFM imaging of living D. geothermalis E50051 biofilms in water resulted in repositioning but not in detachment of the surface-attached cells. The irreversibly attached cells did not detach when pushed with a glass capillary but escaped the mechanical force by sliding along the surface. Air drying eliminated the flexibility of attachment, but it resumed after reimmersion in water. Biofilms were evaluated for their strength of attachment. D. geothermalis E50051 persisted 1 h of washing with 0.2% NaOH or 0.5% sodium dodecyl sulfate, in contrast to biofilms of Burkholderia cepacia F28L1 or the well-characterized biofilm former Staphylococcus epidermidis O-47. Deinococcus radiodurans strain DSM 20539T also formed tenacious biofilms. This paper shows that D. geothermalis has firm but laterally slippery attachment not reported before for a nonmotile species.
Bacteria growing as biofilms are more resistant to many antimicrobial agents than free-swimming bacteria, a characteristic of the biofilms resulting in, e.g., persistent infections of the human body and troublesome biofilms in water distribution systems and in industrial processes (7, 17, 20, 32). Paper mills use biocides to control microbial growth, but this has not eliminated biofilms, which may detach from surfaces, impairing operation of the machines or causing defects such as holes and colored spots in the paper products (4, 16, 29, 30). Not all bacteria form biofilms; some machines run with 105 to 108 CFU of free-swimming bacteria ml of circulating water−1 with no process disturbances. We recently showed Deinococcus geothermalis to be an efficient primary biofilm former in paper machine water (30), functioning as an adhesion platform for secondary biofilm bacteria, e.g., Bacillus species (16). D. geothermalis forms thick biofilms on various abiotic surfaces, such as stainless steel and polystyrenes (16), glass, and polyethene, but the mechanism of attachment is not known.
D. geothermalis possesses no flagella or pili for attachment (11) and does not produce large amounts of slime. Bacteria of the genus Deinococcus, including the well-studied D. radiodurans, are known to be highly resistant towards radiation and desiccation (18). According to Makarova et al. (18), it is likely that the extreme radiation resistance evolved in response to chronic exposure to nonradioactive forms of DNA damage, readily inflicted by, e.g., nonstatic environments such as cycles of desiccation and hydration. Such conditions often prevail in biofilms near the air-water interface. Orthologs of almost all known genes involved in different stress responses in other bacteria are present in D. radiodurans (18). All of these properties can contribute to the potential of D. geothermalis for biofouling. We investigated the strength of D. geothermalis attachment and show that the biofilms persisted despite alkali and detergent washings. Microscopic methods revealed that the surface-attached D. geothermalis cells can escape forces applied to remove the cells from the surfaces. The cells escape without detaching, displaying a firm but slippery attachment not reported before for a nonmotile species.
MATERIALS AND METHODS
Bacterial strains.
Isolation of D. geothermalis E50051 and Burkholderia cepacia F28L1 was described previously (30). Staphylococcus epidermidis O-47 (24) was a gift from Friedrich Götz, Tübingen, Germany. D. radiodurans DSM 20539T was obtained from Deutsche Sammlung von Microorganismen und Zellkulturen GmbH, Braunschweig, Germany. The strains were stored at −20°C in 50% (vol/vol) glycerol and resuscitated on tryptic soy agar (Becton Dickinson, Sparks, Md.) at 37°C. Inocula (0.5%, vol/vol) were grown in tryptic soy broth (Becton Dickinson) on a rotary shaker (160 rpm, 37°C, 1 day). D. geothermalis E50051 has been deposited in the culture collection of the Faculty of Agriculture and Forestry, University of Helsinki, as D. geothermalis HAMBI 2411.
Durability test for biofilms.
D. geothermalis E50051 has been shown to produce similar biofilms on stainless steel and polystyrene surfaces (16). Therefore, it was convenient to grow the biofilms in 96-well tissue culture-treated polystyrene plates (Nunclon 167008; Nalge Nunc International, Roskilde, Denmark). Each well, holding 250 μl of tryptic soy broth (0.3 or 3% [wt/vol] dry matter), was inoculated to 0.5% (vol/vol). The plates were incubated for 1 day at 37°C with agitation at 160 rpm (except for S. epidermidis O-47), emptied, and rinsed with 0.9% NaCl. The wells with biofilm were filled with 300 μl of tap water containing NaOH (0.01, 0.1, or 0.2%, wt/vol) or sodium dodecyl sulfate (SDS) (0.2 or 0.5%, wt/vol). Tap water alone was used as a negative control. The plates were shaken (120 rpm, 37°C, 1 h), emptied, and rinsed with water. The biofilm remaining in the well was stained with 330 μl of crystal violet (4 g liter−1 in 20% [vol/vol] methanol) for 5 min, and the nonabsorbed stain was removed by washing three times under a running water tap. Biofilm-bound stain was dissolved in ethanol (330 μl per well, 1 h) and the A595 was measured with a plate reader (iEMS Reader MF; Labsystems, Helsinki, Finland).
FESEM.
In comparison to conventional scanning electron microscopy, in field-emission scanning electron microscopy (FESEM) the use of a field-emission electron source provides improved spatial resolution, the ability to operate at lower electron-accelerating voltages minimizes sample charging and damage, and the reduced penetration of the electrons gives better image of the immediate biological surface (22). For FESEM, the disks (39 mm2) of stainless steel AISI 316 were polished to 1,000 grit with a series of water sanding papers and degreased with acetone. The disks were mounted in a vertical position to walls of high-density polyethene flasks and disinfected, and biofilms grown on the disks as described elsewhere (16). Paper machine circulating water (white water [WW]) medium (16) or R2 broth (similar to R2A from BBL) (containing [per liter] yeast extract, 0.5 g; peptones, 1 g; dextrose, 0.5 g; soluble starch, 0.5 g; K2HPO4, 0.3 g; MgSO4, 0.024 g; and sodium pyruvate, 0.3 g) at pH 7 were used as the media. The disks with biofilms were rinsed three times with phosphate buffer (0.1 M, pH 7.5); fixed with 2% (vol/vol) glutaraldehyde (TAAB, Reading, United Kingdom) in the buffer for 2 h; rinsed three times with the buffer; dehydrated in a series of 50, 70, 94, and 100% ethanol (three times for 5 min each); dried in hexamethyldisilazane (Fluka, Buchs, Switzerland) for 20 min; and sputter coated with platinum for 7 s. The biofilms were examined with a Hitachi S-4300 FESEM at an angle of 0 or 60° and at voltages of 2 to 10 keV.
