Abstract
The Escherichia coli RrmJ (FtsJ) heat shock protein functions as an rRNA methyltransferase that modifies position U2552 of 23S rRNA in intact 50S ribosomal subunits. An in-frame deletion of the rrmJ (ftsJ) gene leads to severe growth disadvantages under all temperatures tested and causes significant accumulation of ribosomal subunits at the expense of functional 70S ribosomes. To investigate whether overexpression of other E. coli genes can restore the severe growth defect observed in rrmJ null mutants, we constructed an overexpression library from the rrmJ deletion strain and cloned and identified the E. coli genes that were capable of rescuing the rrmJ mutant phenotype. Our intention was to identify other methylases whose specificities overlapped enough with that of RrmJ to allow complementation when overexpressed. To our great surprise, no methylases were found by this method; rather, two small GTPases, Obg (YhbZ) and EngA, when overexpressed in the rrmJ deletion strains, were found to restore the otherwise severely impaired ribosome assembly process and/or stability of 70S ribosomes. 50S ribosomal subunits prepared from these overexpressing strains were shown to still serve as in vitro substrates for purified RrmJ, indicating that the 23S rRNA likely was still lacking the highly conserved Um2552 modification. The apparent lack of this modification, however, no longer caused ribosome defects or a growth disadvantage. Massive overexpression of another related small GTPase, Era, failed to rescue the growth defects of an rrmJ strain. These findings suggest a hitherto unexpected connection between rRNA methylation and GTPase function, specifically that of the two small GTPases Obg and EngA.
The explosion in sequencing studies has revealed a substantial number of conserved genes without assigned functions. One of the major challenges of the postgenomic era is that of ascertaining what these genes do. We have decided to focus on analysis of the function(s) of newly identified heat shock proteins. Heat shock proteins are synthesized in large quantities under stress conditions and are thought to enable organisms to survive severe environmental stress such as heat shock, oxidative stress, and viral infections (3). By far the best-characterized heat shock proteins are the molecular chaperones, including the DnaK-DnaJ-GrpE chaperone complex and the GroEL-GroES chaperone team, which function to promote protein folding (17). Other heat shock proteins include transcription factors and proteases that function to degrade misfolded or abnormal proteins (10).
Recently, Blattner and coworkers reinvestigated the heat shock response in Escherichia coli by using gene chip technology (28). Homology searches and sequence analysis revealed that nearly half of the heat-inducible genes discovered in this manner have neither known function nor homology to proteins of known function (28). These proteins represent a largely untapped resource of novel heat shock proteins unrelated to previously studied heat shock proteins. We have initiated the analysis of structure and function of several of these newly identified heat shock proteins, motivated by the possibility of discovering new molecular chaperones or proteins with other interesting functions previously not connected to heat shock response in the cell. This should help us to understand the role of the heat shock response in much greater detail.
We picked three strongly heat-inducible proteins for our studies, Hsp33, Hsp15, and RrmJ (FtsJ). Their extensive conservation ensured that the functional and structural information obtained would be widely applicable. Hsp33 was found to be a novel redox-regulated molecular chaperone (11). Hsp15 was discovered to be an RNA binding protein involved in ribosome recycling (14). The third protein, RrmJ, was the first rRNA methyltransferase found to be under heat shock control (5, 6).
The rrmJ (ftsJ) gene, which was originally identified by Ogura and coworkers (26), is part of the rrmJ-hflB operon. RrmJ is a well conserved 209-residue heat shock protein present in many species in the domains Eubacteria, Archaea, and Eucarya, including humans (5). Upon heat treatment, rrmJ mRNA levels in E. coli increase more than 20-fold (28). The crystal structure of RrmJ, which we have solved to a 1.5-Å level of resolution, shows that RrmJ adopts an S-adenosyl-methionine (AdoMet)-dependent methyltransferase fold comprised of seven β strands with five interspersed α-helices (5). Adjacent to the AdoMet binding site, a distinct cleft is noticeable that is lined with highly conserved, positively charged residues. This could represent the substrate binding site of RrmJ. We and others have shown that RrmJ functions as an AdoMet-dependent rRNA methyltransferase (5, 6). RrmJ methylates 23S rRNA both in vitro and in vivo and creates the highly conserved 2′-O-methyluridine at position 2552 in 23S rRNA (6). In order for RrmJ to efficiently methylate 23S rRNA, the rRNA needs to be folded and present within the mature 50S ribosomal subunits (5). rrmJ deletion mutants show a substantial increase in levels of unassembled 30S and 50S subunits and a significant decrease in that of 70S ribosomes. This ribosome defect is caused by the in-frame deletion of rrmJ and reflects severely impaired ribosome assembly or stability of 70S ribosomes (5, 6). Given the great reduction in levels of functional ribosomes in rrmJ deletion strains, it is not surprising that these cells also display a significant growth defect on plates and in liquid culture (5, 7). With the identification of RrmJ's function as that of an rRNA methyltransferase, we have established an unexpected connection between RNA methylation and the heat shock response.
In this study, we attempted to gain further understanding of the function of RrmJ by identifying proteins that, when overproduced, suppressed the rrmJ deletion phenotype. Apart from rrmJ, we found two additional genes that rescued the ribosome and growth defect observed in rrmJ deletion strains, obg (yhbZ), and engA. Both obg and engA genes encode members of highly conserved small GTPase families, and we show here that Obg (YhbZ) has GTPase activity. We also show that Era, another small GTPase in E. coli that is closely related to EngA, is unable to rescue the rrmJ deletion phenotype.
