Abstract
Treatment of a sodium dodecyl sulfate-polyacrylamide gel with periodic acid-Schiff (PAS) stain or blotting with Galanthus nivalis agglutinin revealed the presence of several glycosylated polypeptides in a partially purified detergent extract of the membrane fraction of Sulfolobus solfataricus. One of the glycoproteins comigrated with the membrane-associated protein-serine/threonine kinase from S. solfataricus, which had been radiolabeled by autophosphorylation with [32P]ATP in vitro. Treatment with a chemical deglycosylating agent, trifluoromethanesulfonic acid, abolished PAS staining and reduced the Mr of the protein kinase from ∼67,000 to ∼62,000. Protein kinase activity also adhered to, and could be eluted from, agarose beads containing bound G. nivalis agglutinin. Glycosylation of the protein kinase implies that at least a portion of this integral membrane protein resides on the external surface of the cell membrane.
Although several reports indicate that proteins resident in members of the so-called “third domain of life,” the Archaea, are the targets of covalent modification by phosphorylation on serine, threonine, and/or tyrosine residues (20, 30, 31, 33-36), little is known concerning the structural or functional characteristics of the protein kinases responsible. Surveys of several archaeal genomes have identified open reading frames whose predicted protein products exhibit faint homology to the most populous group of protein-serine/threonine/tyrosine kinases, the eukaryotic protein kinase superfamily (14, 21, 26, 32). Many archaea also contain open reading frames potentially encoding homologs of two-component histidine kinases (11, 12), variants of which act as protein-serine/threonine/tyrosine kinases in members of the Eucarya (9) and Bacteria (17, 27, 38, 40). However, with the exception of a CheA-like two-component histidine kinase from Halobacterium salinarum (23), it has yet to be determined whether any of these deduced archaeal protein kinases possesses the catalytic properties suggested by homology searches.
Recently, protein-serine/threonine kinase activity was detected in the membrane fraction of the extreme acidothermophilic archaeon Sulfolobus solfataricus (15). Solubilization of this protein kinase activity required detergents such as Triton X-100 or octyl glucoside, indicating that it was associated with an integral membrane protein. By exploiting the ability of the enzyme responsible to renaturate following sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) or two-dimensional electrophoresis, the source of this activity was traced to a polypeptide with an apparent Mr of ∼67,000 (∼67K) that was able to phosphorylate itself or exogenous protein substrates in situ.
Gel filtration chromatography indicated that the protein kinase was an oligomer. While the catalytic polypeptide appeared to behave as a single species during SDS-PAGE, on two-dimensional gels it migrated in a noticeably diffuse manner, even under conditions where it presumably was in a dephosphorylated state (15). Electrophoretic behavior of this type oftentimes is indicative of covalent modification by glycosylation (22), as complex carbohydrate moieties constitute a frequent source of structural microheterogeneity. Given that glycosylation also correlates with membrane localization in eukaryotes and, as far as is currently known, in prokaryotes as well, we investigated whether the membrane-associated protein-serine/threonine kinase from S. solfataricus was a glycoprotein.
MATERIALS AND METHODS
Materials.
Purchased materials included [γ-32P]ATP (NEN Research Products, Boston, Mass.), protein assay reagent (Bio-Rad, Richmond, Calif.), a Gelcode glycoprotein staining kit (Pierce, Rockford Ill.), a digoxigenin (DIG) glycan differentiation kit (Roche Diagnostics GmbH, Mannheim, Germany), and a Glycofree deglycosylation kit (Glyko Inc., Novato, Calif.). Agarose beads containing immobilized Galanthus nivalis agglutinin were from Sigma (St. Louis, Mo.). General laboratory reagents and microbial culture media were from Fisher (Pittsburgh, Pa.) or Sigma.
Routine procedures.
Protein concentrations were determined as described by Bradford (1) with premixed reagent and a standardized solution of bovine serum albumin. SDS-PAGE was performed as described by Laemmli (13). Gels were stained for protein with Coomassie brilliant blue as described by Fairbanks et al. (7). 32P-labeled phosphoproteins were visualized by electronic autoradiography with a Packard (Meriden, Conn.) Instantimager. Protein kinase activity was assayed at 65°C with [γ-32P]ATP, using myosin light-chain (MLC) peptide as the phosphoacceptor substrate, as described previously (15).