AFM.
This study utilized the oscillating probe techniques of atomic force microscopy (AFM) instead of the conventional contact mode of operation. In the contact mode a sharp tip is scanned over a sample while a constant interatomic force between the tip and the sample surface is maintained, and the movement of the tip in the z direction is measured and converted to a topography image. The contact mode imaging exerts forces large enough to distort delicate specimens. Oscillating the scanning probe during imaging reduces the destructive forces and enhances sensitivity and reproducibility (13). The oscillating probe techniques of this study used an acoustic wave (AAC mode) or an external magnetic field (MAC mode) to drive the cantilever at resonance frequency, and the proximity of the sample surface was revealed by changes in the cantilever amplitude. The system was operated in constant-amplitude mode, meaning that the oscillating tip approached the surface until a given amplitude reduction was reached during each period of the oscillation. The topography images in this study resulted from the cantilever movement in the z direction. The amplitude images resulted from the feedback loop required to keep the amplitude reduction constant. The phase images show the phase shift that occurred between the free oscillation of the tip and the oscillation during surface touching, with the latter depending on the viscoelastic properties of the sample surface and on the adhesive potential between the sample and the tip.
Biofilms were grown in WW or R2 broth (1 day, 45°C, 160 rpm) on autoclaved coverglasses or on pieces of AISI 316 stainless steel (3 by 3 cm; polished to 600 grit, washed with detergent, degreased with acetone, and rinsed with sterile water). Biofilms were rinsed with distilled water and either imaged with a PicoSPM scanning probe microscope (Molecular Imaging Corp., Phoenix, Ariz.) in distilled water or allowed to air dry for 15 min and imaged in air. Air-dried biofilms were imaged in AAC mode with Pointprobe type FM-W silicon cantilevers (Nanosensors, Wetzlar, Germany). Free cantilever oscillation amplitudes ranged from 50 to 200 nm, and images were recorded at amplitude damping of 5 to 20%.
The MAC mode has advantage for measurements in liquid environments due to the direct drive of the cantilever tip via magnetic field (13), eliminating the need to drive the cantilever mounting mechanism. This increases the cantilever control, enabling operation at much smaller amplitudes (13). Consequently, the vertical force influencing the sample during the imaging is lower, thus reducing surface deformations, a crucial factor with soft organic materials. MAC mode imaging was performed with MAClever type II cantilevers (Molecular Imaging). Free cantilever oscillation amplitudes for imaging in liquid ranged from 5 to 50 nm, and images were recorded at amplitude damping of 2 to 5%.
Probing of biofilms with a micromanipulator.
Biofilms were grown in R2 broth (1 day, 45°C, 160 rpm) on sterile glass (Lab-Tek 178565 two-chambered coverglass for tissue culture; Nunc Inc., Naperville, Ill.), rinsed, submerged in sterilized tap water, and imaged with an inverted Zeiss Axiovert 100 microscope with a 40× phase objective (Zeiss LD Achroplan; numerical aperture, 0.6). A Micromanipulator 5171 (Eppendorf, Hamburg, Germany) with an Eppendorf Femtotips 5242 glass capillary and an Eppendorf Transjector 5246 was used to probe the surface-attached cells. Images were captured with a SenSys digital camera (Photometrics, Tucson, Ariz.) and Image Pro Plus software version 4.1 (Media Cybernetics, Silver Spring, Md.).
Motility analysis.
Soft R2A plates (0.25% agar) were inoculated by piercing with a platinum rod and incubated at 37°C for 3 days, and spread zones were measured. B. cepacia F28L1 and S. epidermidis O-47 served as motile and nonmotile controls. Motility was analyzed also by time lapse microscopy. Biofilm grown on a coverglass with photoetched grids (Electron Microscopy Sciences, Fort Washington, Pa.) was rinsed with sterilized tap water and sealed with a coverslip. A living biofilm colony in tap water was photographed every 40 min for 6 h with a Nikon Eclipse E800 microscope using a 100× oil immersion objective (Plan Fluor; numerical aperture, 1.3) and phase illumination (only during photography to avoid heat damage). Movement of the cells was measured from the photographs.
RESULTS
Durability of biofilms to cleaning.
The tenacity of biofilms of D. geothermalis E50051 grown in the wells of microtiter plates was compared to that of biofilms of D. radiodurans DSM 20539T, B. cepacia F28L1, and S. epidermidis O-47 with different washing procedures. The results in Table 1 show that the 1-day-old biofilms formed by the paper machine strain D. geothermalis E50051 were not detectably removed from the polystyrene surface by 1 h of washing with 0.2% NaOH. The concentration of SDS required for partial (29%) removal was 0.5%. Biofilms of D. radiodurans strain DSM 20539T were also partially (28%) removed by 0.5% SDS, but 0.1% NaOH also caused partial (72%) removal. Biofilms of the paper machine biofouling strain B. cepacia F28L1 were partially (39%) removed by washing with 0.1% NaOH and almost completely removed by 0.2% SDS. Biofilms of the clinical strain S. epidermidis O-47, a well-studied biofilm former, were removed by the lowest tested concentrations of both NaOH (0.01%) and SDS (0.2%). Biofilms were also grown on surfaces of glass tubes, washed with 0.15% NaOH or 0.5% SDS for 1 h, stained with crystal violet, and inspected visually. Biofilms of S. epidermidis O-47 and B. cepacia F28L1 were partially or completely removed from the glass surface, whereas biofilms of D. geothermalis E50051 were not visibly removed, similar to the results with the polystyrene surfaces shown in Table 1. The results thus indicate firm attachment of D. geothermalis E50051 to abiotic surfaces as well as firm cell-to-cell attachment in biofilms. The inefficacy of the 1-h washing with 0.2% NaOH at 37°C in removing any biofilm of D. geothermalis E50051 indicates that the alkaline washing routinely used in process industry, e.g., in paper machines, likely fails to clean surfaces biofouled with D. geothermalis E50051. The biofilms of D. radiodurans DSM 20539T also resisted the washing procedures, with only partial removal of the biofilms. D. geothermalis and D. radiodurans thus shared the ability to generate biofilms more recalcitrant than biofilms of B. cepacia F28L1 or S. epidermidis O-47 to NaOH or detergent washings.