MATERIALS AND METHODS
Bacterial strains and media.
E. coli strains were grown at 37°C in Luria-Bertani medium with ampicillin (100 μg/ml) as needed. MacConkey agar was used to screen for suppressor mutants of rrmJ (ftsJ). The strains and plasmids that were used are listed in Table 1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| BL21(DE3) | F−ompT hsdSB(rB− mB−) gal dcm(DE3) | Novagen |
| MG1655 | rph-1 | Lab collection |
| HB23 | MG1655 zgi-203::Tn10 TCrrrmJ (ftsJ) Δ567 | 5 |
| HB24 | MG1655, zgi-203::Tn10, TCr | 5 |
| JT36 | HB23(pJT36) | This study |
| JT37 | HB23(pJT37) | This study |
| JT87 | HB23(pJT87) | This study |
| JT99 | HB23(pJT87) | This study |
| JT130 | HB23(pJT130) | This study |
| JT197 | HB23(pHKP60) | This study |
| Plasmids | ||
| pJT36 | pCR2.1/obg | This study |
| pJT37 | pCR2.1/engA | This study |
| pJT87 | pUC18/engA | This study |
| pJT99 | pUC18/obg | This study |
| pJT130 | pET11a/obg | This study |
| pJT147 | pET11a/engA | This study |
| pHKP60 | pACYC/era | Donald Court |
Genomic library construction.
Genomic DNA from the rrmJ deletion strain HB23 was prepared as previously described (31). The genomic DNA was partially digested by using Sau3A. Sau3A concentrations ranged from 0.8 to 8 U per 100 μl of digest; each digest mixture contained 55 μg of genomic DNA. Aliquots of the digested products were analyzed on 1% TAE agarose gels, and the sample showing the best partial digest was purified using the QIAquick PCR purification kit (QIAGEN). Then, ligation of the fragments into pUC18 BamHI/BAP vectors (Amersham Pharmacia Biotech) was performed.
Erase-A-Base reaction.
This procedure was carried out according to the manufacturer's protocol (Promega). The Erase-A-Base reaction was allowed to proceed for a total of 6 min, with samples removed at 30-s intervals. The samples were then purified by using QIAquick columns (QIAGEN) and electroporated into the rrmJ deletion strain HB23, and the transformants were screened on MacConkey plates. The Erase-A-Base reaction was allowed to proceed for 1 min, and the transformants that were derived from the reaction were then screened a second time on MacConkey plates to verify colony size. Then, 12 plasmids were extracted for sequencing.
Cloning of obg (yhbZ) and engA.
The primers used for amplifying the obg gene from pJT99 and engA gene from pJT87 by PCR were as follows: obg forward primer, 5′ catatgaagtttgttgatgaagcatcg 3′; obg reverse primer, 5′ ggatccttaacgcttgtaaatgaactcaacgc 3′; engA forward primer, 5′ catatgcgttgtctgatgatttataaaaatgagg 3′; and engA reverse primer, 5′ ggatccttatttatttttcttgatgtgcttcatc 3′.
PCR and subsequent cloning steps were performed with the TA cloning kit (Invitrogen) and yielded the following constructs: pJT36 (pCR2.1; obg) and pJT37 (pCR2.1; engA). The inserts were excised and ligated into pET11a to generate pJT130 (pET11a; obg) and pJT147 (pET11a; engA). The overexpressing plasmids were then transformed into E. coli BL21 cells. The correct sequences of the constructs were confirmed by sequencing using the ABI automated sequencer (Perkin-Elmer).
Purification of Obg (YhbZ).
E. coli BL21 cells harboring pJT130 were grown at 37°C to an optical density at 600 nm of 0.8. Then, protein expression was induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). At 4 h after the induction procedure, the cells were harvested by centrifugation (10 min, 4,000 × g, 4°C) and were resuspended in 40 ml of lysis buffer (40 mM HEPES-KOH [pH 7.5], 400 mM KCl, 1 mM dithiothreitol) supplemented with 1 tablet of Boehringer Complete protease inhibitor mix and 2 mM phenylmethylsulfonyl fluoride (PMSF). The cells were lysed with a French Press (2 cycles, 14,000 lb/in2). Native Obg was then purified according to a published Era purification protocol (23) with several modifications. The cell lysate was centrifuged at 48,000 × g for 30 min at 4°C, and the cleared supernatant was applied to a 40-ml Q-Sepharose column (Pharmacia) that was equilibrated in lysis buffer. Obg was eluted by applying a 400-ml gradient in lysis buffer with KCl concentrations ranging from 400 to 1 M. Obg eluted at about 500 mM KCl. Obg-containing fractions were pooled and dialyzed against 20 mM K2HPO4-10% glycerol (pH 6.8) and loaded onto a 40-ml hydroxylapatite column (Bio-Rad). Obg was eluted by applying a 360-ml gradient with 20 to 400 mM KH2PO4-10% glycerol (pH 6.8). Obg eluted at about 130 mM KH2PO4. Highly purified Obg fractions were then pooled and dialyzed against storage buffer (40 mM HEPES-KOH [pH 7.5], 20 mM KCl, 5 mM MgCl2, 20% glycerol). An extinction coefficient of 1.3 for a 1 mg/ml solution at an optical density of 280 was used to determine the exact protein concentration (9).