Preparation of the DE-52 fraction from S. solfataricus
The DE-52 fraction, a partially purified detergent extract from the membrane fraction of S. solfataricus, was prepared as described by Lower et al. (15). Briefly, S. solfataricus was grown in continuous culture with vigorous aeration at 75°C in de Rosa's standard medium (3) with the level of yeast extract increased to 2 g/liter. Kanamycin sulfate (20 mg/liter) was added daily. Cells were harvested and stored at −20°C until needed.
Frozen S. solfataricus (20 g [wet weight]) was thawed and resuspended in 2 volumes of 20 mM morpholineethanesulfonic acid (MES) (pH 6.5) containing 0.5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 10 μg of DNase I/ml. The cells were lysed by two passages through a French pressure cell at 12,000 lb/in2. The lysate was centrifuged at 1,000 × g for 10 min at 4°C. Next, the supernatant liquid was centrifuged at 100,000 × g for 75 min at 4°C. The pellet was washed and resuspended in 40 ml of 20 mM sodium acetate (pH 5.0) containing 0.5 M NaCl and particulate material collected by centrifugation at 100,000 × g for 75 min at 4°C. The pellet was resuspended in 40 ml of 20 mM MES (pH 6.5) containing 125 mM NaCl and 25 mM octyl glucoside. The mixture was centrifuged at 100,000 × g for 75 min at 4°C, and the supernatant liquid was retained as the detergent extract.
The detergent extract was diluted fivefold by the addition of 4 volumes of 20 mM MES (pH 6.5) containing 0.5 mM EDTA and applied to a 1.5- by 18-cm column of DE-52 cellulose that had been equilibrated in 20 mM MES (pH 6.5) containing 25 mM NaCl and 12.5 mM octyl glucoside (equilibration buffer). The column was washed with 3 column volumes of equilibration buffer and developed with a linear gradient (150 ml total) of 25 to 500 mM NaCl in equilibration buffer. Fractions (3 ml) were collected and assayed for protein kinase activity. Fractions corresponding to the major peak of protein kinase activity, which eluted at an NaCl concentration of ∼200 mM, were pooled and retained as the DE-52 fraction.
Autophosphorylation of the protein kinase in gel.
Renaturation and autophosphorylation of the membrane-associated protein kinase from S. solfataricus were performed as described by Lower et al. (15). Briefly, after SDS-PAGE, the detergent was removed from the gel by washing with 20% (vol/vol) isopropanol. Proteins were randomized by soaking the gel in 20 mM MES (pH 6.5) containing 6 M guanidine hydrochloride and then allowed to renature by incubation with several changes of 20 mM MES (pH 6.5) containing 0.1% (vol/vol) Triton X-100, 1 mM dithiothreitol (DTT), and 5 mM (each) MnCl2 and MgCl2. The gels were incubated for 1 h at 65°C in 20 mM MES (pH 6.5) containing 0.1% (vol/vol) Triton X-100, 1 mM DTT, and 5 mM (each) MnCl2 and MgCl2 to which 50 μM ATP (15 μCi of [γ-32P]ATP/ml) had been added. The gels were washed extensively in 2% (wt/vol) sodium pyrophosphate to remove excess ATP and dried, and autophosphorylated proteins were visualized by electronic autoradiography.
Radioactive labeling of protein kinase by autophosphorylation.
The DE-52 fraction (75 μg) was incubated for 60 min at 65°C in a volume of 100 μl of 20 mM MES (pH 6.5) containing 50 μM ATP (300 μCi of [γ-32P]ATP/ml), 5 mM MnCl2, 2 mM DTT, and 12.5 mM octyl glucoside. The reaction was terminated by the addition of 3 volumes of ice-cold acetone. The precipitated proteins were collected by centrifugation at 12,000 rpm for 3 min in a microcentrifuge and resuspended in SDS sample buffer.
Staining with PAS stain.