TABLE 1.
Durability of biofilms grown in wells of microtiter platesa
| Biofilm-forming strain | Quantity of biofilm (A595) persisting after treatment for 1 h withb:
|
|||||
|---|---|---|---|---|---|---|
| Tap water only | NaOH (%, wt/vol)
|
SDS (%, wt/vol)
|
||||
| 0.01 | 0.1 | 0.2 | 0.2 | 0.5 | ||
| D. geothermalis E50051 | 1.44 ± 0.05 | 1.35 ± 0.04 | 1.48 ± 0.13 | 1.47 ± 0.07 | 1.47 ± 0.17 | 1.05 ± 0.09 |
| D. radiodurans DSM 20539T | 2.79 ± 0.52 | 1.37 ± 0.18 | 0.77 ± 0.14 | 0.70 ± 0.11 | 2.88 ± 0.34 | 2.07 ± 0.61 |
| B. cepacia F28L1 | 0.76 ± 0.06 | 0.69 ± 0.08 | 0.46 ± 0.01 | 0.40 ± 0.01 | 0.13 ± 0.10 | 0.03 ± 0.01 |
| S. epidermidis O-47 | 0.85 ± 0.10 | 0.05 ± 0.01 | 0.03 ± 0.00 | 0.04 ± 0.01 | 0.11 ± 0.03 | 0.11 ± 0.03 |
The growth conditions required by the different strains for producing biofilms in 1 day at 37°C in the wells of tissue culture-treated polystyrene plates were as follows: 10× diluted tryptic soy broth with agitation 160 rpm for D. geothermalis and B. cepacia and nondiluted tryptic soy broth with agitation for D. radiodurans and with stagnant conditions for S. epidermidis.
Wells were filled with the test solution, agitated 120 rpm at 37°C for 1 h, and rinsed with tap water. The persisting biofilm was stained with crystal violet, and the biofilm-bound stain was dissolved in ethanol. Values are the average A595 ± standard deviation for six parallel wells. Background values from clean wells were subtracted before calculations.
Electron microscopy of D. geothermalis biofilms.
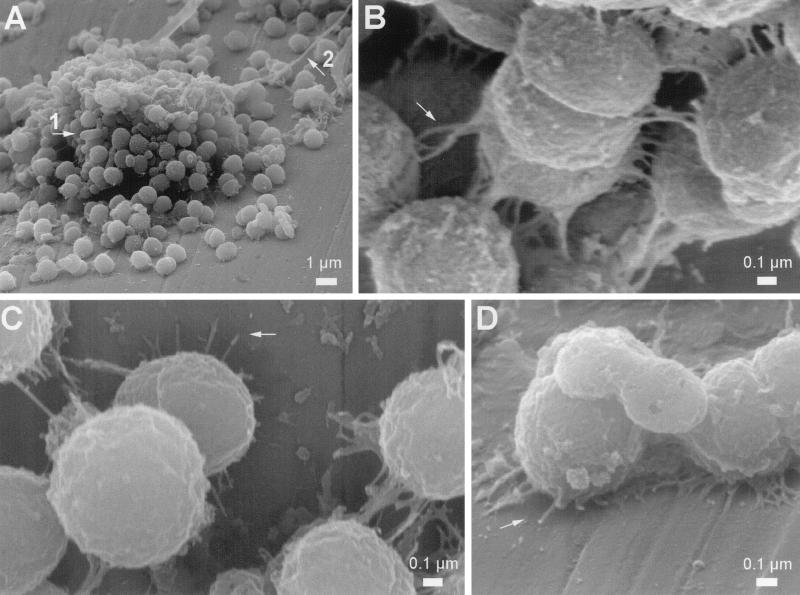
Attachment of D. geothermalis E50051 to AISI 316 stainless steel (the most common construction material for paper machines) and cell-to-cell attachment in the biofilms were examined by FESEM. Figure 1A shows a microcolony on polished stainless steel formed by a pure culture of D. geothermalis E50051 within 24 h in sterilized paper machine circulating water (WW) medium. In the deinococcal microcolonies, or in areas where these compact cell aggregates had grown together to form thicker biofilm (20 to 30 μm), the cells were not embedded in an extensive slime matrix (Fig. 1A to C). FESEM allowed high-resolution imaging of the Deinococcus cells, revealing that thin adhesion threads mediated the cell-to-cell attachment (Fig. 1B). Similar thin (diameter, 15 to 60 nm) threads connected the cells to the fine-polished (1,000 grit) stainless steel surface (Fig. 1C and D). The same result was obtained when the biofilms were grown in R2 broth instead of the WW medium, confirming that the adhesion threads observed by FESEM were produced by D. geothermalis E50051 and did not originate from paper machine fines present in the WW medium. Further studies revealed the adhesion threads to be composed of polysaccharides and of proteins sensitive to subtilisin-type proteases (J. Nuutinen, M. Kolari, and M. S. Salkinoja-Salonen, unpublished data).
FIG. 1.
