Abstract
Rhodobacter capsulatus utilizes two terminal oxidases for aerobic respiration, cytochrome cbb3 and ubiquinol oxidase. To determine the transcription factors involved in terminal oxidase expression, ccoN-lacZ and cydA-lacZ protein fusions were assayed in a variety of regulatory mutants. The results of this and previous studies indicate that cytochrome cbb3 expression is controlled by regB-regA, fnrL, and hvrA and that ubiquinol oxidase expression is controlled by regB-regA, fnrL, hvrA, crtJ, and aerR.
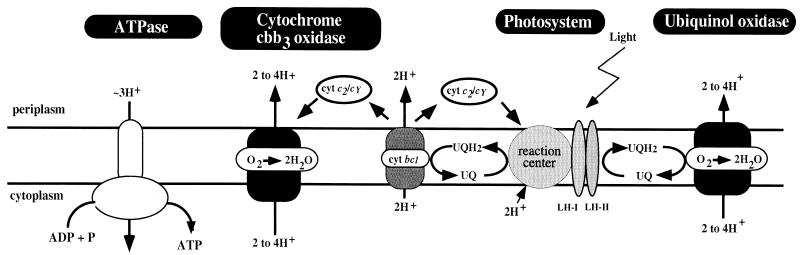
The facultative photosynthetic bacterium Rhodobacter capsulatus demonstrates substantial metabolic plasticity through its ability to grow in response to a variety of different environmental conditions (25). For example, while growing anaerobically in light, R. capsulatus utilizes a photosystem to generate energy. However, when growing in the presence of oxygen, the cells repress synthesis of the photosystem and instead obtain energy by aerobic respiration using two cytochrome oxidases, cytochrome cbb3 and ubiquinol oxidase, as terminal electron acceptors (1, 11, 12, 26) (Fig. 1). Both oxidases reduce oxygen to water in an effort to remove excess reducing power concomitant with proton translocation. The generated proton potential is utilized for multiple cellular processes, including ATP synthesis via ATPase (13-15) (Fig. 1).
FIG. 1.
A model of the electron transport system and ATP synthase in the R. capsulatus membrane. Energy production in the form of ubihydroquinone (UQH2) occurs in the photosystem reaction center. Ubiquinol oxidase utilizes UQH2 to reduce oxygen to water, while cytochrome cbb3 oxidase obtains reducing power from cytochrome c2 or cy via the cytochrome bc1 complex. Electron transfer is coupled to proton translocation at the sites indicated. Protons can reenter the cell via ATP synthase to make ATP. Electrons also shuttle back to the reaction center via cytochrome c2 or cy.
Like many organisms, R. capsulatus has developed a means of ensuring that all available oxygen is used as an electron acceptor before switching to lower-yielding terminal electron acceptors, such as nitrate or dimethyl sulfoxide. The cytochrome cbb3 and ubiquinol oxidases are thought to have different affinities for oxygen. Cytochrome cbb3 is thought to have a low affinity for oxygen, as evidenced by high expression under oxygen-saturating conditions and low expression under oxygen-limiting conditions, while ubiquinol oxidase is thought to have a higher affinity for oxygen due to its low expression under oxygen-saturating conditions and high expression under oxygen-limiting conditions. In order to regulate expression of these terminal electron acceptors in response to oxygen tension, it is necessary that they be differentially regulated by transcription factors that respond to different amounts of oxygen. For example, it was recently demonstrated that the RegB-RegA two-component global signal transduction system from R. capsulatus directly controls aerobic/anaerobic expression of numerous cellular processes, including that of the two terminal oxidases (21). In this study, we reveal that the regulation of terminal oxidase expression in R. capsulatus involves a complex set of regulators beyond that of RegB-RegA. Specifically, we demonstrate that cytochrome cbb3 oxidase expression is regulated by RegB-RegA and FnrL, as well as moderately regulated by HvrA, an activator of various photosynthetic components under low-light conditions. Ubiquinol oxidase expression was found to be regulated by RegB-RegA, FnrL, and HvrA as well as by AerR and CrtJ, which are aerobic repressors of photosystem gene expression.