SDS-polyacrylamide gels were stained for carbohydrate with periodic acid-Schiff (PAS) stain (25) by using a Gelcode glycoprotein staining kit according to the manufacturer's protocols.
Deglycosylation with TFMS.
Chemical deglycosylation with trifluoromethanesulfonic acid (TFMS) (4) was carried out with a Glycofree deglycosylation kit according to the manufacturer's protocols. Briefly, the DE-52 fraction (75 μg) was autophosphorylated as described above, with the exception that the acetone precipitate was resuspended in 0.25% (wt/vol) SDS at a temperature of 65°C and then lyophilized. The lyophilized solid was transferred to a 1-ml glass vial equipped with a Teflon-coated septum cap and cooled on dry ice-ethanol. Next, 50 μl of a 2:1 mixture of TFMS and toluene was added, and the vial was maintained on dry ice-ethanol for an additional 20 s. The vial was then incubated for 4 h at −20°C. Gentle agitation was performed at 5 and 10 min to promote complete dispersal of the lyophilized protein. Next, the mixture was placed on dry ice-ethanol, and 150 μl of 60% (vol/vol) pyridine in water was slowly added to neutralize the TFMS. After 20 s, the mixture was transferred to dry ice for 5 min and then to ice for a further 15 min. Finally, 400 μl of 0.5% (wt/vol) ammonium bicarbonate was added, and the mixture was dialyzed prior to the addition of SDS sample buffer.
Detection of glycoproteins via blotting with DIG-labeled lectins.
Portions of the DE-52 fraction, containing 1 μg of protein each, were mixed with SDS sample buffer and loaded into individual lanes of an SDS-polyacrylamide gel. One lane contained a sample of the DE-52 fraction that had been incubated with [γ-32P]ATP as described above to label the autophosphorylated protein kinase with [32P]phosphate. Other lanes accommodated known glycoproteins serving as positive controls: asialofetuin, fetuin, transferrin, and carboxypeptidase Y. After electrophoresis, the proteins were electrophoretically transferred to an Immobilon P membrane. The membrane was divided into sections, each containing (i) a set of prestained molecular weight standards, (ii) one or more known glycoproteins, and (iii) a sample of the DE-52 fraction. Blotting was then performed with the materials and according to the directions provided in the DIG glycan differentiation kit. Briefly, each membrane section was incubated with blocking solution, washed, and incubated with one of five DIG-labeled lectins: Datura stramonium agglutinin, G. nivalis agglutinin, peanut agglutinin, Sambucus nigra agglutinin, or Maackia amurensis agglutinin. After being washed, membrane sections were then incubated with an anti-DIG antibody conjugated to horseradish peroxidase. Bound lectin was visualized by incubation with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium.
Binding of the protein kinase to lectins immobilized on agarose beads.
The DE-52 fraction (0.185 mg of protein in a volume of 0.5 ml) was supplemented with urea to a final concentration of 2 M and with CaCl2 and MnCl2 to final concentrations of 1 mM each. This material was added to 0.5 ml of swelled agarose beads coated with G. nivalis agglutinin that had been equilibrated in 50 mM morpholinepropanesulfonic acid (MOPS) (pH 7.0) containing 15 mM octyl glucoside, 1 mM CaCl2, 1 mM MnCl2, 50 mM NaCl, and 2 M urea (lectin buffer). After continuous agitation for 30 min, the aqueous phase was collected by centrifugal filtration at 1,000 × g for 2 min. This process was repeated twice more. The final filtrate was referred to as the flo-thru. Next, the agarose beads were pooled together, and adherent proteins were removed by adding lectin buffer containing 500 mM methyl-α-d-mannopyranoside and 1 M NaCl. The eluant liquid was collected by centrifugal filtration at 1,000 × g for 2 min and pooled as the adherent fraction. The load, flo-thru, and adherent fractions each were dialyzed versus 50 mM MES (pH 6.0) containing 0.1% (vol/vol) Triton X-100, concentrated 10-fold by centrifugal ultrafiltration with Centricon 10 devices, and assayed for protein kinase activity as described above.
