Abstract
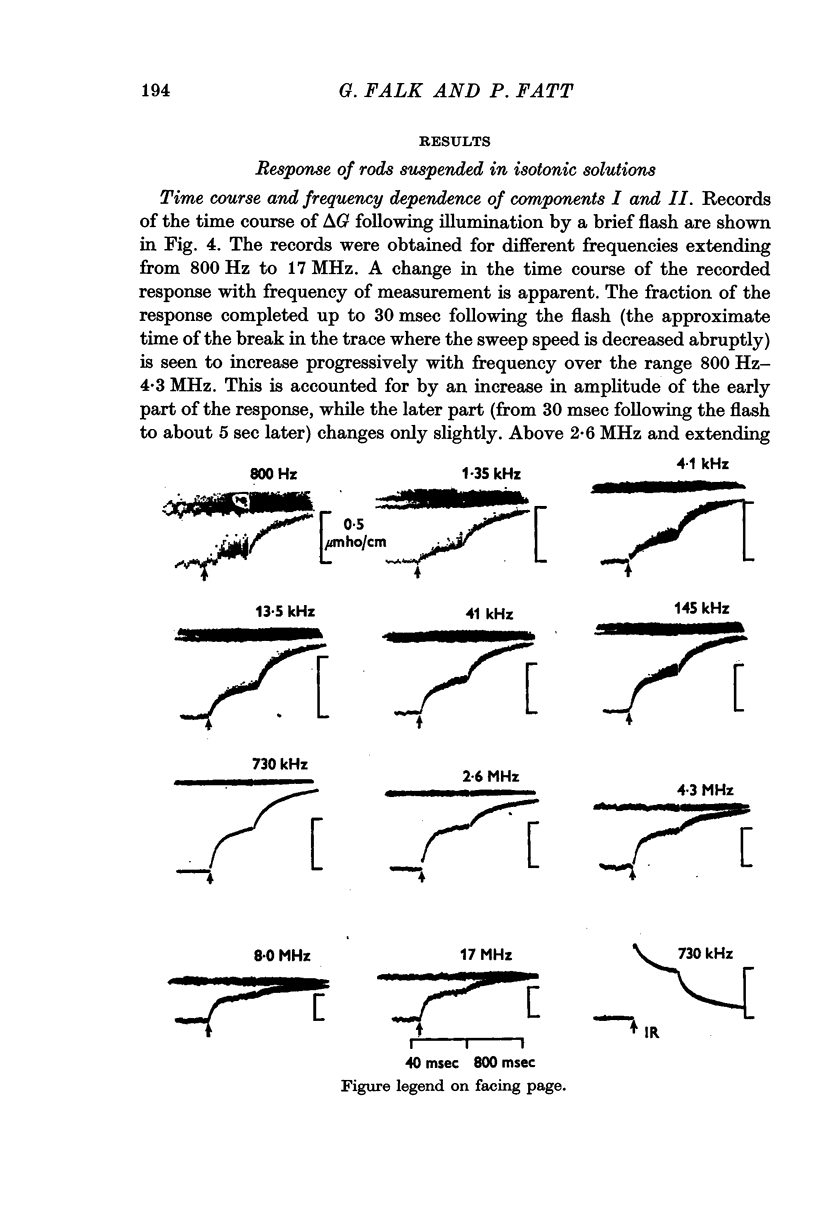
1. Measurements were made of the time course and amplitude of the change in real part of admittance, ΔG, of a suspension of frog rod outer segments, following a flash of light bleaching about 1% of the rhodopsin content of the rods. The measurements, based on the use of a specially designed marginal oscillator, covered the frequency range between 500 Hz and 17 MHz.
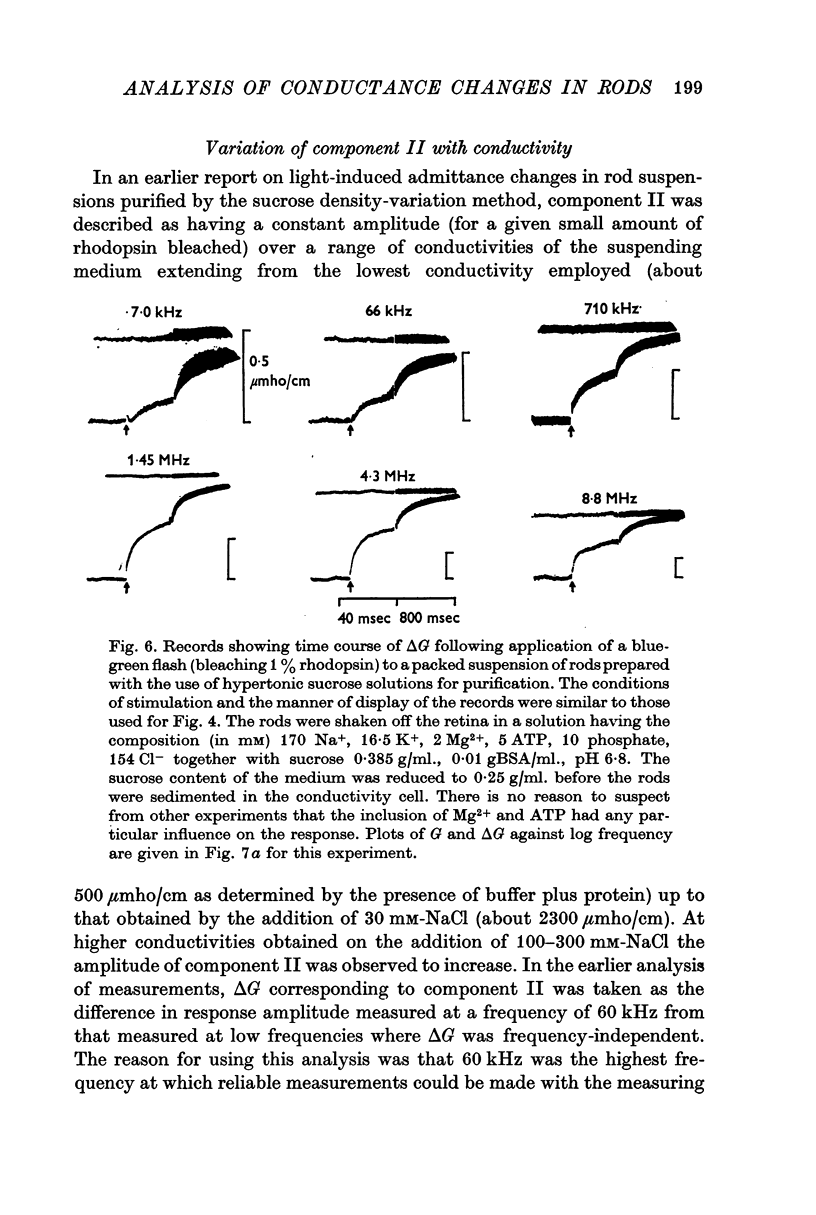
2. The components of response, previously described for rods prepared by a method involving exposure to strongly hypertonic sucrose solutions, are present in similar form when rods are isolated and maintained in isotonic solutions made up with equi-osmotic concentrations of NaCl and sucrose or with Na2SO4.