FESEM analysis of D. geothermalis E50051 biofilms grown on polished (1,000 grit) stainless steel. Laboratory biofilms were grown in sterilized paper machine circulating water medium (1 day, 45°C, 160 rpm). The few rod-shaped dead bacteria and cellulose fibrils adhering to the growing deinococcal colonies (arrows 1 and 2 in panel A) originated from the heat-sterilized medium. Thin adhesion threads mediated the cell-to-cell attachment and connected the cells to the stainless steel surface (arrows in panels B, C, and D).
Imaging of adhesive structures by AFM.
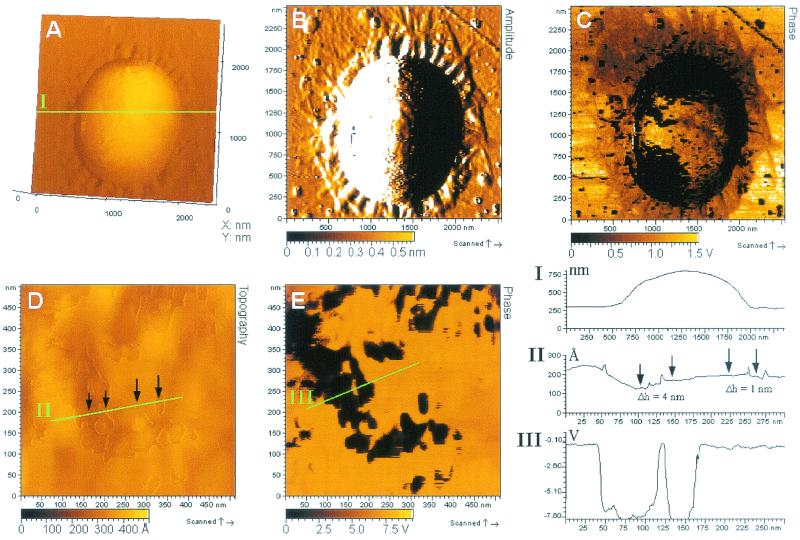
The adhesion threads in living biofilms of D. geothermalis E50051 were examined with AFM in the AAC noncontact mode. Biofilms grown on glass were allowed to air dry for 15 min, which enabled the imaging but kept the cells moist and alive. Figure 2A shows a topography image including a horizontal cross section from a surface-attached cell of D. geothermalis E50051, showing that the unfixed cells retained their spherical shape with a diameter of >1 μm in the short time for air drying. Adhesion threads surrounded the cell in a peritrichous manner. The fine structure of these adhesive threads was better visualized in the amplitude image (Fig. 2B), representing the error signal and thus revealing small changes in topography. The width of the adhesion threads at the periphery of the moist live cells was 120 to 180 nm, i.e., greater than was observed in the FESEM analysis (Fig. 1B to D). This indicates that the glutaraldehyde-fixed biofilms had undergone an extensive shrinkage during the intensive dehydration required for the FESEM. AFM images showed that the adhesion threads stretched out from the cells up to a distance of 5 μm. They were reduced in size with increasing distance from the mother cell, being 40 to 70 nm in width and 5 ± 1 nm in height near the distal end. The phase image (Fig. 2C) shows a phase difference between the adhesive threads and the underlying glass, indicative of different viscoelastic properties. The upper left corners of Fig. 2B and C show that the glass surface had almost continuous coverage by the adhesive material at some sites near the bacterial cell. The AFM phase imaging also revealed several areas of glass surface with no cells but with fragments of similar adhesive threads. These may represent footprints left by formerly detached cells.
FIG. 2.
AFM analysis of D. geothermalis E50051 cells attached on a glass surface. The biofilm was grown in R2 broth (1 day, 45°C, 160 rpm), rinsed with water, allowed to air dry for 15 min, and imaged in the AAC mode. (A, B, and C) Topography, amplitude, and phase images, respectively, of one attached cell. (D and E) Close-up topography and phase images, respectively, of the surface of the same cell. Green lines indicate the cut positions of the horizontal cross sections I, II, and III.
The amplitude image (Fig. 2B) and the phase image (Fig. 2C) show patchiness of the D. geothermalis E50051 cell surface. A close-up image from the same cell shows only slight changes in surface topography (Fig. 2D), whereas the corresponding phase image (Fig. 2E) reveals regions with high phase contrast. Cross sections II and III from the cell surface show that the lower regions in the topography image (Fig. 2D) correspond to the dark regions in the phase image, whereas the 1- to 4-nm-higher regions correspond to the bright regions in the phase image. The very large phase difference corresponding to only a small topographic height difference indicates regions differing in viscoelasticity of the cell surface material. It is possible that the higher regions represent a surface layer which had peeled off locally, exposing an inner surface layer of different material (dark areas in the phase images in Fig. 2C and E). Regular pentagonal structures of ca. 30 nm in diameter were detected in the inner surface layer (not shown in Fig. 2). These structures, which are only faintly visible due to the limitations caused by the spherical shape of the cells, may represent a surface of S-layer proteins on D. geothermalis E50051. All of the above-mentioned results were for biofilms grown on a glass surface. Similar adhesion threads, surface heterogeneity, and surface structures were observed in biofilms grown on stainless steel in R2 broth or on glass in WW medium, i.e., irrespective of the growth medium or the attachment surface.
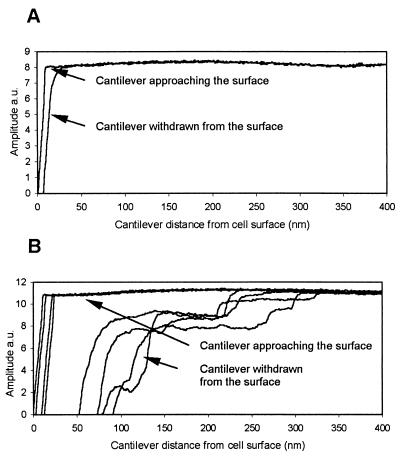
Adhesion forces of the different surface areas were probed with AFM by applying amplitude-versus-distance spectroscopy (Fig. 3). Each analysis location (n = 40) was selected based on the AFM phase image; the tip was driven in the vicinity of the sample surface and then withdrawn, with continuous monitoring for changes of the tip amplitude caused by surface forces. In 80% of the measurements a clear delay in detachment of the AFM tip from the surface of the black areas was detected (Fig. 3). This phenomenon was not observed with the bright areas (Fig. 3). This indicates that the black areas in the phase images were more adhesive towards the silicon tip. Surfaces of the attached Deinococcus cells were thus composed of layers differing in their adhesivity.