RESULTS
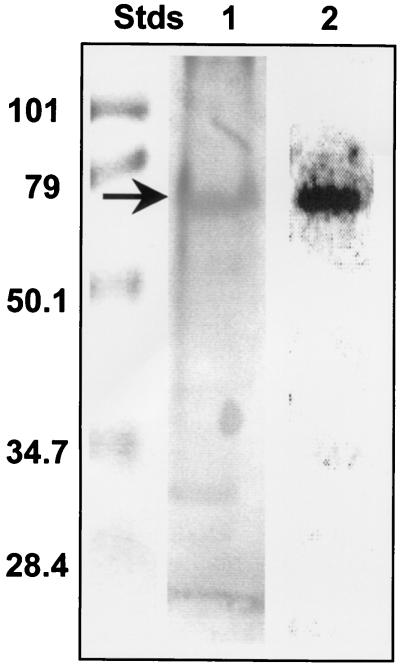
When an SDS-polyacrylamide gel of a DE-52 fraction, a partially purified detergent extract of the membrane fraction from S. solfataricus, was probed for carbohydrate with PAS stain (Fig. 1), several glycosylated proteins were visible. One of these coincided with the position of a polypeptide with an Mr of ∼67K which autophosphorylated itself in gel following renaturation and incubation with [γ-32P]ATP in situ (Fig. 1). Previous studies in our laboratory had established that this polypeptide possessed protein-serine/threonine kinase activity (15). The ∼67K glycoprotein also was visible in preparations of the protein kinase that had been subjected to further purification by gel filtration chromatography (data not shown).
FIG. 1.
A glycoprotein with an apparent Mr of ∼67K comigrates with the membrane-associated protein kinase from S. solfataricus. Portions of a partially purified detergent extract of the membrane fraction from S. solfataricus, the DE-52 fraction, containing 20 μg of protein each, were applied to an SDS-polyacrylamide gel along with a set of prestained molecular weight standards (Stds). After electrophoresis, the gel was divided into sections. One section of the gel was stained with PAS. In a duplicate section, proteins in the gel were renatured and incubated with [γ-32P]ATP. The Mr values (in thousands) of the molecular weight standards are given at left. Lane 1 shows the results of PAS staining, while lane 2 shows an electronic autoradiogram of the gel incubated with [γ-32P]ATP. The position of the autophosphorylated protein kinase is indicated by the arrow. For further details, see Materials and Methods.
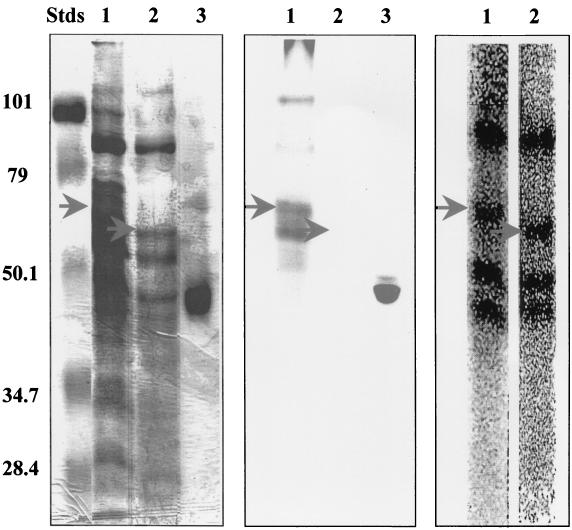
Treatment of the DE-52 fraction with the chemical deglycosylating agent TFMS (4) prior to SDS-PAGE abolished all PAS-detectable carbohydrate (Fig. 2). Since this harsh chemical treatment abolished the ability to renature protein kinase activity following SDS-PAGE, the protein kinase was autophosphorylated prior to TFMS treatment and electrophoresis with [γ-32P]ATP. While a few additional polypeptides also became phosphorylated during this preincubation, their electrophoretic mobilities differed markedly from that of the 32P-labeled protein kinase, allowing the latter's position to be readily tracked by autoradiography. Pretreatment with TFMS resulted in the apparent disappearance of the autophosphorylated protein kinase with the accompanying appearance of a new 32P-labeled species with an apparent Mr of ∼62K (Fig. 2). None of the other 32P-labeled phosphoproteins present in the DE-52 fraction were visibly affected by treatment with TFMS. It therefore was concluded that the ∼62K phosphoprotein represented a form of the autophosphorylated protein kinase whose electrophoretic mobility had increased as a result of the removal of bound carbohydrate.