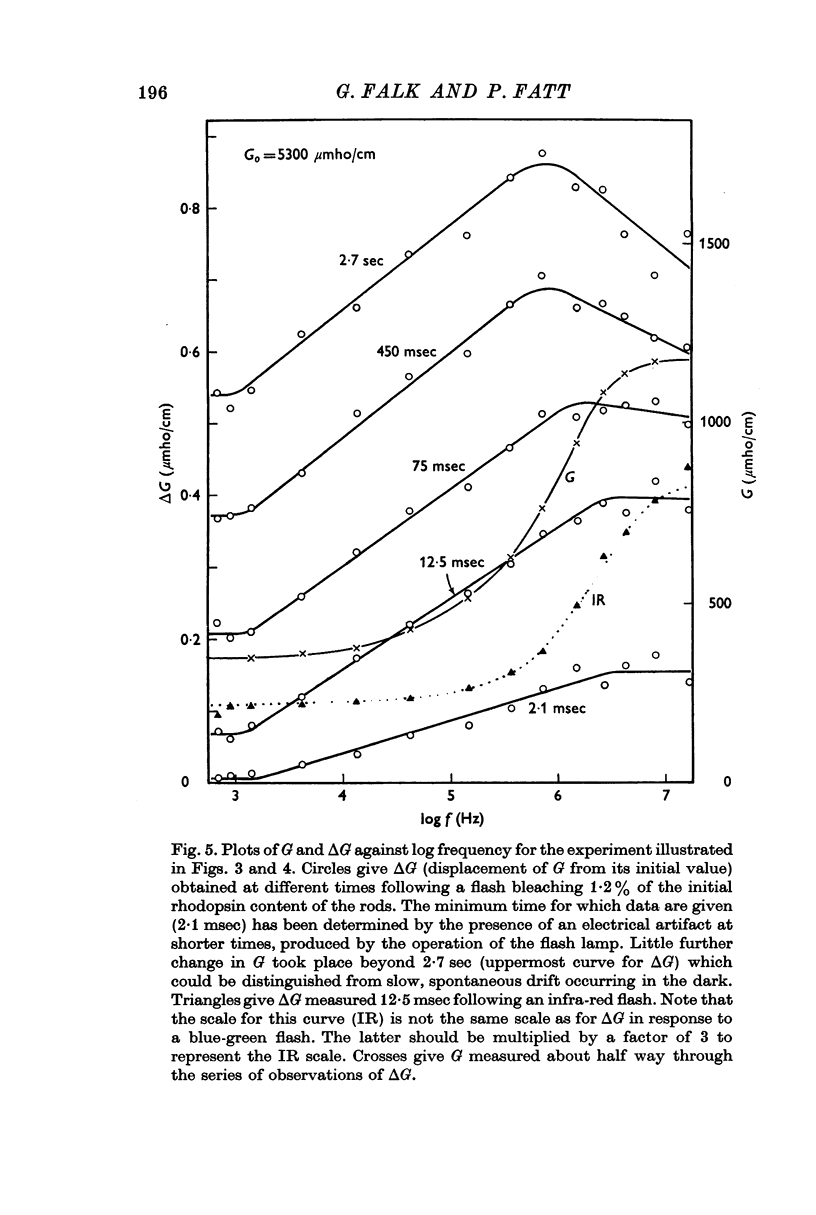
3. Component I, identified as a slowly developing positive ΔG apparent at very low frequencies, is frequency-independent up to the characteristic frequency of admittance for the suspension, fY (about 2 MHz for rods suspended in a solution having the conductivity of Ringer solution), but decreases at still higher frequencies.
4. Component II, identified as a rapidly developing positive ΔG which appears only above a critical frequency about 2·5 decades below fY, increases approximately logarithmically with frequency to reach a limiting amplitude in the region of fY.
