Abstract
The flow of genes among prokaryotes plays a fundamental role in shaping bacterial evolution, and restriction-modification systems can modulate this flow. However, relatively little is known about the distribution and movement of restriction-modification systems themselves. We have isolated and characterized the genes for restriction-modification systems from two species of Salmonella, S. enterica serovar Paratyphi A and S. enterica serovar Bareilly. Both systems are closely related to the PvuII restriction-modification system and share its target specificity. In the case of S. enterica serovar Paratyphi A, the restriction endonuclease is inactive, apparently due to a mutation in the subunit interface region. Unlike the chromosomally located Salmonella systems, the PvuII system is plasmid borne. We have completed the sequence characterization of the PvuII plasmid pPvu1, originally from Proteus vulgaris, making this the first completely sequenced plasmid from the genus Proteus. Despite the pronounced similarity of the three restriction-modification systems, the flanking sequences in Proteus and Salmonella are completely different. The SptAI and SbaI genes lie between an equivalent pair of bacteriophage P4-related open reading frames, one of which is a putative integrase gene, while the PvuII genes are adjacent to a mob operon and a XerCD recombination (cer) site.
Restriction-modification (RM) systems are nearly ubiquitous among bacteria (both eubacteria and archaea). To date, the only complete bacterial genome sequences lacking candidate RM systems are from the obligate intracellular parasites Chlamydia and Rickettsia. Chlamydia trachomatis is the only known cellular organism that lacks an S-adenosyl-l-methionine (AdoMet) synthetase (63), and a type II RM system with separately active endonuclease and AdoMet-dependent methyltransferase proteins could be dangerous in this context. The other bacterial genomes have between 1 and 22 predicted RM systems (74, 75). Even bacteria with the smallest genomes, Mycoplasma and Ureaplasma, have made room for these systems. RM systems provide a defense against DNA bacteriophages, as revealed both by direct experiment and indirectly by the fact that many bacteriophages take specific countermeasures against RM systems (9). In addition to initiating the destruction of some foreign DNAs, RM systems may also promote recombination (6, 51) and improve the spread of some genes by separating them from linked deleterious alleles (4, 42), thus playing both positive and negative roles in modulating the flow of genes among prokaryotes. In the case of type II RM systems, selfish behavior also contributes to their ubiquity (24, 45, 46).
RM system ubiquity is consistent with the selectable phenotypes just summarized, but these phenotypes only help to explain why the systems are maintained once they have entered a given bacterium. It is less clear how these systems move throughout the microbial biosphere. To this end, it would be useful to track the movement of a given RM system by finding very closely related systems in different bacterial host genera. In a truly orthologous RM system pair, all genes in each system would have an ortholog in the other system, the systems would have the same specificity, and the relative gene positions would be conserved. Restriction endonucleases exhibit extreme structural and sequence diversity and are often unrelated to one another even in systems having related methyltransferases and regulatory proteins. For example, a recent BLAST alignment of the corresponding proteins from the PvuII and BamHI RM systems yielded expect scores of 7 × 10−15 for the methyltransferases and 4 × 10−9 for the regulatory C proteins (described below), but 5,156 for the endonucleases. One of the rare exceptions to this generalization is provided by the EcoRI and RsrI systems, from Escherichia coli and Rhodopseudomonas sphaeroides, respectively, which both cleave the sequence G∧AATTC at the indicated position. Their endonucleases are very closely related (50% identity), but their methyltransferases are not (30, 64, 65).
Within a given genus, there are many examples of fully orthologous RM systems in different species; we provide another such example in this report. However, there are very few known examples of such orthologous RM systems in two or more distinct host genera. One such rare case involves NgoPII from the eubacterium Neisseria gonorrhoeae and MthTI from the archaeon Methanobacterium thermoformicicum (48, 66). A second such example is provided by EcoHK31I from E. coli and EaeI from Enterobacter agglomerans (36).
We report here on a pair of orthologous RM systems from distinct genera, one of which is the PvuII system. The PvuII RM system is produced by a strain of the enteric gram-negative organism Proteus vulgaris and cleaves the sequence CAG∧CTG as shown (21). Its methyltransferase acts on the internal cytosine in that sequence (10), generating N4-methylcytosine (13). The regulation of this system has been studied and involves an autogenous regulatory protein with unusual properties, called C•PvuII (1, 68, 69, 73). PvuII is also the first RM system for which X-ray crystallography yielded structures for both the endonuclease and the methyltransferase (5, 16, 22). The methyltransferase, M•PvuII, is particularly interesting, because it is circularly permuted relative to the majority of AdoMet-dependent methyltransferases (19, 28, 39).
As described below, a search of the unfinished microbial genome database revealed an apparent RM system in Salmonella enterica Paratyphi A that is very closely related to the PvuII system. Although Proteus and Salmonella both belong to the family Enterobacteriaceae and show some evidence of past horizontal exchanges (52), they are well separated within that family, so the discovery of closely related RM systems (including closely related endonucleases) was unexpected.
We report here completion of the sequence characterization of the PvuII plasmid pPvu1, the isolation and characterization of the genes for the SptAI RM system, and sequence analysis of the genes for the previously identified SbaI RM system. We determine the basis for the inactivity of the SptAI RM system and discuss the fact that these systems are all adjacent to genes associated with genetic mobility.
MATERIALS AND METHODS
Strains and plasmids.
All work was done in E. coli TOP10 cells (InVitrogen, Carlsbad, Calif.), which have the genotype F− mcrA Δ(mrr-hsdRMS-mcrBC) φ80 lacZΔM15 ΔlacX74 deoR recA1 araΔ139 Δ(ara-leu)7697 galU galK rpsL (Strr), endA1 nupG. mcrBC+ host strains would have been killed by producing methyltransferases such as M•PvuII (10, 53, 55, 67). The plasmids used were based on the vectors pCRII-TOPO (InVitrogen), pKK232-8 (Pharmacia), and pACYC184 (15). Strains of Salmonella or Proteus were obtained from the American Type Culture Collection, and the strain numbers are given in each case.
Cloning the SptAI and SbaI RM systems.
We obtained S. enterica serovar Paratyphi A chromosomal DNA from American Type Culture Collection (Manassas, Va.; ATCC strain 9150) and used PCR to amplify the SptAI system. The oligonucleotide primers that were used matched the released S. enterica serovar Paratyphi A genome sequence: 5′-ATTAATGGTCCAATGGTGGGC and 5′-CCCGCTATTTTTAACGAAATAAGCC. The PCR product was ligated to pCRII-TOPO and introduced by transformation into E. coli strain TOP10. An insert-containing clone was fully sequenced with vector-specific primers.
Restriction and modification assays.