Ubiquinol oxidase expression.
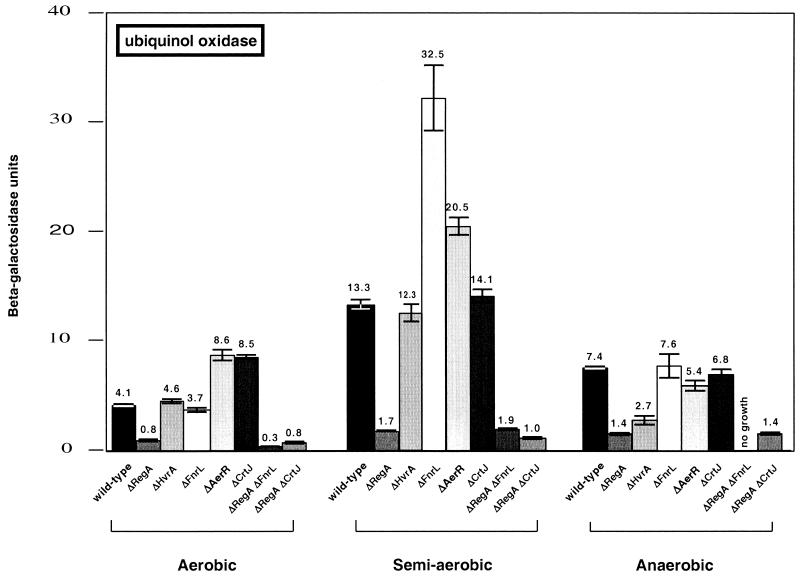
Expression of ubiquinol oxidase was assayed using a translational lacZ fusion to the first gene in the cydAB operon that contains 920 bp of DNA upstream from cydA (21). This plasmid was mobilized as described previously (24) into regA (19), crtJ (20), hvrA (3), aerR (20), and fnrL (20) single-mutant strains, as well as regA-crtJ (20) and regA-fnrL (20) double-mutant strains. Each of the constructed strains was tested for β-galactosidase activity under aerobic, semiaerobic, and anaerobic (photosynthetic) growth conditions as reported by Buggy and Bauer (2).
The expression pattern observed for ubiquinol oxidase in wild-type R. capsulatus was similar to that reported by Swem et al. (21) (Fig. 2). Specifically, expression was lowest under aerobic conditions, intermediate under anaerobic conditions (1.8-fold higher), and highest (3.2-fold higher) under semiaerobic growth conditions. The effect of a disruption of regA was also similar to that reported by Swem et al. (21), in which expression was significantly lower than for the wild type (by 81 to 87%) under all tested growth conditions.
FIG. 2.
β-Galactosidase analysis of aerobic, semiaerobic, and anaerobic photosynthetic ubiquinol oxidase gene expression patterns in the wild-type parent strain SB1003 and various regulatory mutants, as indicated below each bar. The values represent averages of at least three independent assays (standard deviations indicated by the error bars). Units of activity refer to the number of micromoles of o-nitrophenyl-β-d-galactopyranoside hydrolyzed per minute per milligram of protein.
We also assayed the effect of disrupting hvrA on expression of ubiquinol oxidase. HvrA is a member of the HNS family of histone-like DNA-binding proteins and is cotranscribed with RegA (3). Gel shift experiments indicate that HvrA may cooperatively interact with phosphorylated RegA (10). In the hvrA mutant strain, there is a 64% reduction in anaerobic ubiquinol oxidase expression compared to the 81% reduction observed for the regA mutant. Interestingly, HvrA has no effect on expression aerobically or semiaerobically, though RegA does.
In Escherichia coli, Fnr is one of several regulators of terminal oxidase gene expression (4). Since R. capsulatus contains a homolog of Fnr (27), we constructed a mutation in the chromosomal copy of fnrL and tested the mutant strain for its effect on terminal oxidase gene expression. The bar graph in Fig. 2 shows that there is no effect of disrupting fnrL on ubiquinol oxidase expression when the cells are grown strictly aerobically or anaerobically. In contrast, there is a reproducible 2.5- to 3-fold increase in ubiquinol oxidase expression from that in the wild type under semiaerobic growth conditions. This pattern has also been observed for cytochrome o oxidase expression in E. coli, where fnr mutations only show a significant effect under semiaerobic growth conditions (22).