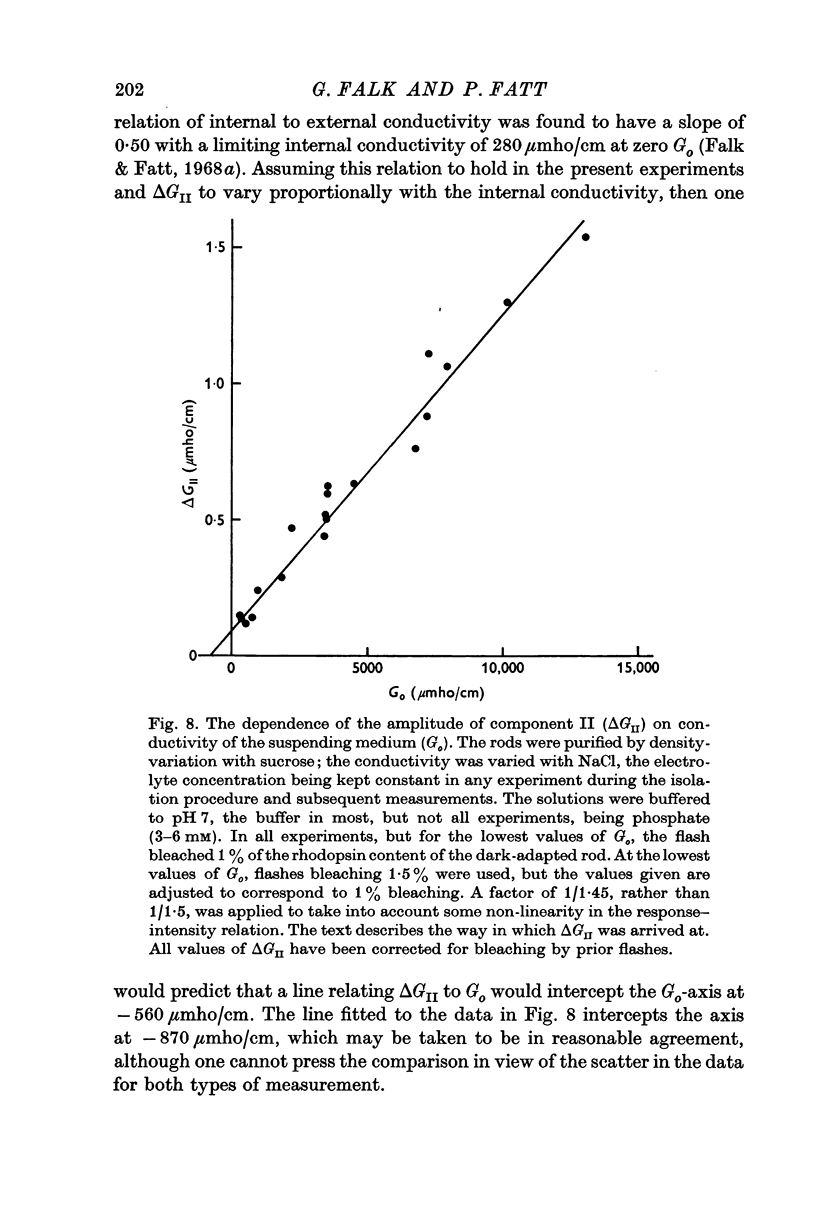
5. The amplitude of component II, ΔGII, measured in the region of fY, varies linearly with the conductivity of the suspending medium, Go, under conditions in which the conductivity of the rod interior is also a linear function of the external conductivity. The relation for a flash bleaching 1% of the rhodopsin content of the dark-adapted rod is [Formula: see text]