To test for in vitro activities of restriction endonucleases or modification methyltransferases, appropriate E. coli strains were grown overnight in Luria-Bertani (LB) medium, 1.5 ml was pelleted by centrifugation for 10 min at 15,000 × g, and the pellets were washed in the appropriate activity buffer. The pellets were resuspended in 5 ml of the same buffer for both restriction endonuclease methyltransferase assays: 10 mM Tris-HCl (pH 7.9), 50 mM NaCl, 1 mM dithiothreitol, and 100 μg of bovine serum albumin per ml. Cells were then opened by sonication in six 1-min pulses at maximal output with a Heat Systems-Ultrasonics model W185 sonifier with the cup horn probe. Cell debris was removed by centrifugation for 10 min at 15,000 × g. For digestion assays, various amounts of cell extract were incubated for 15 min at 37°C with 1 μg of bacteriophage λ DNA. Commercial PvuII endonuclease (5 U; New England Biolabs) was used as a positive control for endonuclease assays or to challenge modified DNAs in some protection assays. Digests were resolved on 1% agarose-Tris-borate-EDTA (TBE) gels and stained with ethidium bromide.
To test for in vivo activities of restriction endonucleases or modification methyltransferases, bacteriophage λvir was grown on appropriate E. coli strains. Typically, 5 ml of an overnight E. coli culture grown in MMLB (LB medium containing 10 mM MgSO4 and 0.2% [wt/vol] d-maltose) was pelleted and resuspended to an A600 of 0.5 in 10 mM MgSO4. The suspended cells were infected with ∼106 PFU of λvir. This mixture was incubated for 15 min at 37°C and then mixed with 2.5 ml of MMLB containing 0.8% agar and poured onto a fresh MMLB plate. After 6 h of growth at 37°C, the plates were overlaid with 5 ml of cold “λ Dil” (10 mM Tris-HCl [pH 7.5], 10 mM MgSO4) and incubated overnight at 4°C. Cell debris was pelleted from the pooled liquid, and the supernatant was stored with a few drops of CHCl3 at 4°C. After spot titering 10-μl portions of a dilution series to obtain approximate numbers of PFU per milliliter, triplicate plates were set up at phage concentrations chosen to give 50 to 500 plaques per plate. A fresh overnight culture (0.1 ml) of the appropriate E. coli strain was incubated with the appropriate amount of λvir for 15 min at 37°C and then spread onto MMLB plates. Plaques were counted after overnight incubation at 37°C.
CAT assays.
Assays for chloramphenicol acetyltransferase (CAT) activity used the Quan-T-CAT kit (Amersham-Pharmacia), following the manufacturer's instructions. Briefly, 225-μl samples are taken when the LB-grown culture reaches an A600 of 0.3, 0.4, and 0.5. Cells in these samples are pelleted, resuspended in 180 μl of 0.1 M Tris-HCl (pH 7.9), and are then disrupted by six freeze-thaw cycles with dry ice-ethanol and a 37°C bath. Disruption is followed by 10 min at 65°C to inactivate a deacetylating enzyme; CAT itself is stable in response to this treatment, which we confirmed by using purified CAT enzyme. After removal of cell debris by centrifugation (10 min at 15,000 × g), the extract is assayed for CAT activity and for protein content. Protein measurements used the Micro BCA kit (Pierce), with bovine serum albumin as the standard. The assay measures transfer of [3H]acetyl groups from [3H]acetyl-coenzyme A (CoA) (2 to 10 Ci/mmol, 0.5 μCi/assay) to biotinylated deoxychloramphenicol. The deoxychloramphenicol is then separated from unused acetyl-CoA by adherence to streptavidin-linked polystyrene beads, and incorporation is determined by liquid scintillation counting. Each measurement thus uses three independent extracts from the same culture.
Nucleotide sequence determination.
All nucleotide sequence determinations used the BigDye Terminator Cycle Sequencing Ready Reaction kit (ABI, Foster City, Calif.), following the manufacturer's instructions. Reaction products were resolved and characterized on an ABI Prism model 310 Genetic Analyzer. Oligonucleotide primers were typically ∼20 nucleotides long and were obtained from GenoSys-Sigma (The Woodlands, Tex.).
Nucleotide sequence accession number.
The GenBank accession number for the complete SptAI sequence is AF306456.
RESULTS
Discovery of the SptAI RM system and comparison to the PvuII RM system.
We routinely search the microbial genome database (www.ncbi.nlm.nih.gov/cgi-bin/Entrez/genom_table_cgi) by using the NCBI BLAST server (2, 29) in an attempt to find new members of the C protein family of transcriptional regulators among RM systems (3, 11, 27, 59, 68, 69, 72, 73). One such match, in S. enterica serovar Paratyphi A (Genome Sequencing Center, personal communication; genome.wustl.edu/gsc/Projects/bacterial/paratyphi/paratyphi.shtml), was flanked by methyltransferase and restriction endonuclease genes, both of which surprisingly showed very close relationship to another RM system cloned in this laboratory—PvuII (10). The new system was named SptAI in consultation with REBASE (60).
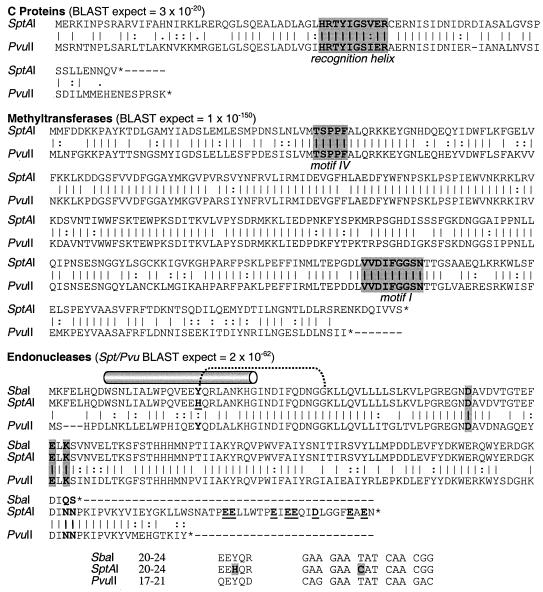
The lower two maps in Fig. 1 show that the bidirectional genetic organization of the PvuII and SptAI systems is conserved, with the methyltransferase gene diverging from the genes for the regulator and endonuclease (which are cotranscribed in the PvuII system) (73). Figure 2 compares the amino acid sequences of the corresponding open reading frames (ORFs) from these two systems. Ignoring the C-terminal gaps due to differing protein lengths, the two methyltransferases show 76% identity, while the two regulatory C proteins are identical at 60% of positions. Most striking is the high similarity between the endonuclease genes (58% identity with gaps only at the termini; 69% identity for the region between the gaps).
FIG. 1.
Comparison of the regions flanking three RM systems. The PvuII, SptAI, and EcoO109I systems are shown (gray ORFs), together with the flanking regions. The PvuII and SptAI systems are oriented so that their homologous genes are aligned, while the EcoO109I system is oriented so that the flanking bacteriophage P4-related genes are in the same orientation as those flanking the SptAI system.
FIG. 2.
Comparison of the SptAI and PvuII RM systems. Alignments of corresponding ORFs were generated by BLAST (2). The expect scores are the base 2 logarithms of the ratio of the number of matching residues to the total number of residues in the database (47); accordingly, these scores will change with the size of the database. A vertical line indicates identity; a colon indicates a conservative substitution. Regions of particular significance are shaded. For the C proteins, shading indicates the recognition helix of the predicted helix-turn-helix motif (69); for the methyltransferases, shading indicates the most highly-conserved of the nine sequence motifs (39); and for the endonucleases, shading indicates the amino acids involved in coordinating the Mg2+ ion required for catalysis (26). The endonuclease alignment also shows both the long α-helix (cylinder) that forms the subunit interface and the hydrogen bonding (dotted line) between the side chain hydroxyl of Tyr19 and the backbone carbonyl of Gly37 (in R•PvuII).