FIG. 3.
Amplitude-versus-distance curves representing the forces acting on the AFM tip as it approached and was withdrawn from the bright (A) and dark (B) areas of the cell surface of D. geothermalis E50051 visible in the AFM phase image (Fig. 2C). The approach of the tip towards the cell surface resulted in a similar amplitude change in both areas, whereas during withdrawal from the dark surface areas (panel B, four example curves) the tip experienced attractive forces, which retarded the return to the free oscillation amplitude. a.u., arbitrary units.
Slippery attachment of D. geothermalis.
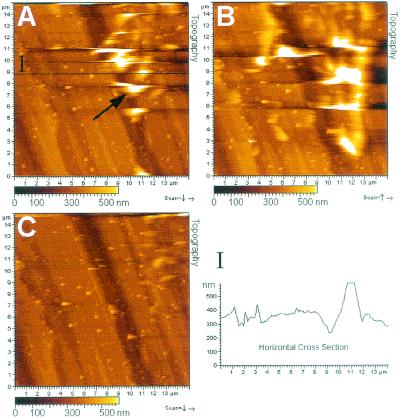
To study the adhesion threads in the fully hydrated state, live biofilms of D. geothermalis E50051 on stainless steel were examined in water using the MAC mode, i.e., magnetically driven noncontact mode AFM. The topography of the polished stainless steel surface was clearly visible (Fig. 4), similar to the case for the air-dried samples, but no sharp image of the attached D. geothermalis E50051 cells was obtained. Instead, the scanning caused movement of the cells, resulting in images where cells were seen as irregularly shaped white spots (Fig. 4A). Subsequent scannings (Fig. 4B and C) moved the cells ahead of the tip along the surface, repositioning the cells but not detaching them from the surface. When a larger area was rescanned, the cells reappeared for the duration of two or three scans but slid towards the sides and finally disappeared outside the scan area. The MAC mode was also used to image attached cells of D. geothermalis E50051 on a glass surface in water. Repositioning of the cells on the glass surface occurred similarly (images not shown) to what was observed on stainless steel (Fig. 4), indicating that the repositioning was not dependent on the substratum.
FIG. 4.
AFM analysis of D. geothermalis E50051 cells attached to stainless steel. The biofilm was grown in R2 broth (1 day, 45°C, 160 rpm) and imaged in the MAC mode in water. The cells are seen as white spots like the one indicated by an arrow in the topography image in panel A. Cross section I confirms the white spots as deinococcal cells. The images in panels A, B, and C are from three subsequent scannings at the same location and show that one scan repositioned the live cells by 1 to 5 μm (panel A versus panel B) but did not detach these cells from the surface. The next scan caused sliding of the cells outside the scan area (C).
The biofilms of D. geothermalis E50051 on stainless steel (Fig. 4) were next drained, allowed to air dry for 15 min, and then reexamined in the AAC mode in air. Images of the attached cells were sharp, similar to those in Fig. 2 showing the air-dried attached cells on glass. Continuous scanning caused no repositioning of the cells or changes in the cell surface morphology due to probe-cell interactions. The biofilm was then reimmersed in water, and the examination in the AAC mode was repeated. The attached cells in water withdrew from the scanning probe similarly to what was observed with the MAC mode before the air drying (Fig. 4). This indicates that short air drying deprived the adhesive polymers of their flexibility in a reversible manner, so that the flexibility was resumed after reimmersion in water. It is possible that dehydration caused shrinking and loss of flexibility of the adhesive fibrils, leading to increased lateral forces between the fibrils and structural heterogeneities of the substratum, thus yielding immobilization. The results also show that repositioning of the cells was not a magnetic effect, as it occurred in the two different imaging modes of AFM used. To summarize, the AFM results show that D. geothermalis E50051 attached to stainless steel and glass by a mechanism of slippery attachment, which allows the irreversibly attached cells to slide along the surface in response to external pushing forces.
Mechanical repositioning of attached cells.
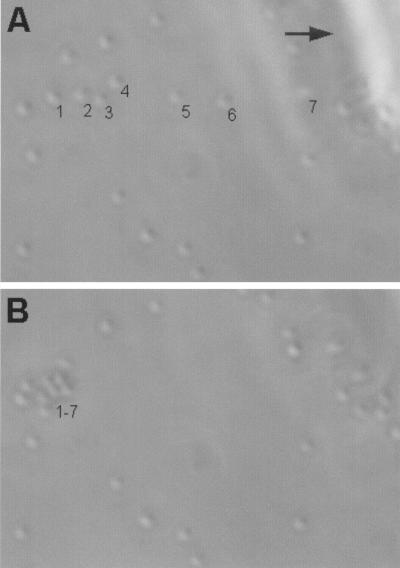
To scrutinize the slippery attachment of D. geothermalis E50051, the attached cells were probed with the glass capillary of a microinjector. Planktonic cells were removed from biofilms grown on glass surfaces by rinsing with sterilized tap water before microscopy. Brownian motion distinguished the few remaining planktonic cells or the reversibly adhering cells from the cells irreversibly attached to the glass surface. Figure 5 shows that mechanical pushing with tip of the glass capillary resulted in repositioning, but not in detachment, of the individual attached cells of D. geothermalis E50051. Repositioning of small cell clusters also occurred. The cells were next probed by placing the tip of the glass capillary adjacent to an individual cell and using a transjector to blow water towards the attached cell. No movement or detachment of the cells occurred. The applied capillary pressure was approximately 7 × 105 Pa, indicating that the lateral forces immobilizing D. geothermalis E50051 to the glass surface resisted a pressure difference beyond 7 × 105 Pa.
FIG. 5.