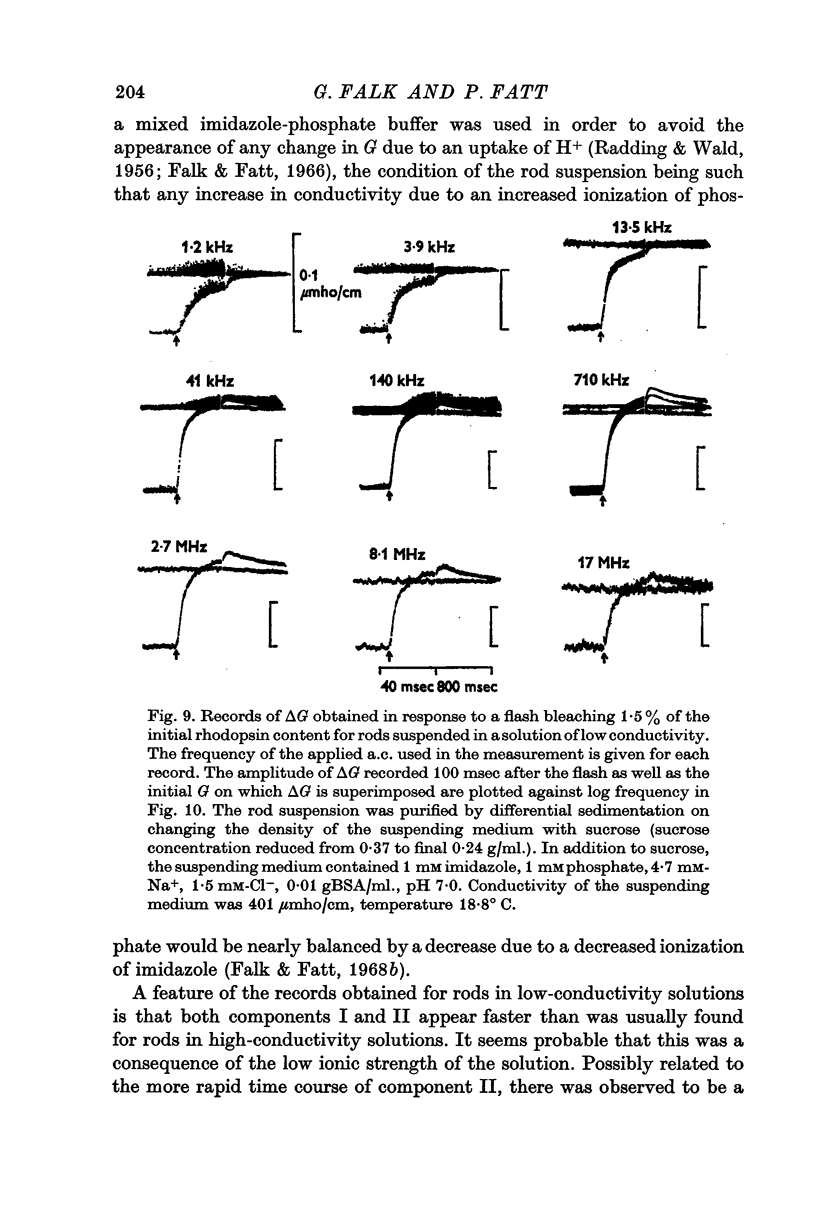
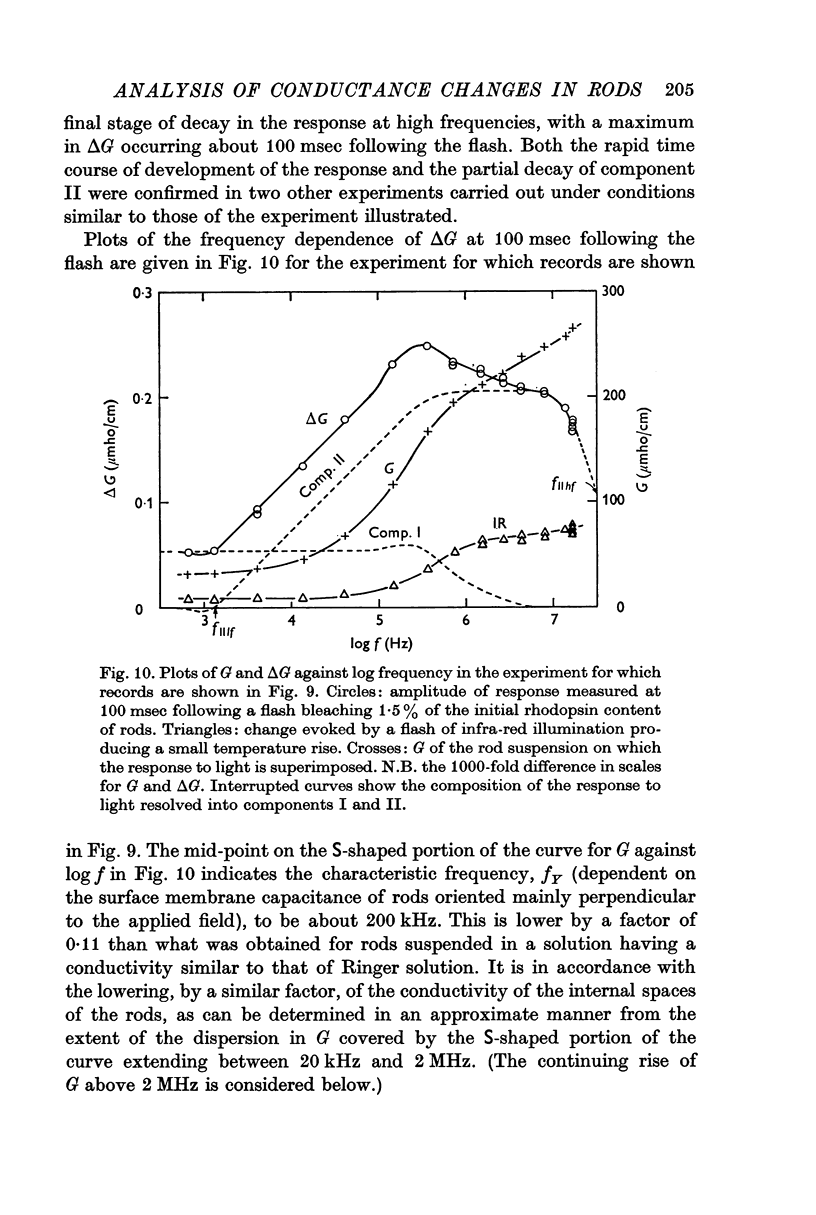
6. Measurements made on rods suspended in a low-conductivity solution, which has the effect of reducing the conductivity of the rod interior to about one ninth its value for rods suspended in Ringer solution, reveal a decline in component II for frequencies above 8 MHz.