To confirm the nucleotide sequence, since the original is a survey sequence with only 2× coverage and no finishing, we obtained S. enterica serovar Paratyphi A chromosomal DNA from American Type Culture Collection (from the same strain as that used for the genomic sequence) and used PCR to amplify the SptAI system. An insert-containing clone was chosen and fully sequenced with vector-specific primers and primer “walking” (GenBank accession no. AF306456). Only one significant discrepancy was noted from the preliminary genomic sequence made available by the Genome Sequencing Center—a frameshift that would interrupt the putative regulatory C gene is not actually present. In the following sequence, the “T” in parentheses is present in the reported draft genome sequence, but is absent from our clone: 5′-CAAGGTCTG(T)TCTCAAGAAGCC. The corrected clone sequence was used for Fig. 1 and 2.
The PvuII and SptAI RM systems thus appear to be fully orthologous, suggesting that comparison of their genetic contexts would be informative with respect to the genetic mobility of an RM system. Before proceeding to such analysis, it remained to determine whether the SptAI system is active and, if so, whether it has the same specificity as the PvuII system.
Activities of the SptAI system.
We tested the activity of the SptAI clones in several ways, beginning by showing that the methyltransferase (M•SptAI) is active. First, we found that the purified plasmid clone DNA was itself resistant to PvuII digestion in vitro due to self-modification (protection of the pCRII-TOPO vector's four PvuII sites in vivo by its own insert-specified methyltransferase [data not shown]). Second, in the presence of AdoMet, extracts from cells carrying SptAI clones (but not from vector-bearing control strains) protected bacteriophage λ DNA from subsequent digestion with PvuII endonuclease (R•PvuII [data not shown]). Third, bacteriophage λvir grown on cells containing SptAI clones (but not those grown on vector-bearing control cells) plated with high efficiency on cells producing the PvuII RM system (Table 1). Together, these three results indicate that the SptAI RM system modifies DNA in such a way as to protect CAGCTG sequences from digestion by R•PvuII.
TABLE 1.
In vivo modification of bacteriophage λ by the SptAI RM system
| Plating straina | Apparent PFU/ml of lysate from strain carrying the plasmidb
|
|
|---|---|---|
| pBSH005 (none) | pMAN100 (SptAI) | |
| pBSH005 (none) | (6.5 ± 0.8) × 109 | (5.1 ± 1.5) × 109 |
| pPvuRM3.4CYC (PvuII) | (6.5 ± 1.2) × 106 | (1.3 ± 0) × 1010 |
| pMAN100 (SptAI) | (9.9 ± 1.1) × 109 | (1.4 ± 0.1) × 1010 |
| PvuII/none | 1.0 × 10−3 | 2.5 × 100 |
| SptAI/none | 1.5 × 100 | 2.7 × 100 |
One of two bacteriophage λ lysates was plated on each of these three strains. All strains are E. coli TOP10 (In Vitrogen) containing the indicated plasmid, specifying the RM system shown in parentheses. The strain with no RM system included an irrelevant E. coli gene (yhhK) cloned into the same vector as the SptAI system (pCRII [In Vitrogen]), yielding pBSH005. The strain producing PvuII carried a pACYC184-based plasmid clone.
Numbers indicate average PFU per milliliter of lysate (± standard deviation) from triplicate. The lysates were prepared on host strains containing either no RM system or the SptAI system, as indicated in parentheses in the column heading.
It should be noted that this protection against R•PvuII is consistent with the possibility, but does not prove, that the SptAI and PvuII methyltransferases have identical specificities. Given their close sequence similarity (Fig. 2), the two methyltransferases are almost certainly both generating N4-methylcytosine (12, 32, 39). In theory, however, M•SptAI might have a broader specificity than M•PvuII. If so, the increased breadth is not simply relaxation of specificity for the outermost nucleotides in the recognized sequence, since the plasmid clone carrying the SptAI system is digested at nearly all AGCT sites by AluI (not shown); AluI recognizes the central 4 bp (underlined) of the PvuII substrate (CAGCTG), and digestion by AluI is blocked by PvuII methylation (13, 58).
In contrast to the methyltransferase, there is no obvious activity corresponding to the SptAI restriction endonuclease (R•SptAI). This is based on the lack of apparent cleavage activity when extracts of cells containing the cloned SptAI system were incubated with bacteriophage λ DNA, and the lack of apparent restriction when unmodified λvir was plated onto E. coli cells containing the cloned SptAI system (Table 1). In both cases, E. coli cells producing the cloned PvuII system were used as positive controls and behaved as expected.
Functionality of the SptAI control system.
There are several possible explanations for the lack of R•SptAI activity. The three most likely are that the sptAICR promoter region is inactive, that the regulator C•SptAI is inactive, or that the endonuclease itself is inactive. In the PvuII RM system, C•PvuII is absolutely required for transcription of the gene for R•PvuII—the C protein binds to conserved sequences called C-boxes (59) that overlap the promoter for a bicistronic sptAICR transcript, and the complex appears to activate transcription over 20-fold (73).
In comparing the sptAICR promoter regions of the PvuII and SptAI systems, the SptAI C-boxes have two alterations relative to PvuII and three changes relative to the consensus. Specifically, the downstream occurrence of the tandem GACTnAnAGTC consensus (8, 73) is changed to GATCtAtTGTC; this generates a substrate site for the Dam DNA methyltransferase (7, 41), although it is not yet known if Dam methylation has any effect on SptAI expression. The apparent −10 promoter hexamer of the sptAICR promoter is conserved relative to PvuII, but the −35 hexamer and putative UP element of PvuII are substantially altered in SptAI. If either the promoter or the C-boxes are impaired, then the autogenous activation circuit would fail, and transcription of both sptAIC and sptAIR would be poor.
To test the activity of the sptAICR promoter region, we supplied C•PvuII from a second plasmid. The C proteins from organisms as different as Proteus vulgaris and Bacillus amyloliquefaciens cross-complement (27), so a possible defect in C•SptAI should be complemented by the closely related C•PvuII protein, while a defect in the sptAICR promoter or C-boxes (or in R•SptAI itself) would not. We saw no sign of SptAI restriction, even in the presence of C•PvuII (Table 2). However, we found that the defect is not in the sptAICR promoter. We subcloned this promoter region (from −139 to +149 relative to the predicted start of transcription) into plasmid pKK232-8, placing it upstream of a promoterless CAT (cat) gene. This plasmid, in E. coli strain TOP10, yielded a very large increase in CAT activity when C•PvuII was provided from a second, compatible plasmid: there were 3.2 ± 1.0 U of CAT activity per μg of protein in cells carrying a mutant pvuIIC and 1,446 ± 198 U/μg in cells carrying wild-type pvuIIC (average ± standard error [see Materials and Methods for assay]). This indicates that the sptAICR promoter can be activated in trans by the C protein from the PvuII RM system.