Polysome profiles.
Cell lysates of strains HB23, HB24, JT87, and JT99 were prepared as previously described (5). Polysome profiles were obtained by loading 70 μg of lysate onto 10 to 50% sucrose gradients in 10-100 buffer (10 mM MgCl2 and 100 mM NH4Cl in 20 mM HEPES-KOH [pH 7.5]-4 mM β-mercaptoethanol) and subsequent ultracentrifugation (2°C, w2t (setting for angular velocity) = 2.87 × 1011 rad2/s) using an SW40Ti rotor in a Beckman L8-70 ultracentrifuge. The sucrose gradients were analyzed as previously described (5).
GTPase activity assay.
Initial rates were measured by monitoring the Obg-catalyzed conversion of [α-32P]GTP into [α-32P]GDP at 37°C with thin-layer chromatography (12). The reaction mixture (20 μl) contained 0.1 μM [α-32P]GTP (100 Ci/mmol), 99.9 μM GTP, 40 mM HEPES-KOH [pH 7.0], 5 mM MgCl2, 100 mM KCl, and 10% glycerol. The reaction was started by the addition of 2 μM Obg, and samples were incubated at 37°C. At defined time points (5, 15, 30, and 60 min) after the start of the incubation reaction, 3-μl aliquots were taken and the reaction was stopped by the addition of 2 μl of quenching solution containing 100 mM GTP, 100 mM GDP, and 100 mM EDTA and by incubation on ice. The 5-μl samples were then spotted onto a polyethyleneimine cellulose apparatus (Selecto Scientific), and thin-layer chromatography was performed in 0.5 M LiCl2-2 M formic acid at room temperature. Quantification of [α-32P]GTP and [α-32P]GDP was performed using a PhosphorImager (Molecular Dynamics) and MolecularAnalyst software.
Methylation assay.
To determine the methylation state of 50S ribosomal subunits in HB23 strains that overexpress Obg or EngA, 50S ribosomal subunits from both JT36 (HB23 harboring pCR2.1; obg) and JT37 (HB23 harboring pCR2.1; engA) as well as HB23 and HB24 were prepared as previously described (5) and used as substrates for an RrmJ in vitro methylation assay (5). RrmJ (0.1 μM) was incubated with 50 μM 3H-methyl AdoMet (80 μCi/ml) in methylation buffer (50 mM HEPES-KOH [pH 7.5], 85 mM NH4Cl, 3 mM MgCl2, 2 mM β-mercaptoethanol), and the methylation reaction was started by adding 50S subunits (5 μM) of the various strains. At 150 min after the start of the incubation reaction, 8-μl aliquots were taken and the methyl incorporation rate was determined as previously described (5). The experiments were performed in triplicate.
RESULTS AND DISCUSSION
Isolation of suppressors of an rrmJ (ftsJ) deletion mutant.
RrmJ (ftsJ) deletion strains show a severe growth disadvantage on plates and in liquid culture. This is most likely because of their ribosome defect, which appears to be due to the absence of the highly conserved uridine methylation at position 2552 in 23S rRNA of E. coli (5, 6). MG1655 strains harboring an in-frame deletion of rrmJ (HB23) were previously shown to display a severe growth defect on MacConkey agar, showing hardly any growth after an overnight incubation and only residual growth after 48 h (5). The rrmJ+ isogenic wild-type strain (HB24), on the other hand, displays robust growth on MacConkey agar. This phenotype was used to screen for overproduction suppressors. In order to avoid repeated recloning of the rrmJ locus and to obtain other genes able to suppress the phenotype when overexpressed, a genomic library, starting with DNA from the rrmJ null mutant (HB23), was constructed as described in Materials and Methods. The library was electroporated into the rrmJ deletion strain (HB23), and selection was performed on MacConkey agar. Complementation of ΔrrmJ was determined by the ability of the clones to restore the growth of large colonies on MacConkey agar plates after an overnight incubation at 37°C. To test whether the rescue was due to the presence of a plasmid clone or to chromosomal mutations capable of suppressing the rrmJ null mutant, DNAs from the clones which had restored the growth of the rrmJ deletion strains were repurified and retransformed into the rrmJ null mutant strain HB23. All of the isolated plasmids were capable of conferring rescue, confirming that the restoration of growth was indeed linked to the plasmids and was not due to chromosomal suppressors.
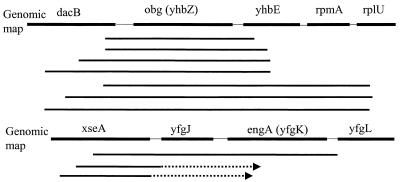
A total of 18 individual clones were sequenced. Sequence analysis showed that 15 clones that were able to rescue the rrmJ deletion phenotype contained a 1.1-kb open reading frame encoding the obg (yhbZ) gene product and three clones contained a 1.5-kb open reading frame encoding the engA (yfgK) gene product (Fig. 1).
FIG. 1.
obg (yhbZ) and engA (yfgK) clone maps. Solid bold lines represent clones sequenced from both ends, while dashed lines indicate clones sequenced from one end; arrows indicate the direction of sequencing. A total of 15 clones that showed rescue of the rrmJ deletion phenotype contained the 1.1-kb obg (yhbZ) gene (redundant clones not shown), while 3 contained the 1.5-kb engA (yfgK) gene.
The information required for the rescue of the rrmJ (ftsJ) mutant phenotype is at the protein level.