In addition to the above tested “global regulators” that are found in many photosynthetic and nonphotosynthetic species, we also tested whether two aerobic repressors of the photosystem, CrtJ and AerR, are also involved in controlling ubiquinol oxidase gene expression. Analysis of ubiquinol oxidase expression indicates that aerR and crtJ mutants exhibit a twofold increase in expression aerobically and no effect anaerobically. This is very similar to the effect on bch, crt, and puc expression that is also observed upon disruption of these regulators (5, 8).
We also addressed the issue of dominance by constructing regA-fnrL and regA-crtJ double mutants. The ubiquinol oxidase expression pattern exhibited by the regA-crtJ double mutant was the same as that observed with the regA mutant under all three growth conditions. The regA mutant phenotype also prevailed in the regA-fnrL mutant when grown aerobically and semiaerobically. However, under anaerobic (photosynthetic) conditions, the regA-fnrL mutant showed a rather unexpected phenotype of no growth.
Cytochrome cbb3 oxidase expression.
To assay the expression of cytochrome cbb3 oxidase, a translational lacZ fusion to the first gene in the ccoNOPQ operon was constructed (pDSccoN2) that contained 466 bp of DNA upstream of ccoN (20). This plasmid was mobilized into the same set of regulatory mutants and assayed in the same manner as described above for ubiquinol oxidase.
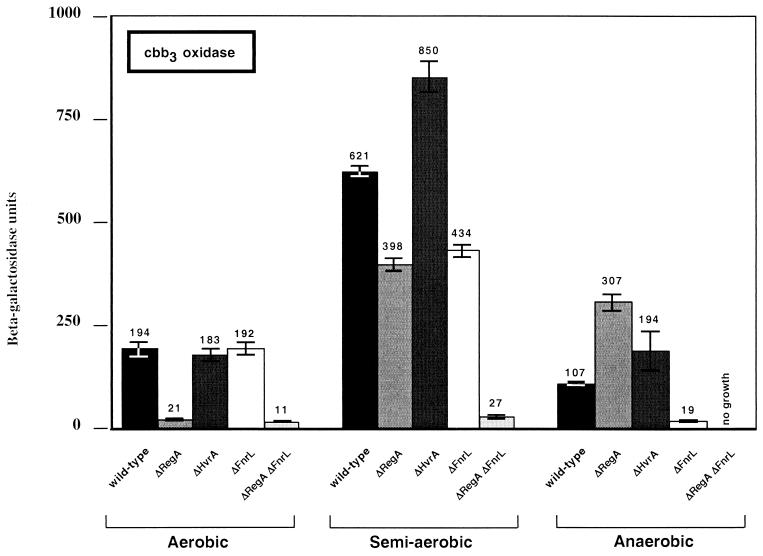
The expression pattern that we observed for cbb3 oxidase in wild-type R. capsulatus was also similar to that reported by Swem et al. (21) (Fig. 3). Specifically, expression was highest under semiaerobic conditions, intermediate under aerobic conditions, and lowest under anaerobic growth conditions.
FIG. 3.
β-Galactosidase analysis of aerobic, semiaerobic, and anaerobic photosynthetic cytochrome cbb3 oxidase gene expression patterns in the wild-type parent strain SB1003 and various regulatory mutants as indicated below each bar. The values represent averages of at least three independent assays. Units of activity refer to the number of micromoles of o-nitrophenyl-β-d-galactopyranoside hydrolyzed per minute per milligram of protein.