TABLE 2.
Effects of C•PvuII on restriction by the SptAI RM system
| Plating straina | Active PvuII gene(s)b | Apparent PFU/ml of lysate from strain carrying the plasmidc
|
|
|---|---|---|---|
| pBSH005 (none) | pMAN100 (SptAI) | ||
| pMAN100 + pACYC184 | None | 7.0 × 105 (1.0) | 3.6 × 107 (1.0) |
| pMAN100 + pRPS400 | C | 8.0 × 105 (1.1) | 2.1 × 107 (0.6) |
| pMAN100 + pPvuRM2.6CYC | C | 1.0 × 106 (1.4) | 2.2 × 107 (0.6) |
| pPvuRM3.4CYC | R, C, M | 4.2 × 103 (0.006) | 1.8 × 107 (0.5) |
One of two bacteriophage λ lysates was plated on each of these four strains. All strains are E. coli TOP10 (In Vitrogen) containing the indicated plasmid(s).
Abbreviations: C, regulatory gene pvuIIC; M, methyltransferase gene pvuIIM; and R, restriction endonuclease gene pvuIIR.
Numbers indicate apparent PFU per milliliter of lysate (normalized value in parentheses), calculated from plating a dilution series of two lysates. The lysates were prepared on host strains containing either no RM system or the SptAI system, as indicated in the column heading. The system (none or SptAI) is indicated at the top of each column. pMAN100 contains the entire SptAI RM system, pRPS400 and pPvuRM2.6CYC are both pACYC184-based plasmids that carry the intact pvuIIC gene and part of the pvuIIM gene, and pPvuRM3.4CYC is a pACYC184-based plasmid that carries the entire PvuII RM system (used as a positive control for restriction). pBSH005 carries the irrelevant E. coli gene yhhK.
We next determined whether the C•SptAI protein itself was active in a complementation test. This was done by cloning the sptAIC ORF into a pET28b vector (Novagen, Madison, Wis.) and testing its ability to prevent transformation by the intact PvuII RM system carried on a compatible (pACYC184-based) plasmid. In the case of PvuII, C protein must accumulate in order to get expression of the restriction endonuclease, and this requirement serves to delay endonuclease expression after an RM system first enters a new cell, so there is time for methylation of the new host's DNA. As a result, cells that already contain an active C protein cannot be transformed by an intact RM system on which that C protein can act, because the resulting premature expression of endonuclease leads to lethal autorestriction (46, 73). In our experiment, cells containing pET28b that carried either pvuIIC (positive control) or sptAIC (test) could not be transformed by the PvuII RM system, while the strain in which pET28b was carrying pvuIIM (negative control) was efficiently transformed (not shown). This means that sptAIC specifies an active C protein that can act on the PvuII system.
Inactivity of R•SptAI.
Another possible explanation for the lack of R•SptAI activity is a defect in that protein itself. The most pronounced difference between R•SptAI and R•PvuII is the 27-amino-acid carboxyl-terminal tail present on the former (Fig. 2). Interestingly, this tail may be the result of a single base change (underlined)—at the position of the TAA termination codon of R•PvuII, R•SptAI has a TCA serine codon and continues until reaching two consecutive TAA codons. The R•SptAI tail is highly acidic, with an aspartate and seven glutamates, but no basic amino acids, and this might interfere with binding the negatively charged DNA substrate. To test the possibility that the C-terminal tail has abolished R•SptAI activity, we repeated the PCR amplification of the entire system, but this time used a primer that eliminates the tail by changing the TCA→TAA as referred to above. When this truncated system was introduced into E. coli TOP10 cells, we still saw no R•SptAI activity, either by restriction of λvir or by digestion of λ DNA in cell extracts (data not shown).
Aside from the nonconserved termini, the PvuII and SptAI endonucleases differ at 47 of 155 positions, although some of these are conservative changes. All 47 of these differences occur in both our PCR-amplified clone and in the original genomic DNA sequence, so they do not result from amplification errors. To implicate the change(s) responsible for R•SptAI inactivity, we sought to compare the amino acid sequences of R•SptAI and R•SbaI. SbaI is an active RM system discovered in Salmonella enterica serovar Bareilly that has the same specificity as the PvuII system (40). It seemed likely that the SbaI system would be closely related to the SptAI system and could reveal which changes between R•SptAI and R•PvuII are irrelevant to activity. We obtained S. enterica serovar Bareilly from the laboratory in which the SbaI system had been discovered, isolated chromosomal DNA, and used SptAI-specific PCR primers to attempt an amplification. We obtained a product of the expected size, cloned it, and determined its nucleotide sequence.
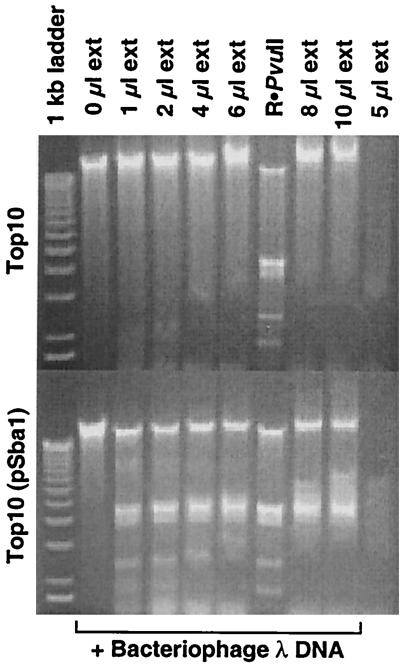
Working with S. enterica serovar Paratyphi A and S. enterica serovar Bareilly DNA in the same laboratory and given the risks of sample cross-contamination, we wished to demonstrate that we hadn't inadvertently amplified the SptAI system. We feel confident that our SbaI clone is authentic for two reasons. First, the nucleotide sequence differs from that of SptAI at seven positions (between the divergent methyltransferase and endonuclease stop codons). Second, and more convincing, the SbaI clone produces an active endonuclease, as indicated by in vitro digestion of bacteriophage λ DNA with whole-cell extracts of the E. coli strain. The cleavage pattern generated by R•SbaI is indistinguishable from that generated by R•PvuII (Fig. 3).
FIG. 3.
Activity of R•SbaI. To test for activity of the SbaI endonuclease, E. coli TOP10 with and without the cloned SbaI RM system was grown overnight in LB medium, pelleted, and opened by sonication as described in Materials and Methods. Various amounts of centrifugally cleared cell extract were incubated for 15 min at 37°C with 0.5 μg of bacteriophage λ DNA. PvuII endonuclease (5 U; New England Biolabs) was used in one lane to provide the expected digestion pattern. Digests were resolved on 1% agarose-TBE gels and stained with ethidium bromide.
Since the R•PvuII and R•SbaI endonucleases are active, while R•SptAI is not, the inactivity presumably results from a sequence change unique (among these three proteins) to R•SptAI. Aside from the carboxyl terminus, which is poorly conserved among all three proteins, there is only one SptAI-specific change, highlighted in Fig. 2. Based on the known crystallographically defined structure of R•PvuII (5, 16), this Y22H alteration in R•SptAI is expected to be within helix A (indicated by a cylinder in Fig. 2), which forms the dimerization interface. This interface, in R•PvuII, is a kinked (“banana”) helix, and the kink is provided by a proline that is conserved in all three proteins. If this Y22H mutation causes a dimerization defect, then R•SptAI should not be dominant negative with respect to R•PvuII expressed in the same cell, and we confirmed the absence of dominant-negative effects with pvuIIR on a low-copy vector (pACYC184) and sptAIR on a higher-copy vector (pCRII; data not shown).