FIG. 2.
Treatment with TFMS increases the electrophoretic mobility of the membrane-associated protein kinase from S. solfataricus. Portions of the DE-52 fraction, each containing 75 μg of protein, were incubated with [γ-32P]ATP to label the autophosphorylated protein kinase with [32P]phosphate. One portion was then treated with TFMS, a chemical deglycosylating agent. Both treated and untreated protein mixtures were then subjected to SDS-PAGE. The left panel shows the SDS-polyacrylamide gel after staining with Coomassie blue. The middle panel displays the results obtained with PAS staining. The panel on the right shows an electronic autoradiogram of lanes 1 and 2. Lanes 1 and 2 contain DE-52 fraction prior to and following treatment with TFMS, respectively, while lanes 3 contain horseradish peroxidase, a glycoprotein serving as a positive control for staining with PAS. The upper arrows indicate the position of the autophosphorylated protein kinase prior to treatment with TFMS. The lower arrows indicate the position of the new phosphoprotein that appeared following treatment with TFMS. The Mr values (in thousands) of the prestained molecular weight standards (Stds) are indicated at left. For further details, see Materials and Methods.
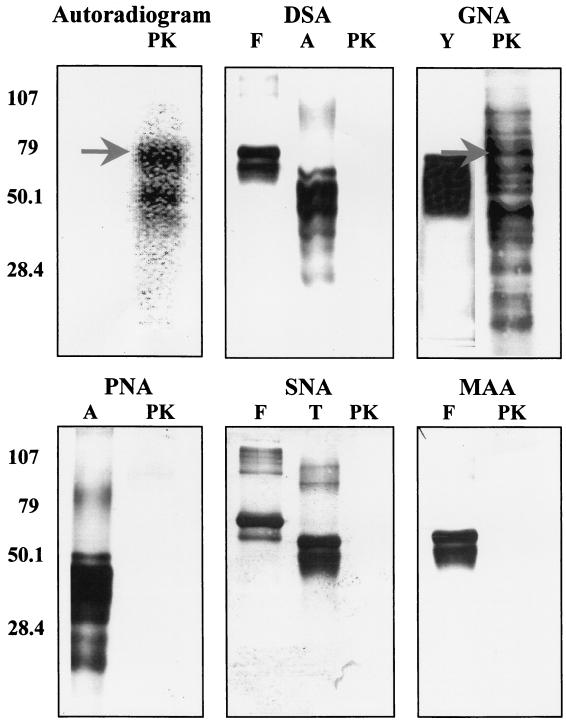
To gain further insight into the nature of the carbohydrate moieties present on the membrane-associated protein-serine/threonine kinase and other glycoproteins from S. solfataricus, samples of the DE-52 fraction were blotted onto an Immobilon P membrane following SDS-PAGE. Sections of the membrane were then probed with one of the following of lectins: G. nivalis agglutinin, Sambuca nigris agglutinin, M. amurensis agglutinin, peanut agglutinin, or D. stramonium agglutinin. Only one of these, G. nivalis agglutinin, was observed to bind to the glycoproteins in the DE-52 fraction (Fig. 3). The noticeably larger number of glycoproteins visualized by lectin blotting than by staining with PAS presumably reflects the much higher sensitivity of the former method. Notably, one of the glycoproteins visualized by blotting with G. nivalis agglutinin appeared to comigrate with the membrane-associated protein kinase, which had been radiolabeled by autophosphorylation in the presence of [γ-32P]ATP prior to SDS-PAGE.
FIG. 3.