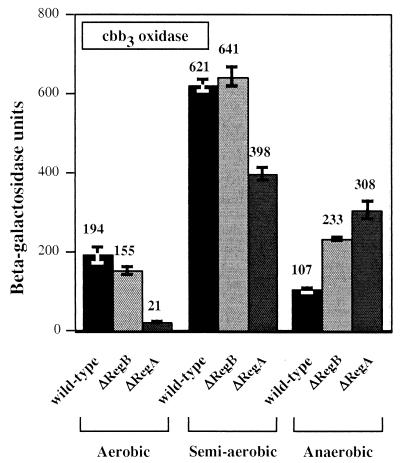
The effect of disrupting regA was also similar to that reported by Swem et al. (21) in that expression was significantly reduced under aerobic conditions, partially reduced under semiaerobic conditions, and increased twofold under anaerobic conditions. This suggests that perhaps dephosphorylated RegA functions as an activator and that phosphorylated RegA functions as a repressor of cbb3 oxidase expression. To test this possibility, we also assayed cbb3 oxidase expression in a regB mutant strain that would just contain dephosphorylated RegA (Fig. 4). In this strain, we observed that the regB mutant has no phenotype under aerobic and semiaerobic growth conditions, which are conditions where RegB should not exhibit significant phosphorylation activity (6). This is contrasted by a rather significant reduction in expression observed upon disruption of regA under these conditions. Under anaerobic conditions, the regB mutant displays an expression level that is intermediate for wild-type cells and for a regA mutant.
FIG. 4.
β-Galactosidase analysis of aerobic, semiaerobic, and anaerobic photosynthetic cytochrome cbb3 oxidase gene expression gene expression patterns in the wild-type parent strain SB1003, as well as in regA- and regB-disrupted strains. The values represent averages of at least three independent assays. Units of activity refer to the number of micromoles of o-nitrophenyl-β-d-galactopyranoside hydrolyzed per minute per milligram of protein.
The effect of an hvrA disruption on cbb3 oxidase expression is different from that observed with ubiquinol oxidase. In this case, there is no effect on aerobic gene expression, but there is a reproducible increase in semiaerobic expression. This indicates that HvrA may have a repressing effect on cbb3 oxidase expression.
One of the significant effects on cbb3 oxidase expression occurs upon disruption of fnrL. In this case there is no effect on aerobically grown cells, a slight (30%) reduction under semiaerobically grown cells, and an 82% reduction in anaerobically grown cells. The effect of FnrL is even more obvious in semiaerobically grown cells when the fnrL mutation is present in conjunction with a regA mutation. In this case the effect is a 96% reduction in expression.
The crtJ and aerR regulatory mutants were also assayed for effects on ccoN::lacZ expression. Neither of these mutants exhibited a significant difference in expression from that found in the wild type under any growth conditions (data not shown).
The involvement of multiple regulators.
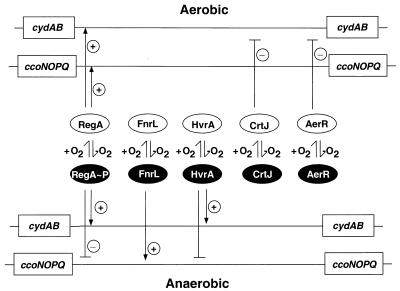
There are two primary conclusions that can be derived from the results of this study. One conclusion is that R. capsulatus uses multiple regulators to ensure preferential and complete use of O2 as an electron acceptor by varying the level of cytochrome cbb3 oxidase and ubiquinol oxidase expression in response to changes in oxygen tension (Fig. 5). The second conclusion is that many transcription factors involved in controlling photosystem gene expression are involved in differentially controlling respiratory gene expression.
FIG. 5.
Regulatory scheme for control of the terminal oxidase operons in R. capsulatus. In response to oxygen availability, the regulators provide negative or positive transcriptional control to coordinate enzyme synthesis for optimal growth. The regulators provide control as follows: (i) RegB-RegA provides regulation in response to both aerobiosis and anaerobiosis, (ii) FnrL provides regulation in response to anaerobiosis, (iii) HvrA provides regulation in response to anaerobiosis, (iv) CrtJ provides regulation in response to aerobiosis, and (v) AerR provides regulation in response to aerobiosis. The operons code for the following genes: cydAB, ubiquinol oxidase; and ccoNOPQ, cytochrome cbb3 oxidase. Positive control (transcriptional activation) (+) and negative control (transcriptional repression) (−) of the genes are indicated.