Sequences flanking the RM systems.
Having demonstrated that the complete PvuII and SptAI/SbaI systems were orthologous, we next examined their flanking sequences in an attempt to understand the history of and basis for their movement between bacterial genera. The chromosomally located SptAI genes lie between two ORFs that are closely related to the integrase and polarity-suppression protein (Int and Psu, respectively) of bacteriophage P4. P4 is a defective lysogenic bacteriophage, dependent on a helper bacteriophage for most capsid components (37). The SbaI system lies between the same two genes as the SptAI system.
The PvuII genes are carried on a 4,677-bp low-copy-number plasmid named pPvu1 (10). Roughly two-thirds of this plasmid had been sequenced previously in this laboratory (14, 68, 70) (GenBank accession no. M77223). We have now completed the sequence determination for pPvu1 (GenBank accession no. AF305615); this is the first complete plasmid native to the genus Proteus, based on a search of the NCBI Entrez Genomes bacterial plasmid database (www.ncbi.nlm.nih.gov/PMGifs/Genomes/eub_p.html). We had previously noted an apparent substrate site for the XerCD recombinase (20, 23, 25), beginning just 17 bp from the termination codon of the R•PvuII gene, within a small ORF of unknown function named pvuIIO (Fig. 1), and this site may confer recombinational mobility on the PvuII system (14).
In addition, the previously uncharacterized region of pPvu1 contains a mob operon, which should increase intercellular movement of this plasmid by allowing it to use the transfer machinery of other, conjugative plasmids. The mob operon comprises four genes, mobA to -D, with the usual arrangement of mobC preceding mobA, followed by mobB and mobD, with alternative reading frames within mobA (49, 61, 62). This characteristic gene arrangement strengthens the individual gene assignments based on sequence comparison, although the mob operon is not yet proven to be functional.
Since pPvu1 contains a mob operon, we also looked to see if the PvuII genes were present in Proteus vulgaris isolates other than the one in which it was originally found (ATCC 13315) (21). To this end, we made opposing primers that matched, respectively, the pvuIIM and pvuIIR genes and which generate an ∼1-kbp band following PCR amplification when DNA from ATCC 13315 is used. We obtained no positive results for any of the strains tested: ATCC 6380, 6897, 8427, 33420, and 49132—all P. vulgaris—and ATCC 7002 (Proteus mirabilis) and 33519 (Proteus penneri).
DISCUSSION
The SptAI RM system.
It is interesting that the SptAI system has a modification-positive, restrictionless (Mod+ Res−) phenotype. From the point of view of the individual cell, the worst outcome would be a lethal Mod− Res+ phenotype, and many RM systems carefully regulate their genes to avoid even transient Mod− Res+ states (27, 38, 50, 57, 73). However, bacterial populations might be expected to vary their production of RM systems to yield a mixed population that includes Mod− Res− cells. Rare bacteriophage escapees of restriction in a cell Mod+ Res+ for a particular RM system become protectively methylated and would not be subject to restriction by that RM system. In a mixed population, some fraction of these escapees would lose their methylation during growth in one of the Mod− Res− cells and would again be susceptible to restriction (54). The protective effect would depend on population density, relative numbers of each cell type, and bacteriophage numbers, but there is substantial evidence for this type of RM system variation (17, 18, 35, 71).
However, the worst possible outcome for phage defense, on a whole-population level, would be variation leading to a Mod+ Res− phenotype, as seen with SptAI, because the efficient production of modified bacteriophage on such cells would undermine the effectiveness of the restriction endonuclease in any Res+ cells in the population. Furthermore, whatever selfish behavior may be exhibited by an RM system is lost in a Mod+ Res− cell, so neither phage defense nor selfishness explains the persistence of such systems. Nonetheless, in a study of Helicobacter pylori, several such examples were found (33). It is unclear why Mod+ Res− phenotypes appear to be so common.
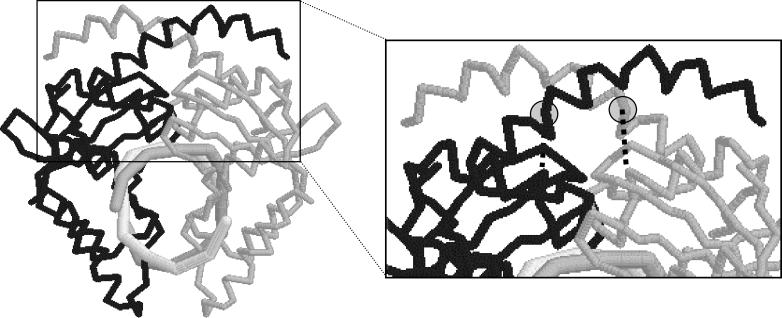
In the case of SptAI, the Res− phenotype is due to a defect in the endonuclease, probably a single T→C transition leading to a Tyr19→His substitution in the subunit dimerization interface (Fig. 2, bottom). The altered nucleotide sequence shows no obvious features one would expect to see in a region designed to be highly polymorphic or to phase vary (43). It is not clear why the Tyr19→His substitution would abolish activity. In the structure for R•PvuII (e.g., 1EYU.pdb), Tyr19 points away from the interface, and its hydroxyl forms a hydrogen bond to the backbone carbonyl of Gly37 on the same chain (Fig. 4). Substitution of His at that position would abolish the bond to Gly37, and this might make the subunit interface more flexible. The observation that coexpression of R•SptAI and R•PvuII leads to no apparent diminution of R•PvuII activity indicates that the R•SptAI mutation is not dominant negative, consistent with (though not proving) a defect in subunit dimerization.
FIG. 4.
Role of Tyr19 in R•PvuII. On the left is the polypeptide backbone of R•PvuII (from PDB 1EYU; rendered by Chime software, MDL, Inc.). The DNA helical axis is perpendicular to the page. The two subunits of this homodimeric protein are in different shades. The subunit interface is generated by proline-kinked α-helices at the amino termini (top, in orientation shown). The Tyr19 side chain is indicated by the circle on each subunit. The dotted line indicates the hydrogen bond between the Tyr19 hydroxyl and the backbone carbonyl of Gly37; the oxygen-oxygen distance is 2.7 Å. In R•SptAI, which is inactive, this Tyr has been replaced by His (Fig. 2).
Plasmid pPvu1.
We have completed the sequence for pPvu1, the first complete sequence for a Proteus plasmid. This plasmid was originally isolated by cloning the PvuII RM system (10), and we previously demonstrated the presence of a functional p15A-type origin of replication and rom gene (14). We report here that most of the remainder of this 4,675-bp plasmid is taken up by a set of mob genes. This means that the PvuII RM system genes are the only genes on pPvu1 that have a function other than plasmid maintenance and transfer. In this respect, pPvu1 resembles some other plasmids such as pEC156 (44).