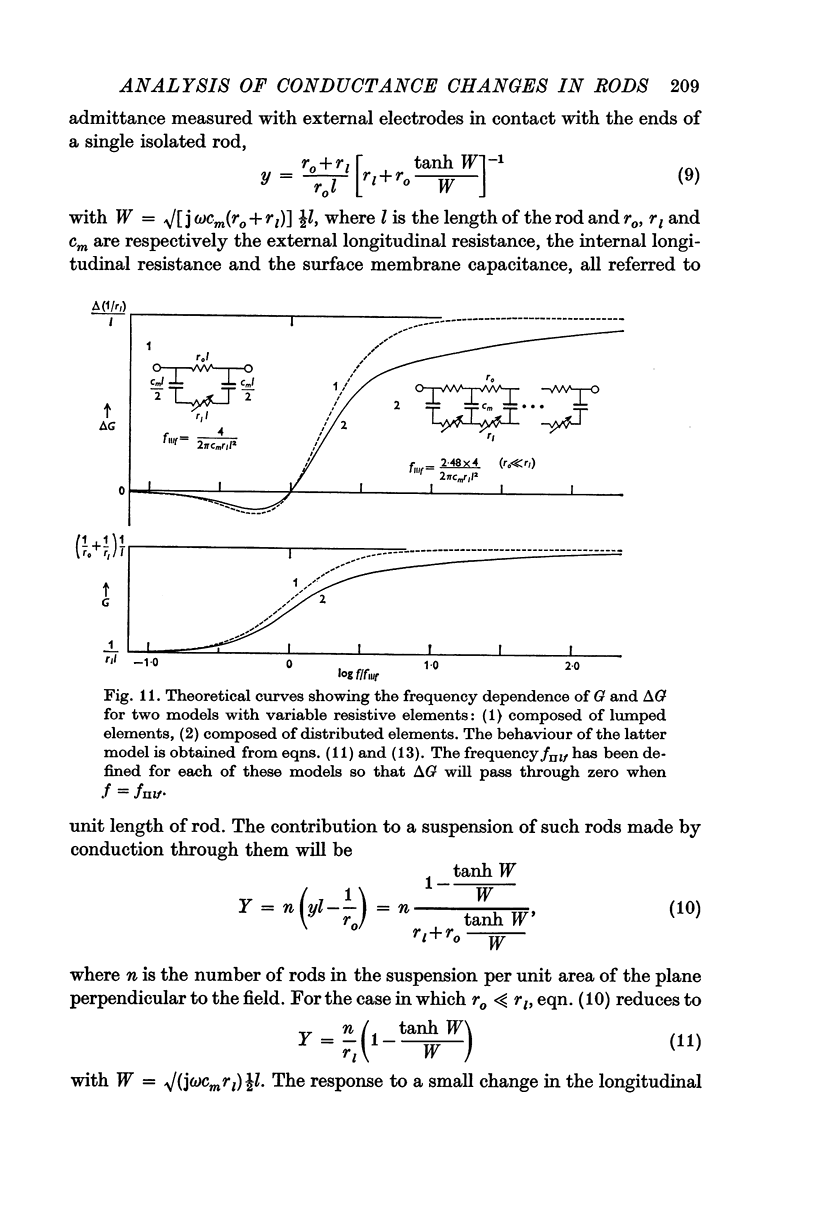
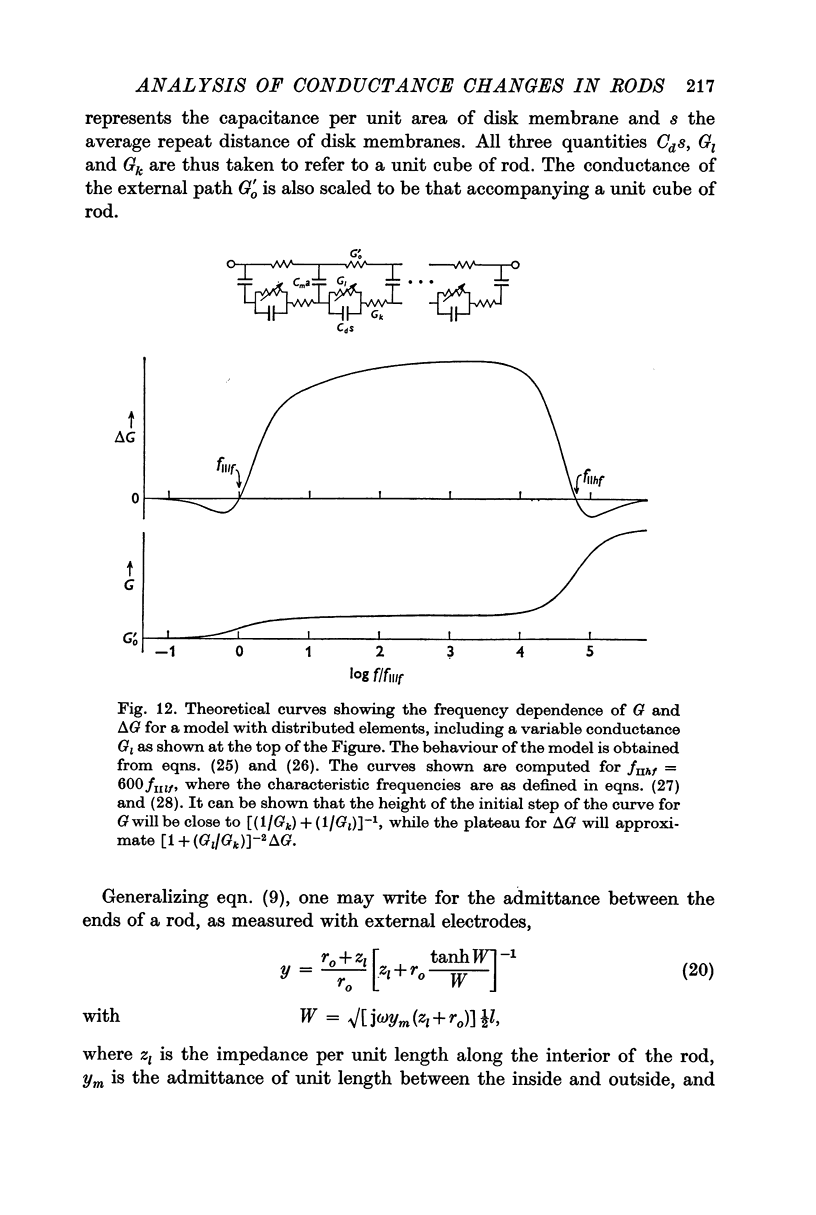
7. To explain the frequency dependence of component II and its dependence on conductivity, it is proposed that component II arises from a light-induced increase in conductance of the disk membranes which obstruct the longitudinal flow of current through the rod interior except at very high frequencies.