The terminal oxidases utilized by E. coli follow an expression pattern similar to that for the terminal oxidases of R. capsulatus. The E. coli cytochrome o and cytochrome d oxidases have different affinities for oxygen, with cytochrome o oxidase having a low affinity for oxygen (Km = 1.4 to 2.9 μM) and cytochrome d having a high affinity for oxygen (Km = 0.23 to 0.38 μM) (18). Therefore, the cell utilizes cytochrome o oxidase for aerobic respiration when oxygen is plentiful. As oxygen becomes depleted, the cell utilizes cytochrome d for respiration because its higher affinity for oxygen allows it to function even when oxygen is scarce. In accordance with this, it has been demonstrated that the cell regulates an inverse expression of these oxidases in response to changes in oxygen tension (22).
The actual affinities of the R. capsulatus cytochrome cbb3 and ubiquinol oxidases for oxygen have not yet been established. However, as is the case for E. coli (22), both of the R. capsulatus oxidases are expressed maximally under semiaerobic conditions. Also like E. coli, the aerobic and anaerobic expression patterns of the two R. capsulatus oxidases are reciprocal (Fig. 2 and 3). This pattern of expression suggests that cytochrome cbb3 is the low-affinity oxidase and that ubiquinol is the high-affinity oxidase. In order to maintain the reciprocal pattern of expression of the terminal oxidases, the cell must regulate transcription of each oxidase according to oxygen availability.
It has been demonstrated that the RegB-RegA regulatory system plays a key role in the transcriptional regulation of the terminal oxidases in R. capsulatus. In addition to RegA, R. capsulatus utilizes a homolog of E. coli Fnr to differentially control terminal oxidase gene expression. In E. coli Fnr functions as a repressor of cytochrome o oxidase semiaerobically but has no effect anaerobically. For cytochrome d oxidase, Fnr switches roles by functioning as an activator semiaerobically and as a repressor anaerobically (4). Similarly, the fnrL mutant in R. capsulatus also has no effect anaerobically on ubiquinol oxidase expression but acts as a repressor semiaerobically (Fig. 2). Conversely, FnrL acts as an activator of cbb3 oxidase expression both semiaerobically and anaerobically (Fig. 3). The phenotype of the regA-fnrL double mutant in R. capsulatus is intriguing though presently unexplainable. In addition to the substantially lowered terminal oxidase expression observed in these mutants, there is also the lack of growth under photosynthetic (anaerobic) conditions. The exact explanation for the nongrowth phenotype observed has yet to be uncovered, but it may be noted that an arcA-fnrL double mutant in E. coli also demonstrates insignificant expression of cytochrome d oxidase regardless of the availability of oxygen (9).
In addition to the regulators with functional similarities to E. coli oxidase regulators, there are other “photosystem specific” oxygen-responsive regulators utilized by R. capsulatus. Specifically, aerobic repression of ubiquinol oxidase appears to be promoted by the photopigment repressors CrtJ and AerR. These two repressors are known to be responsible for aerobic repression of bacteriochlorophyll, carotenoid, light harvesting, and reaction center gene expression (5, 8, 17). Our observation that CrtJ and AerR also aerobically repress expression of ubiquinol oxidase (Fig. 2) further supports the recent observation that R. capsulatus coordinates many different aerobic and anaerobic processes, such as photosynthesis (16), respiration (21), nitrogen fixation (7), carbon fixation (23), and hydrogen utilization (7). This appears to be mediated in part through the use of global response regulators such as RegB-RegA, as well as by CrtJ and AerR, which have overlapping photosynthesis and nonphotosynthesis target genes.