Mobility of type II RM systems.
There appears to be an association between the genes for RM systems and genes that increase genetic mobility. The simplest explanation is that the original association of RM and mobility genes is a random occurrence, with those RM systems that happen to associate with mobility loci being overrepresented in samplings of extant systems purely as a consequence of the increased mobility. There may be some additional effect resulting from the fact that restriction endonuclease-mediated cleavage can itself stimulate recombination (4, 42), although it's not yet clear that the genes for RM systems change their immediate genetic contexts more readily than other genes.
Carriage of restriction genes by defective prophages appears to be fairly common. For example, the McrA methylation-dependent restriction enzyme is specified by the defective ɛ14 prophage in many strains of E. coli (56). However it is particularly striking that another type II RM system has been found in an exactly analogous position to the SptAI and SbaI genes—as illustrated in Fig. 1, the EcoO109I system is carried between the P4 Int and Psu genes in a strain of E. coli (31). Aside from their analogous locations, the SptAI and EcoO109I genes are not closely related. The orthologous systems EcoHK31I from E. coli and EaeI from Enterobacter agglomerans are also adjacent to P4 Int-like ORFs, but there is no Psu ortholog present (36). This phenomenon of a common location for disparate RM systems is similar to one seen in Haemophilus, not known to involve defective prophages, where unrelated systems are found inserted next to the gene for valyl-tRNA synthetase (34).
Only one isolate of P. vulgaris tested contained a PvuII RM system. We made no systematic survey of Salmonella isolates, but two species (S. enterica serovar Paratyphi A and S. enterica serovar Bareilly) contain PvuII-like systems, while no evidence for such systems was found by BLAST analysis of the genome sequences (incomplete, January 2002) of four other Salmonella species: S. enterica subsp. enterica serovar Dublin, S. enterica serovar Enteritidis, S. enterica serovar Typhi, and S. enterica serovar Typhimurium LT2.
Based on activity assay, there is a PvuII-type system in the gram-positive sporulating organism Bacillus alvei (BavI) (REBASE, 1992 posting date [http://rebase.neb.com/cgi-bin/refget?1523]); however, repeated attempts to isolate this system via PCR with a variety of SptAI-specific primers were unproductive (data not shown). It would be interesting to know if the BavI system failed to amplify due to numerous synonymous or conservative changes, or if instead it represents an unrelated system that gained the PvuII specificity via convergent evolution.
Nevertheless, the results presented here show that essentially identical RM systems can appear in completely distinct genetic contexts (comparing the PvuII and SptAI/SbaI systems) and that completely unrelated RM systems can appear in virtually identical genetic contexts (comparing the SptAI/SbaI and EcoO109I systems). These isolated snapshots of RM systems cannot support the detailed analysis needed to understand RM system mobility, although they lay important foundations. A broader and more systematic isolation and context characterization of conspecific RM systems is needed to understand the distribution of these gatekeepers of microbial gene exchange.
Acknowledgments
M.N. and J.R.B. contributed equally to this work.
We thank the Genome Sequencing Center, Washington University, St. Louis, for communication of DNA sequence data prior to publication and K. Mise (National Institute of Hygeinic Sciences, Tokyo, Japan) for S. enterica serovar Bareilly.
This research was supported by the U.S. National Science Foundation under grant MCB-9904523.
REFERENCES
- 1.Adams, G. M., and R. M. Blumenthal. 1995. Gene pvuIIW: a possible modulator of PvuII endonuclease subunit association. Gene 157:193-199. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anton, B. P., D. F. Heiter, J. S. Benner, E. J. Hess, L. Greenough, L. S. Moran, B. E. Slatko, and J. E. Brooks. 1997. Cloning and characterization of the BglII restriction-modification system reveal a possible evolutionary footprint. Gene 187:19-27. [DOI] [PubMed] [Google Scholar]
- 4.Arber, W. 2000. Genetic variation: molecular mechanisms and impact on microbial evolution. FEMS Microbiol. Rev. 24:1-7. [DOI] [PubMed] [Google Scholar]
- 5.Athanasiadis, A., M. Vlassi, D. Kotsifaki, P. A. Tucker, K. S. Wilson, and M. Kokkinidis. 1994. Crystal structure of PvuII endonuclease reveals extensive structural homologies to EcoRV. Struct. Biol. 1:469-475. [DOI] [PubMed] [Google Scholar]
- 6.Barcus, V. A., and N. E. Murray. 1995. Barriers to recombination: restriction. Cambridge University Press, Cambridge, United Kingdom.
- 7.Barras, F., and M. G. Marinus. 1989. The great GATC: DNA methylation in E. coli. Trends Genet. 5:139-143. [DOI] [PubMed] [Google Scholar]
- 8.Bart, A., J. Dankert, and A. van der Ende. 1999. Operator sequences for the regulatory proteins of restriction modification systems. Mol. Microbiol. 31:1277-1278. [DOI] [PubMed] [Google Scholar]
- 9.Bickle, T. A., and D. H. Krüger. 1993. Biology of DNA restriction. Microbiol. Rev. 57:434-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumenthal, R. M., S. A. Gregory, and J. S. Cooperider. 1985. Cloning of a restriction-modification system from Proteus vulgaris and its use in analyzing a methylase-sensitive phenotype in Escherichia coli. J. Bacteriol. 164:501-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks, J. E., P. D. Nathan, D. Landry, L. A. Sznyter, P. Waite-Rees, C. L. Ives, L. S. Moran, B. E. Slatko, and J. S. Benner. 1991. Characterization of the cloned BamHI restriction modification system: its nucleotide sequence, properties of the methylase, and expression in heterologous hosts. Nucleic Acids Res. 19:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bujnicki, J. M., and M. Radlinska. 1999. Molecular evolution of DNA-(cytosine-N4) methyltransferases: evidence for their polyphyletic origin. Nucleic Acids Res. 27:4501-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butkus, V., S. Klimasauskas, L. Petrauskiene, Z. Maneliene, A. Lebionka, and A. A. Janulaitis. 1987. Interaction of AluI, Cfr6I and PvuII restriction-modification enzymes with substrates containing either N4-methylcytosine or 5-methylcytosine. Biochim. Biophys. Acta 909:201-207. [DOI] [PubMed] [Google Scholar]
- 14.Calvin-Koons, M. D., and R. M. Blumenthal. 1995. Characterization of p Pvu1, the autonomous plasmid from Proteus vulgaris that carries the genes of the PvuII restriction-modification system. Gene 157:73-79. [DOI] [PubMed] [Google Scholar]
- 15.Chang, A. C. Y., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng, X., K. Balendiran, I. Schildkraut, and J. E. Anderson. 1994. Structure of PvuII endonuclease with cognate DNA. EMBO J. 13:3927-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Bolle, X., C. D. Bayliss, D. Field, T. van de Ven, N. J. Saunders, D. W. Hood, and E. R. Moxon. 2000. The length of a tetranucleotide repeat tract in Haemophilus influenzae determines the phase variation rate of a gene with homology to type III DNA methyltransferases. Mol. Microbiol. 35:211-222. [DOI] [PubMed] [Google Scholar]
- 18.Dybvig, K., R. Sitaraman, and C. T. French. 1998. A family of phase-variable restriction enzymes with differing specificities generated by high-frequency gene rearrangements. Proc. Natl. Acad. Sci. USA 95:13923-13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fauman, E. B., R. M. Blumenthal, and X. Cheng. 1999. Structure and evolution of AdoMet-dependent methyltransferases, p. 3-38. In X. Cheng and R. M. Blumenthal (ed.), S-Adenosylmethionine-dependent methyltransferases: structures and functions. World Scientific Publishing, Singapore.