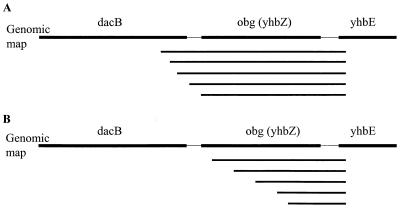
The most obvious interpretation of the obtained results was that the proteins encoded respectively by the obg (yhbZ) and engA (yfgK) genes were responsible for rescuing the phenotype of an rrmJ deletion strain. An alternative explanation to these results, however, was that the rescue occurs at the DNA level. One possibility, for instance, is that a DNA site, which when present in multiple copies is capable of titrating out a repressor controlling the expression of another methyltransferase that might substitute for RrmJ, is present in or near these open reading frames. To determine if the open reading frames were directly responsible for the observed rescue, we created subclones of one of the obg (yhbZ)-containing clones by using the Erase-a-base method (Promega). Using this method, an increasing number of bases were removed from the 3′ end of the obg (yhbZ) clone. These deletion derivatives were then transformed into the rrmJ deletion strain (HB23), and the transformants were plated onto MacConkey agar. A selected number of clones that failed to rescue (tiny colonies) and clones that were able to rescue the growth defect of HB23 (ΔrrmJ) (big colonies) were sequenced. Only the clones that contained the intact obg gene were capable of rescuing the phenotype (Fig. 2A). Deletion of as few as 23 bp from the obg gene was sufficient to completely abolish the ability of the clones to rescue the severe growth defect of the rrmJ deletion strain (Fig. 2B). This suggested that the expression of an intact Obg protein is necessary for the efficient rescue of the phenotype.
FIG. 2.
obg (yhbZ) subclones sequenced from both ends with the Erase-A-Base system. (A) All clones that conferred rescue of the rrmJ deletion phenotype contained the complete obg open reading frame. (B) All clones that did not rescue the rrmJ deletion growth defect did not contain the entire obg gene. obg is shown in complement, and deletions were from the 3′ end of the gene.
Overexpression of Obg and EngA rescues the growth and ribosome defects of rrmJ (ftsJ) deletion strains.
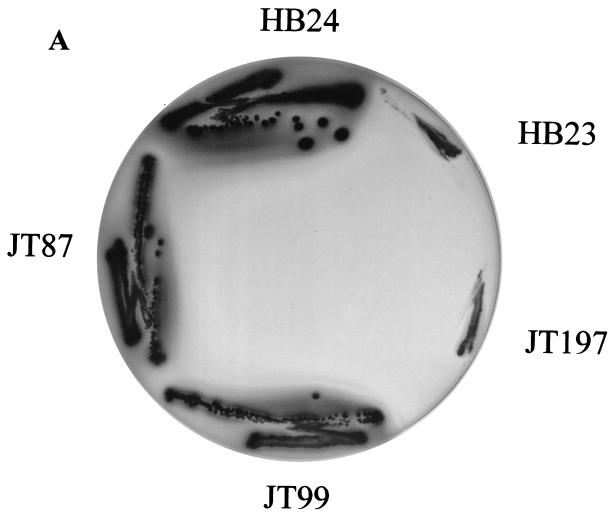
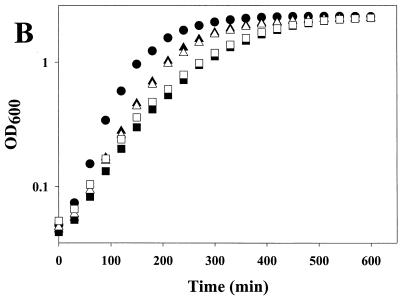
The engA and obg genes without any flanking sequences were amplified by PCR and cloned into pUC18 to generate the plasmids pJT87 and pJT99, respectively. The overexpressing plasmids were then introduced into the rrmJ deletion strain HB23, creating the strains JT87 and JT99, respectively. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of total protein extracts prepared from strains containing these plasmids revealed that JT87 overexpressed the 56-kDa protein EngA while JT99 overexpressed the 43.3-kDa Obg protein. The growth of the HB24, HB23, JT87, and JT99 strains was first compared at different temperatures (30°C, 37°C, and 43°C) on plates. The wild-type strain (HB24) grew rapidly under all conditions tested. In contrast, the growth of the rrmJ deletion strain HB23 was severely impaired under all temperature conditions. At 30°C, small colonies of HB23 (ΔrrmJ) appeared on MacConkey agar but only after 2 days of incubation (see Fig. 3A). The impaired growth of HB23 (ΔrrmJ) was largely alleviated when the plasmids carrying engA (pJT87) or obg (pJT99) were introduced into this mutant strain. The presence of these plasmids led to a substantial increase in growth rates at all temperatures tested (Fig. 3B; data not shown for growth at 30°C and 43°C). Next, the ribosome profiles of HB23, HB24, JT87, and JT99 cells were examined using sucrose gradient centrifugation under nonstringent salt conditions (10 mM MgCl2, 100 mM NH4Cl). Under these salt conditions, 70S ribosomes were stabilized and only small amounts of 30S and 50S ribosomal subunits were detectable in wild-type E. coli strains (Fig. 4A). The ribosome profiles of rrmJ deletion strains, on the other hand, show an accumulation of unassembled 30S and 50S ribosomal subunits. This accumulation of ribosomal subunits is presumably due to the absence of uridine methylation at position 2552 in 23S rRNA (5, 6). This highly conserved modification is located in the A site of the ribosome, and the absence of the methylase that catalyzes its formation either causes problems in proper 70S assembly or leads to premature disassembly of the mature 70S ribosomes (5). We note that so far, only the absence of the methylase has been shown to cause this effect. Although it seems likely, it remains to be proven that it is the absence of Um2552 that is the direct cause of the ribosome profile differences. It remains possible that RrmJ has a function in addition to its methylation activity. There is precedence for this hypothesis in the literature. The trmA gene which encodes the tRNA(m5U54) methyltransferase is essential for viability, yet the point mutation trmA5, which completely eliminates the tRNA(m5U54) methyltransferase activity, is viable (27). This suggests that the trmA gene product has at least two functions. Dim1p is a yeast methylase responsible for the m62Am62A at the 3′ terminus of the small subunit rRNA. Although the dim1 gene is essential, its methylase activity is not (15, 16).