8. The disk-membrane conductance increase for rods suspended in a solution having the conductivity of Ringer solution is calculated to be 4·3 × 10-11 mho/rhodpsin molecule bleached, a value which is similar to what has been found for ionic channels operated by membrane potential change in the nerve membrane and by synaptic transmitter in the postjunctional membrane.
9. No component of response has been observed which could be reliably attributed to a surface membrane conductance decrease of the type observed in receptor cells in the retina.
Full text
PDF


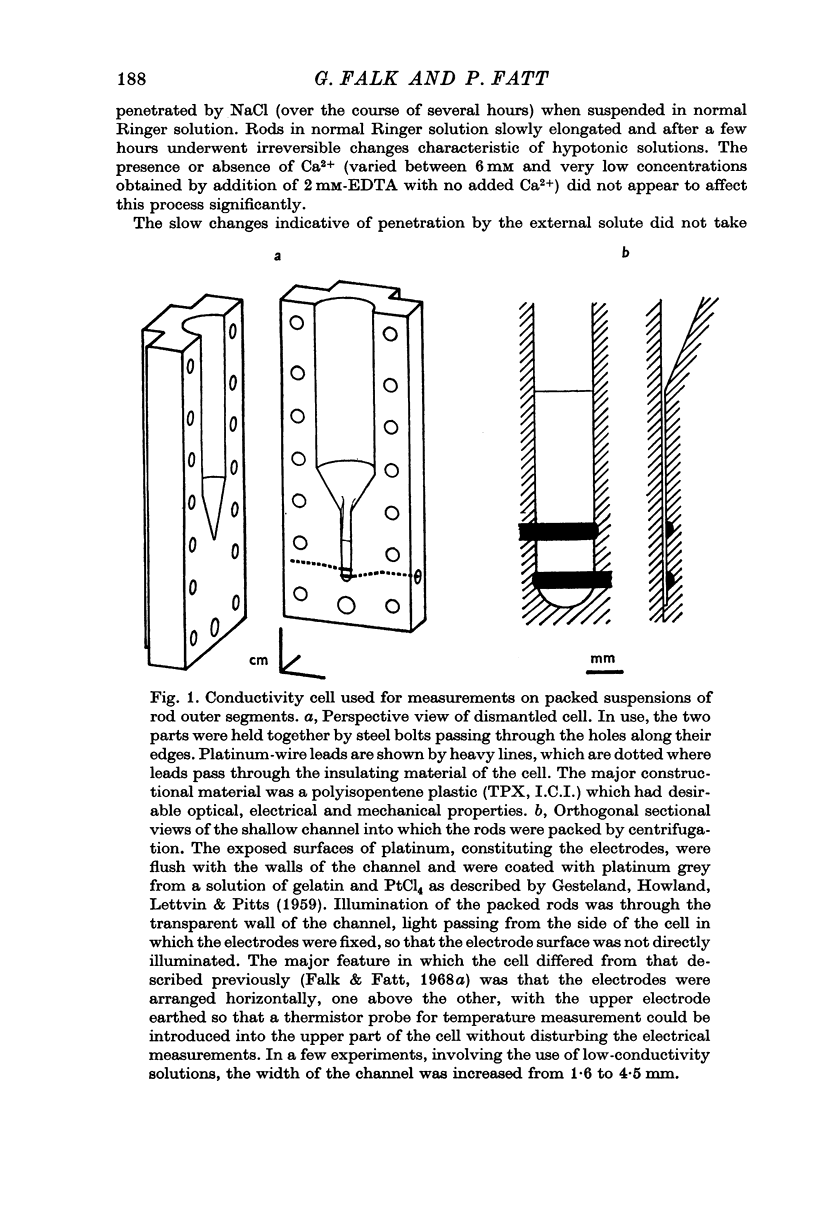

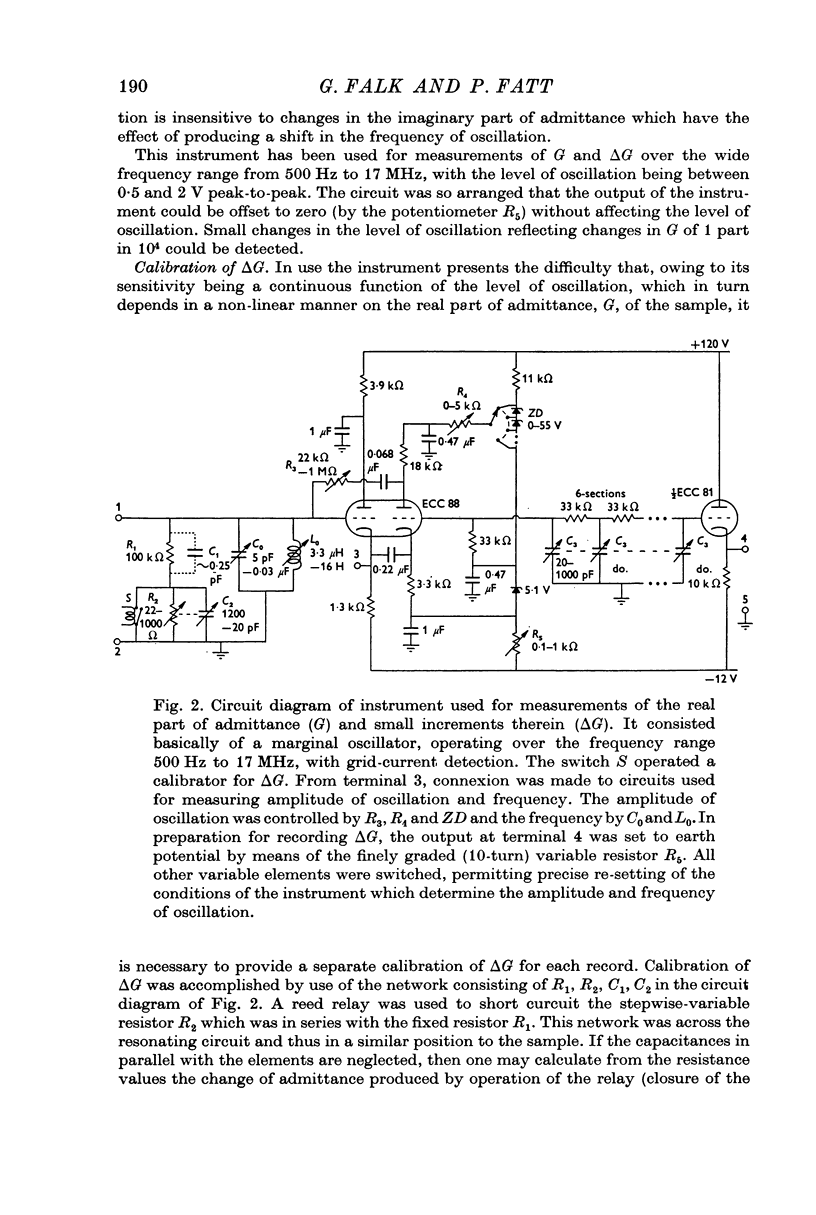
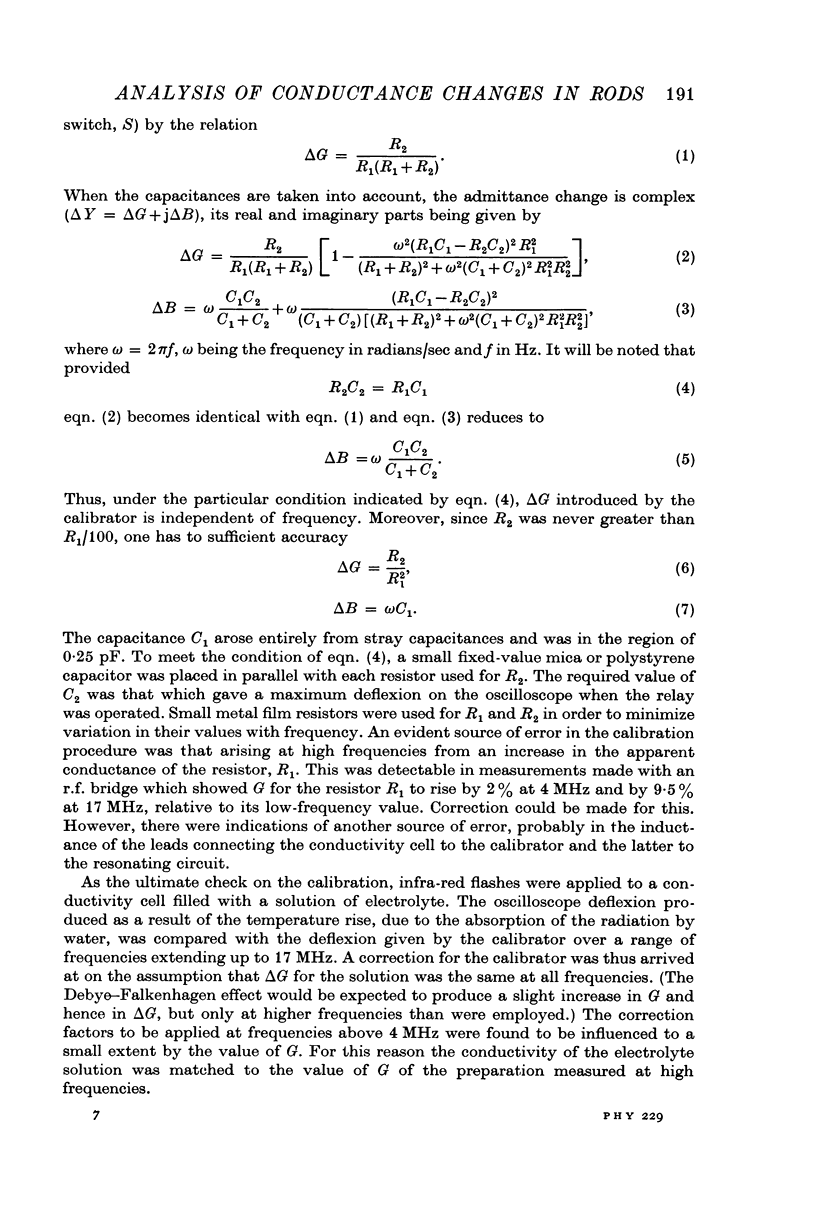

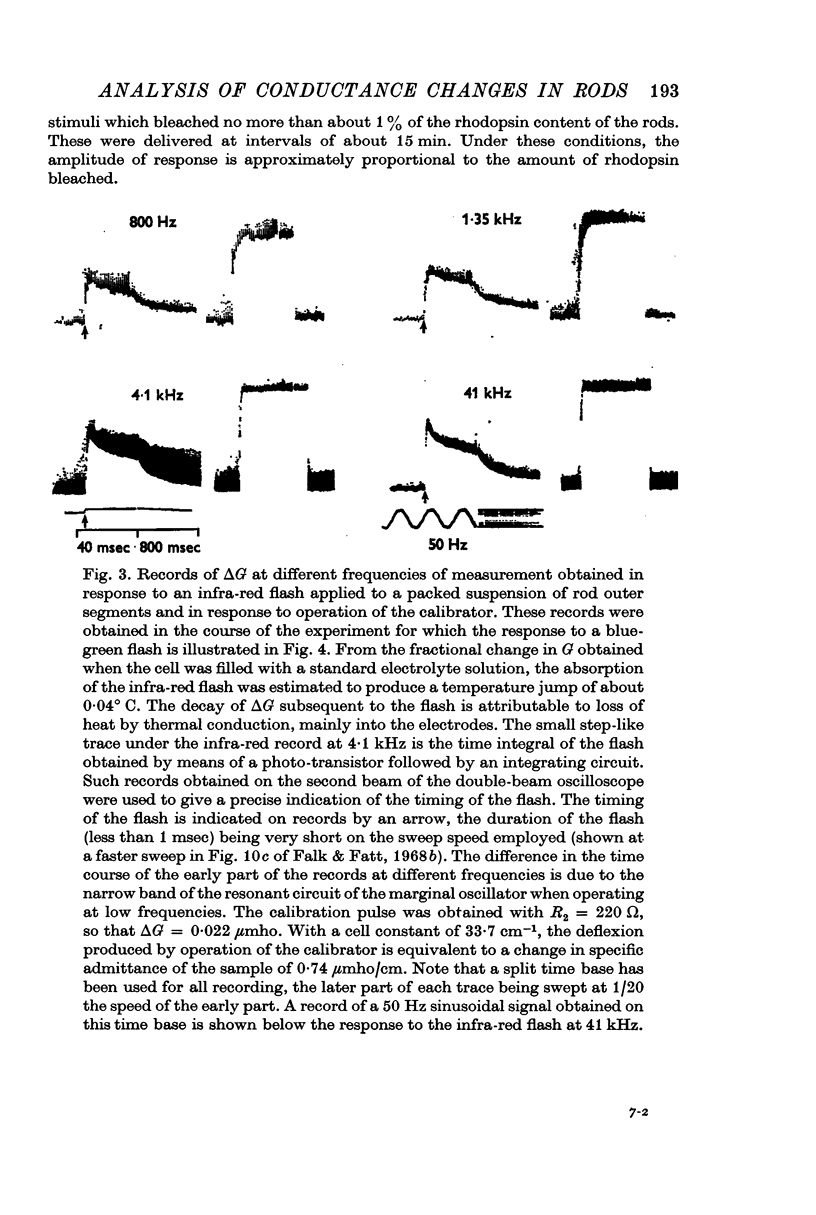