Phase-contrast light microscopy images of D. geothermalis E50051 cells attached on glass surface. (A) Positions of seven numbered cells on the surface (the arrow points to shadow of a glass capillary). (B) New positions of the same cells 1 to 7 after being pushed with the tip of the glass capillary. All seven pushed cells repositioned with no detachment from the surface, indicating a mechanism of slippery attachment. Panel B also shows that all untouched cells retained their positions. More images are shown at http://www.honeybee.helsinki.fi/users/mkolari/deino.html.
The spontaneous motility of D. geothermalis E50051 was assessed by 6 h of time lapse microscopy of a biofilm colony on glass. No migration (<1 μm) of the attached cells occurred. No spreading (<1 mm) of the deinococcal inoculum was observed on soft R2A plates in 72 h. No flagellum-like appendage was visible in the high-magnification FESEM (Fig. 1). The results confirm the nonmotility described as a taxonomic trait of D. geothermalis E50051. However, the present results demonstrate that it possesses a mechanism for firm but slippery attachment, allowing the nonmotile cells to slide over the surfaces to escape external pushing forces (Fig. 4 and 5).
DISCUSSION
This is the first report on the attachment mechanism of D. geothermalis. This gram-positive species is pink pigmented and includes strains that are efficient biofilm formers on abiotic surfaces (16, 30). It produces pink slimes that are detrimental in paper machines (16). We show here that D. geothermalis E50051 can attach to abiotic surfaces by a mechanism of slippery attachment not described before. This mechanism allowed lateral repositioning of the irreversibly attached, inherently nonmotile cells. We also show that D. geothermalis E50051 and the related species D. radiodurans strain DSM 20539T both formed biofilms that were much more durable towards washing with NaOH or anionic surfactant than biofilms formed by B. cepacia F28L1, a paper machine biofilm strain (30), or Staphylococcus epidermidis O-47, a gram-positive biofilm former isolated from a hospital patient (24). B. cepacia F28L1 was chosen as a reference because the species B. cepacia is one of the most frequent contaminants in paper machine water and slimes (29, 30). Biofilms of this species are also involved in persistent human infections (7). S. epidermidis is a notorious nosocomial human pathogen owing to its ability to form biofilm on nonliving materials implanted in the body (7, 15, 21). Intensive studies have shown, e.g., that cell-to-cell adhesion in the growing S. epidermidis biofilm is mediated by PIA, a polysaccharide intercellular adhesin (21, 24). Our results show that the mechanisms of surface attachment and cell-to-cell attachment in D. geothermalis E50051 and D. radiodurans DSM 20539T biofilms were more tenacious than the well-described mechanisms of S. epidermidis. These two Deinococcus species seem to share the ability to form tenacious biofilms, analogously to their sharing of resistance to radiation and desiccation (18).
Exocellular polymeric substances (EPS) are known to play an important role in the accumulation of biofilms (7, 15, 17, 20, 21, 32). The high-resolution FESEM and AFM images in this paper showed the presence of adhesive polymers on the outer surface of D. geothermalis E50051. In contrast to many biofilm studies showing cells embedded in an extensive slime matrix (1, 3, 5, 7, 15, 26, 28), the durable attachment of D. geothermalis E50051 was mediated by small-volume, highly adhesive, pseudopod-like structures of EPS. AFM phase images, expressing properties such as adhesiveness and stiffness, showed that the cell surface was locally heterogeneous in adhesivity, instead of showing even wrapping of the cells into the adhesive material. Auerbach et al. (1) studied unsaturated Pseudomonas putida biofilms and showed EPS mesostructures on the cell surfaces, but in their AFM phase images the cell surfaces appeared to be uniform.
AFM enables high-resolution imaging of bacterial biofilms, as recently reviewed by Dufrêne (8). Biofilms of sulfate-reducing bacteria (SRB), of different pseudomonads, and of acidophilic mine bacteria have been imaged with contact and noncontact mode AFM in air (2, 3, 8, 10, 26). Hydrated biofilms were first examined by Bremer et al. (5). They imaged attached bacterial cells with contact mode AFM in culture medium and observed no cell movement on the copper surface. A review by Beech (2) summarized that the bacterial EPS naturally immobilize the cells to the substratum, and hence there is no need for sample fixation in AFM studies of bacterial biofilms. In contrast, we report here a novel finding that high-resolution imaging of D. geothermalis biofilms in water is prevented because the mechanism of slippery attachment of D. geothermalis allows lateral movement of unfixed attached cells on abiotic surfaces in water. This laterally slippery attachment may protect the cells from detaching, when biofouled stainless steel surfaces are cleaned, e.g., by pressurized water. The flexibility of the attachment was reversibly lost and gained upon dehydration and rehydration. Bacteria of the genus Deinococcus are highly resistant to desiccation (18). The slippery attachment, the desiccation resistance, and the recalcitrance of D. geothermalis biofilms to washing with 0.2% NaOH together may confer a selective advantage for this species when making biofilms on surfaces with high hydraulic flow and varying wet, dry, and wash conditions, such as, e.g., in paper machines. The durability of D. geothermalis E50051 biofilms supports the view based on our earlier results (16) that this bacterium is an important primary biofilm former.
The cells were repositioned by AFM even though the imaging was performed in the MAC mode, which produces smaller shearing forces than the contact mode (13). Grantham and Dove (12) used AFM in the fluid tapping mode to examine attachment of Shewanella putrefaciens on iron-coated silica glass. The adherent cells of S. putrefaciens failed to withstand the low imaging forces in the fluid tapping mode and detached. We observed no detachment, indicating strong vertical adhesion forces between the D. geothermalis E50051 cells and the attachment substrata.