FIG. 3.
Overexpression of Obg (YhbZ) or EngA rescues the growth defect of an rrmJ (ftsJ) deletion strain. (A) HB23 (rrmJ deletion strain), HB24 (wild type), JT87 (HB23 expressing EngA), JT99 (HB23 expressing Obg), and JT197 (HB23 expressing Era) were streaked onto MacConkey agar and incubated at 30°C overnight. HB23 (ΔrrmJ) shows practically no growth, while HB24 displays healthy growth. Both the JT87 and JT99 strains show growth very similar to that observed for the rrmJ+ isogenic wild-type strain. Era overexpression in strain JT197 is unable to rescue the rrmJ deletion phenotype. (B) Growth of HB23 (▪), HB24 (•), JT87 (▴), JT99 (▵), and JT197 (□) were monitored over a period of 600 min at 37°C. Growth rates of HB23 and JT197 were lower than those of the other strains.
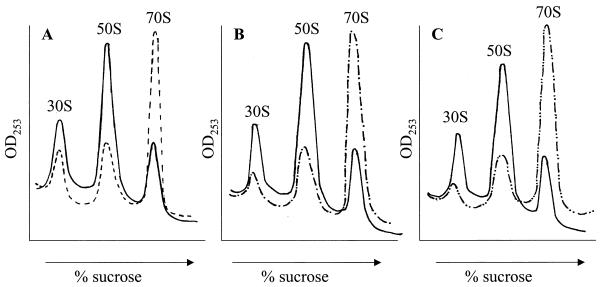
FIG. 4.
Obg (YhbZ) and EngA overexpression rescue the ribosome defect of rrmJ (ftsJ) deletion strains. (A) The polysome profile of HB23 (ΔrrmJ) (solid line) shows an increase in the levels of unassembled 30S and 50S subunits and a decrease in those of intact 70S ribosomes, while that of HB24 (dashed line) shows a wild-type profile consisting of low levels of 30S and 50S ribosomal subunits and a high level of 70S ribosomes. (B) Polysome profile of HB23 (ΔrrmJ) (solid line) in comparison to that of HB23 (ΔrrmJ) overexpressing EngA (dashed line). (C) The polysome profile of HB23 (ΔrrrmJ) (solid line) in comparison to that of HB23 (ΔrrmJ) overexpressing Obg (dashed line).
Lysates obtained from JT87 or JT99, in which the rrmJ deletion was complemented by a plasmid-borne copy of the engA or obg gene, respectively, exhibited a ratio of 70S ribosomes to 50S and 30S subunits that closely resembled that of the wild-type strain (Fig. 4B and C). These results were in excellent agreement with the observed rescue of the growth defects conferred by the respective plasmids and demonstrated that overexpression of either Obg or EngA led to the formation and stabilization of intact 70S ribosomes in rrmJ deletion strains.
Obg (YhbZ) and EngA are members of the family of small GTPases.
BLAST searches (1) revealed that Obg is homologous to members of the Obg/Gtp1 subfamily of small GTP binding proteins. Obg from E. coli shows 43% sequence identity to CgtA from Caulobacter crescentus, 39% sequence identity to Obg from Bacillus subtilis, and 40% sequence identity to its human homologue. Multisequence alignments of Obg with members of the Obg family of GTPases revealed conservation of the GTP binding domains G1 (GxxxxGK[S/T]) in Obg 161-DVGMLGMPNAGKSTFI-176, G2 (YxFTT) in Obg 185-KVADYPFTTLV-195, G3 (DxxG) in Obg 209-FVVADIPG-216, and G4 (NKxD) in Obg 279-WLVFNKID-286 (8). The conserved residues in Obg are shown in boldface. These four critical domains are required for GDP-GTP exchange, GTP-induced conformational changes, and GTP hydrolysis (4), suggesting their functional importance. EngA, on the other hand, showed significant sequence identity (26%) to Era (E. coli Ras-like protein), another family of small GTPases. Very recently, a detailed comparative analysis has been performed that revealed the existence of a total of six families of small GTPases in prokaryotes (Era, Obg, EngA, ThdF, YchF, and YihA) (25).
Members of the Obg family are widely distributed among phyla and were detected in every genome that has been completely sequenced, pointing to strong selective pressure to maintain this family of GTPases. The essential nature of Obg in E. coli (2), Obg in Bacillus subtilis (32) and CgtA, the Obg family member in Caulobacter crescentus (20) corroborates the importance of the Obg family. EngA is essential in Neisseria gonorrhoeae; indeed, the name EngA stands for “Essential Neisserial GTPase” (22).