Numerous glycoproteins from S. solfataricus bind G. nivalis agglutinin. Portions of the DE-52 fraction, containing 1 μg of protein each, were resolved into individual components by SDS-PAGE and transferred to an Immobilon membrane. One lane contained DE-52 fraction in which the protein kinase had been radiolabeled by incubation with [γ-32P]ATP. The top left panel shows an autoradiogram, with the position of the autophosphorylated protein kinase indicated by an arrow. The other panels show the results obtained when membranes were blotted with one of five different lectins: D. stramonium agglutinin (DSA), G. nivalis agglutinin (GNA), peanut agglutinin (PNA), Sambucus nigra agglutinin (SNA), and M. amurensis agglutinin (MAA). Lanes marked PK contain DE-52 fraction. Those marked A, F, T, and Y contain asialofetuin, fetuin, transferrin, and carboxypeptidase Y, respectively, which are known glycoproteins serving as positive controls. The positions and values (in thousands) of the prestained molecular weight standards are indicated by the numbers at left. For further details, see Materials and Methods.
Exploiting the information obtained by lectin blotting, we used agarose beads coated with covalently bound G. nivalis agglutinin as a second means for ascertaining whether the membrane-associated protein kinase was glycosylated. Since this method employed nondenaturing conditions, it afforded an opportunity to monitor the behavior of the protein kinase by assaying its ability to catalyze phosphoryl transfer from [γ-32P]ATP to an exogenous substrate, MLC peptide. MLC peptide is modeled after a phosphorylation site in myosin light chains from smooth muscle (10) that is adventitiously phosphorylated by this archaeal protein kinase (15).
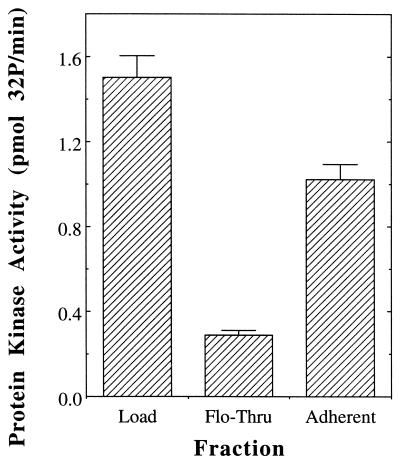
Incubation of the DE-52 fraction with agarose beads coated with G. nivalis agglutinin depleted the vast majority (∼81%) of the protein kinase activity from the solution (Fig. 4). The majority (∼84%) of the activity that had been depleted could be recovered by elution with methyl-α-d-mannopyranoside. The presence of the protein kinase in the adherent fraction also could be detected by autophosphorylation in gel following SDS-PAGE, while analysis of the adherent fraction by both PAS and lectin staining indicated the presence of the ∼67K glycoprotein as well (data not shown). Binding of the protein kinase to the column required the presence of 2 M urea, which may have rendered the carbohydrate moiety(ies) on the enzyme more accessible to the lectin by dissociating protein aggregates, “opening” the conformation of the glycoprotein, or a combination of the two.
FIG. 4.
The membrane-associated protein kinase binds G. nivalis agglutinin. The DE-52 fraction (0.5 ml containing 185 μg of protein) was sequentially incubated with 0.5-ml portions of agarose beads containing bound G. nivalis agglutinin. The beads were pooled together, and adherent proteins were eluted with methyl-α-d-mannopyranoside. Shown are the total protein kinase activity contained in the DE-52 fraction (Load), that remaining after incubation with the lectin agarose (Flo-Thru), and that liberated by incubation with methyl-α-d-mannopyranoside (Adherent). All assays were performed in duplicate and agreed within ±7%. For further details, see Materials and Methods.
DISCUSSION
Staining with PAS and blotting with G. nivalis agglutinin revealed that a partially purified detergent extract of the membrane fraction from the archaeon S. solfataricus contained numerous glycoproteins. As has proved to be the case for many of the small handful of glycoproteins previously characterized from members of the Archaea, including S. solfataricus (6), binding to the aforementioned lectin indicates the presence of terminal mannose residues (29). The lack of detectable binding by Sambucus nigra agglutinin (28) or M. amurensis agglutinin (37) suggests an absence of abundant glycoproteins whose carbohydrate moieties contain terminal sialic acid. Similarly, the lack of detectable binding by D. stramonium agglutinin (39) or peanut agglutinin (19) suggests an absence of terminal galactose moieties.