- 20.Ferreira, H., D. Sherratt, and L. Arciszewska. 2001. Switching catalytic activity in the XerCD site-specific recombination machine. J. Mol. Biol. 312:45-57. [DOI] [PubMed] [Google Scholar]
- 21.Gingeras, T. R., L. Greenough, I. Schildkraut, and R. J. Roberts. 1981. Two new restriction endonucleases from Proteus vulgaris. Nucleic Acids Res. 9:4525-4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong, W., M. O'Gara, R. M. Blumenthal, and X. Cheng. 1997. Structure of PvuII DNA-(cytosine N4) methyltransferase, an example of domain permutation and protein fold assignment. Nucleic Acids Res. 25:2702-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guhathakurta, A., I. Viney, and D. Summers. 1996. Accessory proteins impose site selectivity during ColE1 dimer resolution. Mol. Microbiol. 20:613-620. [DOI] [PubMed] [Google Scholar]
- 24.Handa, N., A. Ichige, K. Kusano, and I. Kobayashi. 2000. Cellular responses to postsegregational killing by restriction-modification genes. J. Bacteriol. 182:2218-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodgman, T. C., H. Griffiths, and D. K. Summers. 1998. Nucleoprotein architecture and ColE1 dimer resolution: a hypothesis. Mol. Microbiol. 29:545-558. [DOI] [PubMed] [Google Scholar]
- 26.Horton, J. R., and X. Cheng. 2000. PvuII endonuclease contains two calcium ions in active sites. J. Mol. Biol. 300:1051-1058. [DOI] [PubMed] [Google Scholar]
- 27.Ives, C. L., A. Sohail, and J. E. Brooks. 1995. The regulatory C proteins from different restriction-modification systems can cross-complement. J. Bacteriol. 177:6313-6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeltsch, A., F. Christ, M. Fatemi, and M. Roth. 1999. On the substrate specificity of DNA methyltransferases. J. Biol. Chem. 274:19538-19544. [DOI] [PubMed] [Google Scholar]
- 29.Jenuth, J. P. 2000. The NCBI. Publicly available tools and resources on the Web. Methods Mol. Biol 132:301-312. [DOI] [PubMed] [Google Scholar]
- 30.Kaszubska, W., C. Aiken, C. D. O'Connor, and R. I. Gumport. 1989. Purification, cloning and sequence analysis of RsrI DNA methyltransferase: lack of homology between two enzymes, RsrI and EcoRI, that methylate the same nucleotide in identical recognition sequences. Nucleic Acids Res. 17:10403-10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kita, K., J. Tsuda, T. Kato, K. Okamoto, H. Yanase, and M. Tanaka. 1999. Evidence of horizontal transfer of the EcoO109I restriction-modification gene to Escherichia coli chromosomal DNA. J. Bacteriol. 181:6822-6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klimasauskas, S., A. Timinskas, S. Menkevicius, D. Butkiene, V. Butkus, and A. Janulaitis. 1989. Sequence motifs characteristic of DNA[cytosine-N4]methylases: similarity to adenine and cytosine-C5 DNA-methylases. Nucleic Acids Res. 17:9823-9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong, H., L. F. Lin, N. Porter, S. Stickel, D. Byrd, J. Posfai, and R. J. Roberts. 2000. Functional analysis of putative restriction-modification system genes in the Helicobacter pylori J99 genome. Nucleic Acids Res. 28:3216-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulakauskas, S., J. M. Barsomian, A. Lubys, R. J. Roberts, and G. G. Wilson. 1994. Organization and sequence of the HpaII restriction-modification system and adjacent genes. Gene 142:9-15. [DOI] [PubMed] [Google Scholar]
- 35.Lacks, S. A., B. M. Mannarelli, S. S. Springhorn, and B. Greenberg. 1986. Genetic basis of the complementary DpnI and DpnII restriction systems of S. pneumoniae: an intercellular cassette mechanism. Cell 46:993-1000. [DOI] [PubMed] [Google Scholar]
- 36.Lee, K. F., P. C. Shaw, S. J. Picone, G. G. Wilson, and K. D. Lunnen. 1998. Sequence comparison of the EcoHK31I and EaeI restriction-modification systems suggests an intergenic transfer of genetic material. Biol. Chem. 379:437-441. [DOI] [PubMed] [Google Scholar]
- 37.Liu, T., S. K. Renberg, and E. Haggård-Ljungquist. 1997. Derepression of prophage P2 by satellite phage P4: cloning of the P4 ɛ gene and identification of its product. J. Virol. 71:4502-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makovets, S., V. A. Doronina, and N. E. Murray. 1999. Regulation of endonuclease activity by proteolysis prevents breakage of unmodified bacterial chromosomes by type I restriction enzymes. Proc. Natl. Acad. Sci. USA 96:9757-9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malone, T., R. M. Blumenthal, and X. Cheng. 1995. Structure-guided analysis reveals nine sequence motifs conserved among DNA amino-methyl-transferases, and suggests a catalytic mechanism for these enzymes. J. Mol. Biol. 253:618-632. [DOI] [PubMed] [Google Scholar]
- 40.Matsui, M., K. Mise, Y. Yoshida, and M. Ishidate. 1986. Production of restriction endonucleases from various Salmonella strains of human origin. Bull. Natl. Inst. Hyg. Sci. (Tokyo) 104:92-96. [PubMed] [Google Scholar]
- 41.Messer, W., and M. Noyer-Weidner. 1998. Timing and targeting: the biological functions of Dam methylation in E. coli. Cell 54:735-737. [DOI] [PubMed] [Google Scholar]
- 42.Milkman, R. 1999. Gene transfer in Escherichia coli, p. 291-309. In R. L. Charlebois (ed.), Organization of the prokaryotic genome. ASM Press, Washington, D.C.