Obg (YhbZ) possesses intrinsic GTPase activity.
The presence of the GTP binding motifs sequentially arranged in Obg strongly suggested that Obg binds GTP. These motifs are very similar to the ones found in Obg-like proteins of Bacillus subtilis and Caulobacter crescentus, the only two members of the Obg family that have been biochemically characterized and have been previously shown to bind GTP (18, 33). Since neither Obg from E. coli nor EngA has been characterized in vitro, we cloned both genes into pET11a overexpressing vectors, transformed the vectors into BL21 strains, overexpressed the proteins, and attempted to purify both GTPases. While EngA could not be purified due to its high tendency toward irreversible aggregation, we established an efficient purification protocol for Obg. The purification of Obg from E. coli involves two chromatography steps, Q-Sepharose anion exchange chromatography followed by use of a hydroxylapatite column. After these two chromatography steps, Obg was more than 99% pure. A total of 37 mg of Obg/liter of cell culture was purified.
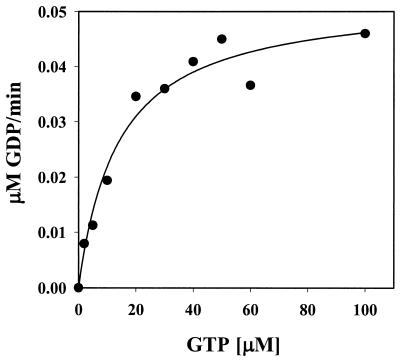
To determine whether Obg is capable of hydrolyzing GTP and to evaluate its kinetic parameters, we measured Obg's ability to convert [α-32P]GTP to [α-32P]GDP by using thin-layer chromatography analysis. Obg converted GTP to GDP in a magnesium-dependent and time-dependent manner. To determine the enzymatic parameters of GTP hydrolysis, the GTP concentration was adjusted from 2 to 100 μM and the reaction products were quantified. Obg's Km for GTP was determined to be 18 μM with a turnover number of 0.02 min−1 (Fig. 5). These enzymatic parameters are very similar to the ones determined for Obg from Bacillus subtilis, whose GTP Km value was reported to be 5.4 μM with a turnover number of 0.006 min−1, and are much slower than the rates obtained for the α subunits of G proteins, which are in the range of 3 to 5 min−1 (13). These experiments clearly showed that Obg is indeed a member of small GTPases in E. coli.
FIG. 5.
Obg (YhbZ) has GTPase activity. The GTPase activity of Obg was determined as described in Materials and Methods. Obg was present at 2 μM, and the GTP concentration was adjusted from 2 to 100 μM. The Km for GTP was determined to be 18 μM, with a turnover number of 0.02 min−1.
How does overexpression of Obg (YhbZ) or EngA influence the assembly or stability of 70S ribosomes?
We showed here that the overexpression of members of two different families of small GTPases (Obg and EngA) is able to support assembly or confer stability for 70S ribosomes which were otherwise severely defective due to the absence of the rRNA methyltransferase RrmJ. One possibility is that overexpression of any GTPase could rescue rrmJ null mutants. There are at least 15 GTPases present in E. coli (8). Only two of these GTPases were identified as suppressors, and each of these two suppressors was isolated multiple times. This suggests that simple overexpression of any GTPase is not sufficient to rescue rrmJ null mutants. To determine whether rescue of the rrmJ deletion phenotype is enabled by the general overexpression of small GTPases, we decided to test Era, another small GTPase. Era is similar to Obg and EngA in being a monomeric GTPase with an unknown essential function. Of all the GTPases in E. coli, Era is the one most closely related to EngA (21) and Caldon et al. place EngA in the Era subfamily (8). Era has recently been found to specifically interact with 16S rRNA and to bind to 30S ribosomal subunits (29). Its C terminus was shown to be essential for RNA binding, and RNA was demonstrated to modulate the GTPase activity of Era (24). In screening for genes that can restore the growth ability of a cold-sensitive mutant Era, it was found that most of the suppressors contained the ksgA gene that codes for a 16S rRNA methylase (19). All these facts point to the possibility that Era is also involved in ribosomal function. The Era clone (pHKP60) under pBAD control was introduced into the rrmJ (ftsJ) deletion strain HB23 to generate strain JT197. Results show that although Era was overexpressed to much higher levels than Obg (YhbZ) and EngA (as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis), the poor growth of HB23 (ΔrrmJ) was not rescued by Era overexpression (see Fig. 3A and B). This is further evidence that simple overexpression of any GTPase is not sufficient to rescue rrmJ mutants, suggesting that the rescue is more specific than can be accounted for by an alteration in GTP pool levels.