Vertical adhesion forces of bacteria have been quantified by using contact mode AFM (10, 23). Fang et al. (10) examined an air-dried biofilm of SRB on mica and measured adhesion forces in the range of −3.9 to −6.8 nN (attractive forces) between the silicon nitride tip and the cells. These vertical forces were insufficient to overcome the cell-substratum interfacial forces and to pull off any of the air-dried SRB cells from the mica surface. Similarly to Fang et al. (10), we observed attractive tip-cell forces locally on the surface of air-dried but moist cells of D. geothermalis, and these vertical forces did not pull off any cells from the glass or stainless steel surfaces. Razatos et al. (23) measured adhesion forces in water. They observed that increasing electronegativity of Escherichia coli cell surface mutants increased the repulsion to the silicon nitride tip, also negatively charged in water (23). These repulsive tip-cell forces caused no detachment or movement of the E. coli cells immobilized on polyethyleneimine-coated glass. When we examined live biofilms of D. geothermalis E50051 in water, the imaging forces caused lateral movement of the unfixed cells and prevented measurement of vertical adhesion forces with the hydrated biofilm. Thus, weaker forces than would be required for pulling off the cells from the substratum were sufficient to cause lateral movement of attached Deinococcus cells.
Our results conform to those of Busscher et al. (6), reasoning that from a physiochemical perspective, the detachment of adhering microbes can be very difficult, while lateral mobility can be relatively easy. The DLVO, or double-layer, theory equates the electrostatic forces and the attractive van der Waals forces acting on an adhering particle (17). Based on the DLVO theory, Busscher et al. (6) showed that microbes attach irreversibly due to the perpendicular interactions between the microbe and a surface but that on an ideally homogenous surface the microbe is never laterally immobilized. In reality, chemical and structural heterogeneities of the surface increase lateral interactions between the adhering microbes and yield immobilization (6). On a smooth surface the lateral interaction energies can be one order of magnitude smaller than the perpendicular adhesion energies (6). In accordance with this, air-dried SRB cells were not pulled off from the substratum by a perpendicular force of 105 N m−2 (10), whereas a lateral shearing stress, generated by fluid flow parallel to the surface, of only 0.1 to 54 N m−2 (depending on the substrata and species tested) has been sufficient to detach attached microbes (25).
Once in initial contact with a surface, microbes develop different types of attachment behaviors (17). Microbial adhesion can be reversible or irreversible, with the latter indicating attachment, where microbes can no longer move perpendicularly away from the surface (6, 17). Reversible adhesion of E. coli cells with residence times of over 2 min on a surface, a behavior called near-surface swimming, has been reported (31). Motile attachment behavior of Pseudomonas fluorescens allows the flagellated cells to move along surfaces in a semiattached condition within the hydrodynamic boundary layer, independent of the flow direction (17). It is assumed that most microbes become irreversibly attached only after a period of unstable, reversible adhesion, during which the cells can show motion (17). Vibrio cholerae and E. coli utilize the flagella to approach and spread across the surface, and anchoring onto the surface requires pili and possibly outer membrane proteins (21). Microbes can attach irreversibly while retaining active motility by mechanisms known as gliding, swarming, twitching, swimming, darting. and sliding (9, 17, 19, 21). Uropathogenic E. coli cells were shown to attach irreversibly and yet actively migrate along solid surfaces (14). In mature biofilms, the biofilm structures have generally been assumed to remain at the same location on a surface until they age and detach. This view was recently challenged by Stoodley et al. (27), who showed downstream migration for the ripple-like biofilm structures of mixed species growing in turbulent flow.
Despite these fascinating observations on different behaviors of attachment, that of nonmotile bacteria is still thought to involve a short residence time on the surface, after which the cells anchor themselves irreversibly in one location on the surface. D. geothermalis possesses no flagella or pili for active motility or attachment (11). We observed that D. geothermalis E50051 attaches irreversibly on abiotic surfaces by a mechanism of firm but slippery attachment mediated by adhesion threads. Friction between the exocellular adhesive material and the substratum was low enough to let the cells laterally escape external pushing forces. We propose to call this behavior on surfaces slippery attachment. It resembles the bacterial surface translocation mechanism called sliding (19) but differs in that the deinococcal cells do not move independently. Our results thus open a new viewpoint to the attachment of nonmotile bacteria to biofilms. It is too early to say whether this kind of slippery attachment is widespread, as this is the first study of attachment mechanisms in biofilms of a species representing the Deinococcus-Thermus phylum of eubacteria.
Acknowledgments
This work was supported by the Viikki Graduate School in Biosciences of Helsinki University, Kemira Chemicals Oy, the National Technology Agency (TEKES), and the Academy of Finland.
We acknowledge use of the Helsinki University electron microscopy facilities at the Institute of Biotechnology. We thank Pentti Väätänen, Juha Mentu, Jyrki Juhanoja, the Viikki Science Library, and the Faculty Instrument Centre for their services.