The utilization of multiple regulators for this process may be explained through their various sensitivities to oxygen that can be observed in this study (Fig. 5). As oxygen becomes depleted, some regulators may lose activity while others gain activity. For this reason it would be necessary for multiple regulators to be utilized so that the cell can optimally coordinate the synthesis of respiratory, photosynthetic, and biosynthetic processes according to the available levels of oxygen. The remaining challenge will be to determine mechanistic details of how the two terminal oxidase promoters are controlled by the aforementioned transcription factors and whether many of the observed effects observed are the direct result of interactions of these regulators with these respiratory promoters.
Acknowledgments
We thank Lee Swem for his helpful comments regarding the manuscript.
This work was supported by National Institutes of Health grants GM 53940 and GM40941.
REFERENCES
- 1.Baccarini Melandri, A., D. Zannoni, and B. A. Melandri. 1973. Energy transduction in photosynthetic bacteria. VI. Respiratory sites of energy conservation in membranes from dark-grown cells of Rhodopseudomonas capsulata. Biochim. Biophys. Acta 314:298-311. [DOI] [PubMed] [Google Scholar]
- 2.Buggy, J., and C. E. Bauer. 1995. Cloning and characterization of senC, a gene involved in both aerobic respiration and photosynthesis gene expression in Rhodobacter capsulatus. J. Bacteriol. 177:6958-6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buggy, J. J., M. W. Sganga, and C. E. Bauer. 1994. Characterization of a light-responding trans-activator responsible for differentially controlling reaction center and light-harvesting-I gene expression in Rhodobacter capsulatus. J. Bacteriol. 176:6936-6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotter, P. A., and R. P. Gunsalus. 1992. Contribution of the fnr and arcA gene products in coordinate regulation of cytochrome o and d oxidase (cyoABCDE and cydAB) genes in Escherichia coli. FEMS Microbiol. Lett. 70:31-36. [DOI] [PubMed] [Google Scholar]
- 5.Dong, C., S. Elsen, L. R. Swem, and C. E. Bauer. 2002. AerR, a second aerobic repressor of photosynthesis gene expression in Rhodobacter capsulatus. J. Bacteriol. 184:2805-2814. [DOI] [PMC free article] [PubMed]
- 6.Du, S., T. H. Bird, and C. E. Bauer. 1998. DNA binding characteristics of RegA. A constitutively active anaerobic activator of photosynthesis gene expression in Rhodobacter capsulatus. J. Biol. Chem. 273:18509-18513. [DOI] [PubMed] [Google Scholar]
- 7.Elsen, S., W. Dischert, A. Colbeau, and C. E. Bauer. 2000. Expression of uptake hydrogenase and molybdenum nitrogenase in Rhodobacter capsulatus is coregulated by the RegB-RegA two-component regulatory system. J. Bacteriol. 182:2831-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsen, S., S. N. Ponnampalam, and C. E. Bauer. 1998. CrtJ bound to distant binding sites interacts cooperatively to aerobically repress photopigment biosynthesis and light harvesting II gene expression in Rhodobacter capsulatus. J. Biol. Chem. 273:30762-30769. [DOI] [PubMed] [Google Scholar]
- 9.Fu, H. A., S. Iuchi, and E. C. Lin. 1991. The requirement of ArcA and Fnr for peak expression of the cyd operon in Escherichia coli under microaerobic conditions. Mol. Gen. Genet. 226:209-213. [DOI] [PubMed] [Google Scholar]
- 10.Kouadio, J. L. 1997. Functional characterization of transcription factors involved in photosynthesis from the purple non-sulfur bacterium Rhodobacter capsulatus. Ph. D. thesis. Indiana University, Bloomington.