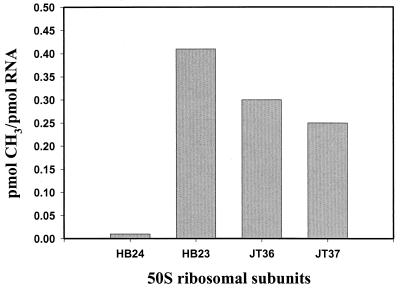
To investigate whether the Obg- and EngA-mediated rescue was due to the activation of a back-up methyltransferase that takes over the function of RrmJ (FtsJ), we analyzed the methylation status of 23S rRNA in rrmJ deletion strains, which overexpressed either Obg or EngA. To do this, we determined the ability of purified RrmJ to methylate the 50S ribosomal subunits prepared from the overexpressing strains. 50S ribosomal subunits prepared from wild-type E. coli strains (HB24) do not serve as a substrate for RrmJ in vitro, because the methylation of U2552 goes to completion in vivo (5, 6) (Fig. 6). RrmJ, on the other hand, methylates 50S ribosomal subunits prepared from rrmJ deletion strains at U2552 quite rapidly in vitro (5, 6). To test whether the 50S ribosomal subunits are methylated or still unmodified in HB23 (ΔrrmJ) strains overexpressing Obg or EngA, 50S ribosomal subunits from both strains were prepared and used as substrates for an RrmJ in vitro methylation assay. The assay was performed several times, and the results were reproducible. As shown in Fig. 6, the ribosomal subunits of both strains were still able to serve as substrates for RrmJ in vitro, indicating that the 23S rRNA is still lacking this important modification. This clearly excluded an indirect effect of the overexpression of both GTPases on the modification of 23S rRNA but suggested that both GTPases exert stabilizing effects on 70S ribosomes, thereby overcoming the effects of the missing modification in the A site of the ribosome. We note that we cannot exclude the possibility that a site different from U2552 is being methylated.
FIG. 6.
50S ribosomal subunits of HB23 (ΔrrmJ) strains overexpressing Obg (YhbZ) or EngA are still substrates for RrmJ. Subunits of 50S from HB24 were not methylated by RrmJ in vitro, while RrmJ efficiently methylated 50S ribosomal subunits prepared from HB23 (ΔrrmJ), JT36 (HB23 [ΔrrmJ] overexpressing Obg), and JT37 (HB23 [ΔrrmJ] overexpressing EngA).
The functions of prokaryotic small GTPases are still poorly defined. Recently, Obg from Bacillus subtilis has been shown to associate with ribosomes during its purification (30). Other bacterial GTP binding proteins have long been associated with translational processes (21). Members of the Obg family have also been found to be physically linked in presumptive operons to genes encoding the ribosomal proteins L21 and L27 in more than 50% of the prokaryotic genomes present in the ERGO database (Integrated Genomics). This type of genetic association very often indicates a functional relationship in prokaryotes. These findings, together with the fact that the Obg proteins are evolutionarily conserved and essential for viability, suggest that these proteins may play a crucial role in ribosomal function. We have now presented evidence that overexpression of Obg and EngA rescues the severe rrmJ mutant phenotype, thereby providing additional evidence that small GTPases are indeed involved in ribosome assembly or stability. These observations raise intriguing possibilities for the role of a GTPase such as Obg in ribosome function.
Acknowledgments
We are indebted to H. Bügl, whose work on RrmJ formed the groundwork of the study presented here. We thank D. Court for helpful discussions and for providing the Era clone.
This work was supported by an NIH grant to J.C.A.B. J.C.A.B. is a PEW scholar, and U.J. is a Burroughs Wellcome Fund scholar.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arigoni, F., F. Talabot, M. Peitsch, M. D. Edgerton, E. Meldrum, E. Allet, R. Fish, T. Jamotte, M. L. Curchod, and H. Loferer. 1998. A genome-based approach for the identification of essential bacterial genes. Nat. Biotechnol. 16:851-856. [DOI] [PubMed] [Google Scholar]
- 3.Becker, J., and E. A. Craig. 1994. Heat-shock proteins as molecular chaperones. Eur. J. Biochem. 219:11-23. [DOI] [PubMed] [Google Scholar]
- 4.Bourne, H. R., D. A. Sanders, and F. McCormick. 1991. The GTPase superfamily: conserved structure and molecular mechanism. Nature 349:117-127. [DOI] [PubMed] [Google Scholar]
- 5.Bugl, H., E. B. Fauman, B. L. Staker, F. Zheng, S. R. Kushner, M. A. Saper, J. C. Bardwell, and U. Jakob. 2000. RNA methylation under heat shock control. Mol. Cell 6:349-360. [DOI] [PubMed] [Google Scholar]
- 6.Caldas, T., E. Binet, P. Bouloc, A. Costa, J. Desgres, and G. Richarme. 2000. The FtsJ/RrmJ heat shock protein of Escherichia coli is a 23 S ribosomal RNA methyltransferase. J. Biol. Chem. 275:16414-16419. [DOI] [PubMed] [Google Scholar]
- 7.Caldas, T., E. Binet, P. Bouloc, and G. Richarme. 2000. Translational defects of Escherichia coli mutants deficient in the Um(2552) 23S ribosomal RNA methyltransferase RrmJ/FTSJ. Biochem. Biophys. Res. Commun. 271:714-718. [DOI] [PubMed] [Google Scholar]
- 8.Caldon, C. E., P. Yoong, and P. E. March. 2001. Evolution of a molecular switch: universal bacterial GTPases regulate ribosome function. Mol. Microbiol. 41:289-297. [DOI] [PubMed] [Google Scholar]
- 9.Gill, S. C., and P. H. von Hippel. 1989. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 182:319-326. [DOI] [PubMed] [Google Scholar]