REFERENCES
- 1.Auerbach, I. D., C. Sorensen, H. G. Hansma, and P. A. Holden. 2000. Physical morphology and surface properties of unsaturated Pseudomonas putida biofilms. J. Bacteriol. 182:3809-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beech, I. B. 1996. The potential use of atomic force microscopy for studying corrosion of metals in the presence of bacterial biofilms. Int. Biodeterior. Biodegrad. 37:141-149. [Google Scholar]
- 3.Beech, I. B., C. W. S. Cheung, D. B. Johnson, and J. R. Smith. 1996. Comparative studies of bacterial biofilms on steel surfaces using atomic force microscopy and environmental scanning electron microscopy. Biofouling 10:65-77. [DOI] [PubMed] [Google Scholar]
- 4.Blanco, M. A., C. Negro, I. Gaspar, and J. Tijero. 1996. Slime problems in the paper and board industry. Appl. Microbiol. Biotechnol. 46:203-208. [Google Scholar]
- 5.Bremer, P. J., G. G. Geesey, and B. Drake. 1992. Atomic force microscopy examination of the topography of a hydrated bacterial biofilm on a copper surface. Curr. Microbiol. 24:223-230. [Google Scholar]
- 6.Busscher, H. J., A. T. Poortinga, and R. Bos. 1998. Lateral and perpendicular interaction forces involved in mobile and immobile adhesion of microorganisms on model solid surfaces. Curr. Microbiol. 37:319-323. [DOI] [PubMed] [Google Scholar]
- 7.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 8.Dufrêne, Y. F. 2001. Application of atomic force microscopy to microbial surfaces: from reconstituted cell surface layers to living cells. Micron 32:153-165. [DOI] [PubMed] [Google Scholar]
- 9.Eberl, L., S. Molin, and M. Givskov. 1999. Surface motility of Serratia liquefaciens MG1. J. Bacteriol. 181:1703-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang, H. H. P., K.-Y. Chan, and L.-C. Xu. 2000. Quantification of bacterial adhesion forces using atomic force microscopy. J. Microbiol. Methods 40:89-97. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira, A. C., M. F. Nobre, F. A. Rainey, M. T. Silva, R. Wait, J. Burghardt, A. P. Chung, and M. S. DaCosta. 1997. Deinococcus geothermalis sp. nov. and Deinococcus murrayi sp. nov., two extremely radiation-resistant and slightly thermophilic species from hot springs. Int. J. Syst. Bacteriol. 47:939-947. [DOI] [PubMed] [Google Scholar]
- 12.Grantham, M. C., and P. M. Dove. 1996. Investigation of bacterial-mineral interactions using fluid tapping mode atomic force microscopy. Geochim. Cosmochim. Acta 60:2473-2480. [Google Scholar]
- 13.Han, W., S. M. Lindsay, and T. Jing. 1996. A magnetically driven oscillating probe microscope for operation in liquids. Appl. Phys. Lett. 69:4111-4113. [Google Scholar]
- 14.Harkes, G., J. Dankert, and J. Feijen. 1992. Bacterial migration along solid surfaces. Appl. Environ. Microbiol. 58:1500-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussain, M., M. H. Wilcox, and P. J. White. 1993. The slime of coagulase-negative staphylococci: biochemistry and relation to adherence. FEMS Microbiol. Rev. 104:191-208. [DOI] [PubMed] [Google Scholar]
- 16.Kolari, M., J. Nuutinen, and M. S. Salkinoja-Salonen. 2001.. Mechanisms of biofilm formation in paper machine by Bacillus species: the role of Deinococcus geothermalis. J. Ind. Microbiol. Biotechnol. 27:343-351. [DOI] [PubMed] [Google Scholar]
- 17.Korber, D. R., J. R. Lawrence, H. M. Lappin-Scott, and J. W. Costerton. 1995. Growth of microorganisms on surfaces, p. 15-45. In H. M. Lappin-Scott and J. W. Costerton (ed.), Microbial biofilms. Plant and microbial biotechnology research series, 5. University Press, Cambridge, United Kingdom.
- 18.Makarova, K. S., L. Aravind, Y. I. Wolf, R. L. Tatusov, K. W. Minton, E. V. Koonin, and M. J. Daly. 2001. Genome of the extremely radiation-resistant bacterium Deinococcus radiodurans viewed from the perspective of comparative genomics. Microbiol. Mol. Biol. Rev. 65:44-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez, A., S. Torello, and R. Kolter. 1999. Sliding motility in mycobacteria. J. Bacteriol. 181:7331-7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morton, L. H. G., D. L. A Greenway, C. C. Gaylarde, and S. B. Surman. 1998. Consideration of some implications of the resistance of biofilms to biocides. Int. Biodeterior. Biodegrad. 41:247-259. [Google Scholar]
- 21.O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 22.Pawley, J. 1997. The development of field-emission scanning electron microscopy for imaging biological surfaces. Scanning 19:324-336. [PubMed] [Google Scholar]
- 23.Razatos, A., Y.-L. Ong, M. M. Sharma, and G. Georgiou. 1998. Molecular determinants of bacterial adhesion monitored by atomic force microscopy. Proc. Natl. Acad. Sci. USA 95:11059-11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rupp, M. E., P. D. Fey, C. Heilmann, and F. Götz. 2001. Characterization of the importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intravascular catheter-associated infection in a rat model. J. Infect. Dis. 183:1038-1042. [DOI] [PubMed] [Google Scholar]
- 25.Rutter, P. R., and B. Vincent. 1988. Attachment mechanisms in the surface growth of microorganism, p. 87-107. In M. J. Bazin and J. I. Prosser (ed.), Physiological models in microbiology. CRC Press, Boca Raton, Fla.
- 26.Steele, A., D. T. Goddard, and I. B. Beech. 1994. An atomic force microscopy study of the biodeterioration of stainless steel in the presence of bacterial biofilms. Int. Biodeterior. Biodegrad. 34:35-46. [Google Scholar]
- 27.Stoodley, P., Z. Lewandowski, J. D. Boyle, and H. M. Lappin-Scott. 1999. The formation of migratory ripples in a mixed species bacterial biofilm growing in turbulent flow. Environ. Microbiol. 1:447-455. [DOI] [PubMed] [Google Scholar]
- 28.Telegdi, J., Z. Keresztes, G. Pálinkás, E. Kálmán, and W. Sand. 1998. Microbially influenced corrosion visualized by atomic force microscopy. Appl. Phys. A 66:S639-S642.
- 29.Väisänen, O. M., E.-L. Nurmiaho-Lassila, S. A. Marmo, and M. S. Salkinoja-Salonen. 1994. Structure and composition of biological slimes on paper and board machines. Appl. Environ. Microbiol. 60:641-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Väisänen, O. M., A. Weber, A. Bennasar, F. A. Rainey, H.-J. Busse, and M. S. Salkinoja-Salonen. 1998. Microbial communities of printing paper machines. J. Appl. Microbiol. 84:1069-1084. [DOI] [PubMed] [Google Scholar]
- 31.Vigeant, M. A. S., and R. M. Ford. 1997. Interactions between motile Escherichia coli and glass in media with various ionic strengths, as observed with a three-dimensional-tracking microscope. Appl. Environ. Microbiol. 63:3474-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watnick, P., and R. Kolter. 2000. Biofilm, city of microbes. J. Bacteriol. 182:2675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]