Our findings indicate that the ∼67K polypeptide previously identified as the source of the membrane-associated protein-serine/threonine kinase activity in S. solfataricus (15) is a glycoprotein. Not only did the autophosphorylated, 32P-labeled protein kinase comigrate with glycoproteins visualized by PAS staining or blotting with G. nivalis agglutinin, but it was also the only phosphoprotein in the mixture whose electrophoretic mobility was altered by treatment with the general chemical deglycosylating agent TFMS. Moreover, by using assays of its activity toward an exogenous peptide substrate as a second, independent means for detection, it was observed that the majority of the protein kinase bound to and could be eluted from G. nivalis agglutinin that had been immobilized on agarose beads.
It is tempting to speculate that the glycosylation of the membrane-associated protein-serine/threonine kinase from S. solfataricus indicates a transmembrane topography. Thus far, glycosylation of archaeal proteins appears to be confined to secreted proteins (8) and to the extracellular domains of membrane proteins (5, 6, 18, 24), a pattern mirroring that observed for eukaryotes. Covalent modification by glycosylation therefore suggests that at least a portion of the membrane-associated protein-serine/threonine kinase from S. solfataricus is exposed on the exterior surface of the cell.
On the other hand, it appears likely that the catalytic domain of the protein kinase resides on the cytoplasmic side of the membrane. Not only would this conform to the pattern observed for the overwhelming majority of membrane-associated protein kinases previously characterized in eucaryal and bacterial organisms, but it represents the explanation most consistent with the observed influence of pH on the catalytic activity of the archaeal protein kinase in vitro. An extreme acidothermophile, S. solfataricus requires an external pH between 1 and 3 for growth (2). However, members of the genus Sulfolobus maintain an internal pH near 6 (16). In vitro, the membrane-associated protein-serine/threonine kinase from S. solfataricus exhibited its highest catalytic activity at pH values between 5.5 and 7.5, while catalytic activity declined to negligible levels at pH values below 4 (data not shown). Although the supporting evidence remains circumstantial in nature, a transmembrane topography would be consistent with the participation of this archaeal protein-serine/threonine kinase in the transduction of extracellular signals.
Acknowledgments
This work was supported by grant number MCB 0077484 from the National Science Foundation.
REFERENCES
- 1.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 2.Brock, T. D., K. M. Brock, R. T. Belly, and R. L. Weiss. 1972. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch. Mikrobiol. 84:54-68. [DOI] [PubMed] [Google Scholar]
- 3.de Rosa, M. A., A. Gambacorta, and J. D. Bullock. 1975. Extremely thermophilic acidophilic bacteria convergent with Sulfolobus solfataricus. J. Gen. Microbiol. 86:156-164. [DOI] [PubMed] [Google Scholar]
- 4.Edge, A. S. B., C. R. Faltynek, L. Hof, L. E. Reichert, Jr., and P. Weber. 1981. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal. Biochem. 118:131-137. [DOI] [PubMed] [Google Scholar]
- 5.Eichler, J. 2000. Novel glycoproteins of the halophilic archaeon Haloferax volcanii. Arch. Microbiol. 173:445-448. [DOI] [PubMed] [Google Scholar]
- 6.Elferink, M. G. L., S.-V. Albers, W. N. Konings, and A. J. M. Driessen. 2001. Sugar transport in Sulfolobus solfataricus is mediated by two families of binding protein-dependent ABC transporters. Mol. Microbiol. 39:1494-1503. [DOI] [PubMed] [Google Scholar]
- 7.Fairbanks, G., T. L. Steck, and D. F. H. Wallace. 1971. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 10:2606-2617. [DOI] [PubMed] [Google Scholar]