- 43.Moxon, E. R., P. B. Rainey, M. A. Nowak, and R. E. Lenski. 1994. Adaptive evolution of highly mutable loci in pathogenic bacteria. Curr. Biol. 4:24-33. [DOI] [PubMed] [Google Scholar]
- 44.Mruk, I., M. Sektas, and T. Kaczorowski. 2001. Characterization of pEC156, a ColE1-type plasmid from Escherichia coli E1585-68 that carries genes of the EcoVIII restriction-modification system. Plasmid 46:128-139. [DOI] [PubMed] [Google Scholar]
- 45.Naito, T., K. Kusano, and I. Kobayashi. 1995. Selfish behavior of restriction-modification systems. Science 267:897-899. [DOI] [PubMed] [Google Scholar]
- 46.Nakayama, Y., and I. Kobayashi. 1998. Restriction-modification gene complexes as selfish gene entities: roles of a regulatory system in their establishment, maintenance, and apoptotic mutual exclusion. Proc. Natl. Acad. Sci. USA 95:6442-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicholas, H. B., Jr., D. W. Deerfield II, and A. J. Ropelewski. 2000. Strategies for searching sequence databases. BioTechniques 28:1174-1178, 1180, 1182. [DOI] [PubMed]
- 48.Nolling, J., and W. M. de Vos. 1992. Characterization of the archaeal, plasmid-encoded type II restriction-modification system MthTI from Methanobacterium thermoformicicum THF: homology to the bacterial NgoPII system from Neisseria gonorrhoeae. J. Bacteriol. 174:5719-5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nomura, N., M. Yamashita, and Y. Murooka. 1996. Genetic organization of a DNA-processing region required for mobilization of a non-self-transmissible plasmid, pEC3, isolated from Erwinia carotovora subsp. carotovora. Gene 170:57-62. [DOI] [PubMed] [Google Scholar]
- 50.Prakash-Cheng, A., and J. Ryu. 1993. Delayed expression of in vivo restriction activity following conjugal transfer of Escherichia coli hsdK (restriction-modification) genes. J. Bacteriol. 175:4905-4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price, C., and T. A. Bickle. 1986. A possible role for DNA restriction in bacterial evolution. Microbiol. Sci. 3:296-299. [PubMed] [Google Scholar]
- 52.Pronk, L. M., and K. E. Sanderson. 2001. Intervening sequences in rrl genes and fragmentation of 23S rRNA in genera of the family Enterobacteriaceae. J. Bacteriol. 183:5782-5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raleigh, E. A. 1992. Organization and function of the mcrBC genes of Escherichia coli K-12. Mol. Microbiol. 6:1079-1086. [DOI] [PubMed] [Google Scholar]
- 54.Raleigh, E. A., and J. E. Brooks. 1998. Restriction modification systems: where they are and what they do, p. 78-92. In F. J. De Bruijn, J. R. Lupski, and G. M. Weinstock (ed.), Bacterial genomes. Chapman & Hall, New York, N.Y.
- 55.Raleigh, E. A., N. E. Murray, H. Revel, R. M. Blumenthal, D. Westaway, A. D. Reith, P. W. J. Rigby, J. Elhai, and D. Hanahan. 1988. McrA and McrB restriction phenotypes of some E. coli strains and implications for gene cloning. Nucleic Acids Res. 15:1563-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raleigh, E. A., R. Trimarchi, and H. Revel. 1989. Genetic and physical mapping of the mcrA (rglA) and mcrB (rglB) loci of Escherichia coli K-12. Genetics 122:279-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Redaschi, N., and T. A. Bickle. 1996. Posttranscriptional regulation of EcoP1I and EcoP15I restriction activity. J. Mol. Biol. 257:790-803. [DOI] [PubMed] [Google Scholar]
- 58.Rice, M. R., and R. M. Blumenthal. 2000. Recognition of native DNA methylation by the PvuII restriction endonuclease. Nucleic Acids Res. 28:3143-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rimseliene, R., R. Vaisvila, and A. Janulaitis. 1995. The eco72IC gene specifies a trans-acting factor which influences expression of both DNA methyltransferase and endonuclease from the Eco72I restriction-modification system. Gene 157:217-219. [DOI] [PubMed] [Google Scholar]
- 60.Roberts, R. J., and D. Macelis. 2000. REBASE—restriction enzymes and methylases. Nucleic Acids Res. 28:306-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scherzinger, E., V. Kruft, and S. Otto. 1993. Purification of the large mobilization protein of plasmid RSF1010 and characterization of its site-specific DNA-cleaving/DNA-joining activity. Eur. J. Biochem. 217:929-938. [DOI] [PubMed] [Google Scholar]
- 62.Scherzinger, E., R. Lurz, S. Otto, and B. Dobrinski. 1992. In vitro cleavage of double- and single-stranded DNA by plasmid RSF1010-encoded mobilization proteins. Nucleic Acids Res. 20:41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stephens, R. S., S. Kalman, C. Lammel, J. Fan, R. Marathe, L. Aravind, W. Mitchell, L. Olinger, R. L. Tatusov, Q. Zhao, E. V. Koonin, and R. W. Davis. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754-759. [DOI] [PubMed] [Google Scholar]
- 64.Stephenson, F. H., B. T. Ballard, H. W. Boyer, J. M. Rosenberg, and P. J. Greene. 1989. Comparison of the nucleotide and amino acid sequences of the RsrI and EcoRI restriction endonucleases. Gene 85:1-13. [DOI] [PubMed] [Google Scholar]
- 65.Stephenson, F. H., and P. J. Greene. 1989. Nucleotide sequence of the gene encoding the RsrI methyltransferase. Nucleic Acids Res. 17:10503.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sullivan, K. M., and J. R. Saunders. 1989. Nucleotide sequence and genetic organization of the NgoPII restriction-modification system of Neisseria gonorrhoeae. Mol. Gen. Genet. 216:380-387. [DOI] [PubMed] [Google Scholar]
- 67.Sutherland, E., L. Coe, and E. A. Raleigh. 1992. McrBC: a multisubunit GTP-dependent restriction endonuclease. J. Mol. Biol. 225:327-358. [DOI] [PubMed] [Google Scholar]
- 68.Tao, T., and R. M. Blumenthal. 1992. Sequence and characterization of pvuIIR, the PvuII endonuclease gene, and of pvuIIC, its regulatory gene. J. Bacteriol. 174:3395-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tao, T., J. C. Bourne, and R. M. Blumenthal. 1991. A family of regulatory genes associated with type II restriction-modification systems. J. Bacteriol. 173:1367-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tao, T., J. Walter, K. J. Brennan, M. M. Cotterman, and R. M. Blumenthal. 1989. Sequence, internal homology and high-level expression of the gene for a DNA-(cytosine N4)-methyltransferase, M•Pvu II. Nucleic Acids Res. 17:4161-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 72.Vasquez, C. C., C. P. Saavedra, and S. E. Pichuantes. 2000. Nucleotide sequence of the gene encoding the BstLVI DNA methyltransferase: comparison with other amino-DNA methyltransferases. Curr. Microbiol. 40:114-118. [DOI] [PubMed] [Google Scholar]
- 73.Vijesurier, R. M., L. Carlock, R. M. Blumenthal, and J. C. Dunbar. 2000. Role and mechanism of action of C•PvuII, a regulatory protein conserved among restriction-modification systems. J. Bacteriol. 182:477-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu, Q., R. D. Morgan, R. J. Roberts, and M. J. Blaser. 2000. Identification of type II restriction and modification systems in Helicobacter pylori reveals their substantial diversity among strains. Proc. Natl. Acad. Sci. USA 97:9671-9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu, Q., S. Stickel, R. J. Roberts, M. J. Blaser, and R. D. Morgan. 2000. Purification of the novel endonuclease, Hpy188I, and cloning of its restriction-modification genes reveal evidence of its horizontal transfer to the Helicobacter pylori genome. J. Biol. Chem. 275:17086-17093. [DOI] [PubMed] [Google Scholar]