- 11.La Monica, R. F., and B. L. Marrs. 1976. The branched respiratory system of photosynthetically grown Rhodopseudomonas capsulata. Biochim. Biophys. Acta 423:431-439. [DOI] [PubMed] [Google Scholar]
- 12.Marrs, B., and H. Gest. 1973. Genetic mutations affecting the respiratory electron-transport system of the photosynthetic bacterium Rhodopseudomonas capsulata. J. Bacteriol. 114:1045-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell, P. 1966. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol. Rev. Camb. Philos. Soc. 41:445-502. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell, P. 1979. Compartmentation and communication in living systems. Ligand conduction: a general catalytic principle in chemical, osmotic and chemiosmotic reaction systems. Eur. J. Biochem. 95:1-20. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell, P. 1961. Coupling of phosphorylation to electron and hydrogen transfer by chemiosmotic type of mechanism. Nature (London) 191:144-148. [DOI] [PubMed] [Google Scholar]
- 16.Mosley, C. S., J. Y. Suzuki, and C. E. Bauer. 1994. Identification and molecular genetic characterization of a sensor kinase responsible for coordinately regulating light harvesting and reaction center gene expression in response to anaerobiosis. J. Bacteriol. 176:7566-7573. (Erratum, 177:3359, 1995.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponnampalam, S. N., and C. E. Bauer. 1997. DNA binding characteristics of CrtJ. A redox-responding repressor of bacteriochlorophyll, carotenoid, and light harvesting-II gene expression in Rhodobacter capsulatus. J. Biol. Chem. 272:18391-18396. [DOI] [PubMed] [Google Scholar]
- 18.Poole, R. K., and W. J. Ingledew. 1987. Pathways of electrons to oxygen, p. 170-200. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol. 1. American Society for Microbiology, Washington, D.C. [Google Scholar]
- 19.Sganga, M. W., and C. E. Bauer. 1992. Regulatory factors controlling photosynthetic reaction center and light-harvesting gene expression in Rhodobacter capsulatus. Cell 68:945-954. [DOI] [PubMed] [Google Scholar]
- 20.Swem, D. L. 2001. Coordination of ubiquinol and cytochrome cbb3 oxidase expression by regulators in R. capsulatus. M.A. thesis. Indiana University, Bloomington. [DOI] [PMC free article] [PubMed]
- 21.Swem, L. R., S. Elsen, T. H. Bird, D. L. Swem, H. G. Koch, H. Myllykallio, F. Daldal, and C. E. Bauer. 2001. The RegB/RegA two-component regulatory system controls synthesis of photosynthesis and respiratory electron transfer components in Rhodobacter capsulatus. J. Mol. Biol. 309:121-138. [DOI] [PubMed] [Google Scholar]
- 22.Tseng, C. P., J. Albrecht, and R. P. Gunsalus. 1996. Effect of microaerophilic cell growth conditions on expression of the aerobic (cyoABCDE and cydAB) and anaerobic (narGHJI, frdABCD, and dmsABC) respiratory pathway genes in Escherichia coli. J. Bacteriol. 178:1094-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vichivanives, P., T. H. Bird, C. E. Bauer, and F. R. Tabita. 2000. Multiple regulators and their interactions in vivo and in vitro with the cbb regulons of Rhodobacter capsulatus. J. Mol. Biol. 300:1079-1099. [DOI] [PubMed] [Google Scholar]
- 24.Young, D. A., C. E. Bauer, J. C. Williams, and B. L. Marrs. 1989. Genetic evidence for superoperonal organization of genes for photosynthetic pigments and pigment-binding proteins in Rhodobacter capsulatus. Mol. Gen. Genet. 218:1-12. [DOI] [PubMed] [Google Scholar]
- 25.Zannoni, D. 1995. Aerobic and anaerobic electron transport chains in anoxygenic phototrophic bacteria, p. 949. In R. E. Blankenship, M. T. Madigan, and C. E. Bauer (ed.), Anoxygenic photosynthetic bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 26.Zannoni, D., B. A. Melandri, and A. Baccarini-Melandri. 1976. Composition and function of the branched oxidase system in wild-type and respiratory mutants of Rhodopseudomonas capsulata. Biochim. Biophys. Acta 423:413-430. [DOI] [PubMed] [Google Scholar]
- 27.Zeilstra-Ryalls, J. H., K. Gabbert, N. J. Mouncey, S. Kaplan, and R. G. Kranz. 1997. Analysis of the fnrL gene and its function in Rhodobacter capsulatus. J. Bacteriol. 179:7264-7273. [DOI] [PMC free article] [PubMed] [Google Scholar]