- 10.Gross, C. A. 1996. Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 11.Jakob, U., W. Muse, M. Eser, and J. C. Bardwell. 1999. Chaperone activity with a redox switch. Cell 96:341-352. [DOI] [PubMed] [Google Scholar]
- 12.Jakob, U., T. Scheibel, S. Bose, J. Reinstein, and J. Buchner. 1996. Assessment of the ATP binding properties of Hsp90. J. Biol. Chem. 271:10035-10041. [DOI] [PubMed] [Google Scholar]
- 13.Kaziro, Y., H. Itoh, T. Kozasa, M. Nakafuku, and T. Satoh. 1991. Structure and function of signal-transducing GTP-binding proteins. Annu. Rev. Biochem. 60:349-400. [DOI] [PubMed] [Google Scholar]
- 14.Korber, P., T. Zander, D. Herschlag, and J. C. Bardwell. 1999. A new heat shock protein that binds nucleic acids. J. Biol. Chem. 274:249-256. [DOI] [PubMed] [Google Scholar]
- 15.Lafontaine, D., J. Delcour, A. L. Glasser, J. Desgres, and J. Vandenhaute. 1994. The DIM1 gene responsible for the conserved m6(2)Am6(2)A dimethylation in the 3′-terminal loop of 18 S rRNA is essential in yeast. J. Mol. Biol. 241:492-497. [DOI] [PubMed] [Google Scholar]
- 16.Lafontaine, D. L., T. Preiss, and D. Tollervey. 1998. Yeast 18S rRNA dimethylase Dim1p: a quality control mechanism in ribosome synthesis? Mol. Cell. Biol. 18:2360-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langer, T., C. Lu, H. Echols, J. Flanagan, M. K. Hayer, and F. U. Hartl. 1992. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature 356:683-689. [DOI] [PubMed] [Google Scholar]
- 18.Lin, B., K. L. Covalle, and J. R. Maddock. 1999. The Caulobacter crescentus CgtA protein displays unusual guanine nucleotide binding and exchange properties. J. Bacteriol. 181:5825-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu, Q., and M. Inouye. 1998. The gene for 16S rRNA methyltransferase (ksgA) functions as a multicopy suppressor for a cold-sensitive mutant of Era, an essential RAS-like GTP-binding protein in Escherichia coli. J. Bacteriol. 180:5243-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maddock, J., A. Bhatt, M. Koch, and J. Skidmore. 1997. Identification of an essential Caulobacter crescentus gene encoding a member of the Obg family of GTP-binding proteins. J. Bacteriol. 179:6426-6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.March, P. E. 1992. Membrane-associated GTPases in bacteria. Mol. Microbiol. 6:1253-1257. [DOI] [PubMed] [Google Scholar]
- 22.Mehr, I. J., C. D. Long, C. D. Serkin, and H. S. Seifert. 2000. A homologue of the recombination-dependent growth gene, rdgC, is involved in gonococcal pilin antigenic variation. Genetics 154:523-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meier, T. I., R. B. Peery, S. R. Jaskunas, and G. Zhao. 1999. 16S rRNA is bound to Era of Streptococcus pneumoniae. J. Bacteriol. 181:5242-5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meier, T. I., R. B. Peery, K. A. McAllister, and G. Zhao. 2000. Era GTPase of Escherichia coli: binding to 16S rRNA and modulation of GTPase activity by RNA and carbohydrates. Microbiology 146:1071-1083. [DOI] [PubMed] [Google Scholar]
- 25.Mittenhuber, G. 2001. Comparative genomics of prokaryotic GTP-binding proteins (the Era, Obg, EngA, ThdF [TrmE], YchF and YihA families) and their relationship to eukaryotic GTP-binding proteins (the DRG, ARF, RAB, RAN, RAS and RHO families). J. Mol. Microbiol. Biotechnol. 3:21-35. [PubMed] [Google Scholar]
- 26.Ogura, T., T. Tomoyasu, T. Yuki, S. Morimura, K. J. Begg, W. D. Donachie, H. Mori, H. Niki, and S. Hiraga. 1991. Structure and function of the ftsH gene in Escherichia coli. Res. Microbiol. 142:279-282. [DOI] [PubMed] [Google Scholar]
- 27.Persson, B. C., C. Gustafsson, D. E. Berg, and G. R. Bjork. 1992. The gene for a tRNA modifying enzyme, m5U54-methyltransferase, is essential for viability in Escherichia coli. Proc. Natl. Acad. Sci. USA 89:3995-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richmond, C. S., J. D. Glasner, R. Mau, H. Jin, and F. R. Blattner. 1999. Genome-wide expression profiling in Escherichia coli K-12. Nucleic Acids Res. 27:3821-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sayed, A., S. Matsuyama, and M. Inouye. 1999. Era, an essential Escherichia coli small G-protein, binds to the 30S ribosomal subunit. Biochem. Biophys. Res. Commun. 264:51-54. [DOI] [PubMed] [Google Scholar]
- 30.Scott, J. M., J. Ju, T. Mitchell, and W. G. Haldenwang. 2000. The Bacillus subtilis GTP binding protein Obg and regulators of the σB stress response transcription factor cofractionate with ribosomes. J. Bacteriol. 182:2771-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silhavy, M. L., T. J. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 32.Trach, K., and J. A. Hoch. 1989. The Bacillus subtilis spo0B stage 0 sporulation operon encodes an essential GTP-binding protein. J. Bacteriol. 171:1362-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welsh, K. M., K. A. Trach, C. Folger, and J. A. Hoch. 1994. Biochemical characterization of the essential GTP-binding protein Obg of Bacillus subtilis. J. Bacteriol. 176:7161-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]