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk G., Fatt P. Conductance changes produced by light in rod outer segments. J Physiol. 1968 Oct;198(3):647–699. doi: 10.1113/jphysiol.1968.sp008631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk G., Fatt P. Isolation of components of admittance change in rod outer segments. J Physiol. 1973 Feb;229(1):221–239. doi: 10.1113/jphysiol.1973.sp010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk G., Fatt P. Passive electrical properties of rod outer segments. J Physiol. 1968 Oct;198(3):627–646. doi: 10.1113/jphysiol.1968.sp008630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk G., Fatt P. Rapid hydrogen ion uptake of rod outer segments and rhodopsin solutions on illumination. J Physiol. 1966 Mar;183(1):211–224. doi: 10.1113/jphysiol.1966.sp007861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B. Microphysiology of the neuromuscular junction; the chemo-receptor function of the motor end-plate. Bull Johns Hopkins Hosp. 1958 Jun;102(6):296–312. [PubMed] [Google Scholar]
- Katz B., Miledi R. Further observations on acetylcholine noise. Nat New Biol. 1971 Jul 28;232(30):124–126. doi: 10.1038/newbio232124b0. [DOI] [PubMed] [Google Scholar]
- RADDING C. M., WALD G. Acid-base properties of rhodopsin and opsin. J Gen Physiol. 1956 Jul 20;39(6):909–922. doi: 10.1085/jgp.39.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda J., Nosaki H., Tomita T. Light-induced resistance changes in single photoreceptors of Necturus and Gekko. Vision Res. 1969 Apr;9(4):453–463. doi: 10.1016/0042-6989(69)90134-5. [DOI] [PubMed] [Google Scholar]