- 8.Goldman, S., K. Hecht, H. Eisenberg, and M. Mevarech. 1990. Extracellular Ca2+-dependent inducible alkaline phosphatase from the extremely halophilic archaebacterium Haloarcula marismorturi. J. Bacteriol. 172:7065-7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris, R. A., K. M. Popov, Y. Zhao, N. Y. Kedishvili, Y. Shimomura, and D. A. Crabb. 1995. A new family of protein kinases—the mitochondrial protein kinases. Adv. Enzyme Regul. 35:147-162. [DOI] [PubMed] [Google Scholar]
- 10.Kennelly, P. J., A. M. Edelman, D. K. Blumenthal, and E. G. Krebs. 1987. Rabbit skeletal muscle myosin light chain kinase. The calmodulin binding domain as a potential active site-directed inhibitory domain. J. Biol. Chem. 262:11958-11963. [PubMed] [Google Scholar]
- 11.Kim, D.-J., and S. Forst. 2001. Genomic analysis of the histidine kinase family in bacteria and archaea. Microbiology 147:1197-1212. [DOI] [PubMed] [Google Scholar]
- 12.Koretke, K. K., A. N. Lupas, P. V. Warren, M. Rosenberg, and J. R. Brown. 2000. Evolution of two-component signal transduction. Mol. Biol. Evol. 17:1956-1970. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 14.Leonard, C. J., L. Aravind, and E. V. Koonin. 1998. Novel families of putative protein kinases in bacteria and archaea: evolution of the “eukaryotic” protein kinase superfamily. Genome Res. 8:1038-1047. [DOI] [PubMed] [Google Scholar]
- 15.Lower, B. H., K. M. Bischoff, and P. J. Kennelly. 2000. The archaeon Sulfolobus solfataricus contains a membrane-associated protein kinase activity that preferentially phosphorylates threonine residues in vitro. J. Bacteriol. 182:3452-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lübben, M., and G. Schäfer. 1989. Chemiosmotic energy conversion of the archaebacterial thermoacidophile Sulfolobus acidocaldarius: oxidative phosphorylation and the presence of an F0-related N,N′-dicyclohexylcarbodiimide-binding proteolipid. J. Bacteriol. 171:6106-6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Min, K.-T., C. M. Hilditch, B. Diederich, J. Errington, and M. D. Yudkin. 1993. Sigma F, the first compartment-specific transcription factor of B. subtilis, is regulated by an anti-sigma factor that is also a protein kinase. Cell 74:735-742. [DOI] [PubMed] [Google Scholar]
- 18.Moens, S., and J. Vanderleyden. 1997. Glycoproteins in prokaryotes. Arch. Microbiol. 168:169-175. [DOI] [PubMed] [Google Scholar]
- 19.Novogrodsky, A., R. Lotan, A. Ravid, and N. Sharon. 1975. Peanut agglutinin, a new mitogen that binds to galactosyl sites exposed after neuraminidase treatment. J. Immunol. 115:1243-1248. [PubMed] [Google Scholar]
- 20.Osorio, G., and C. A. Jerez. 1996. Adaptive responses of the archaeon Sulfolobus acidocaldarius BC65 to phosphate starvation. Microbiology 142:1531-1536. [DOI] [PubMed] [Google Scholar]
- 21.Ponting, C. P., L. Aravind, J. Schultz, P. Bork, and E. V. Koonin. 1999. Eukaryotic signaling domain homologues in archaea and bacteria. Ancient ancestry and horizontal gene transfer. J. Mol. Biol. 289:729-745. [DOI] [PubMed] [Google Scholar]
- 22.Raab, B. 1992. Characterization of endopolygalacturonase (EC 3.2.1.15) from Aspergillus niger as glycoprotein by electrophoretic methods and lectin affino-blotting. Electrophoresis 13:807-808. [DOI] [PubMed] [Google Scholar]
- 23.Rudolph, J., N. Tolliday, C. Schmitt, S. C. Schuster, and D. Oesterhelt. 1995. Phosphorylation in halobacterial signal transduction. EMBO J. 14:4249-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaffer, C., and P. Messner. 2001. Glycobiology of surface layer proteins. Biochimie 83:591-599. [DOI] [PubMed] [Google Scholar]
- 25.Segrest, J. P., and R. L. Jackson. 1972. Molecular weight determination of glycoproteins by polyacrylamide gel electrophoresis in sodium dodecyl sulfate. Methods Enzymol. 28:54-63. [Google Scholar]
- 26.Shi, L., M. Potts, and P. J. Kennelly. 1998. The serine, threonine, and/or tyrosine specific protein kinases and protein phosphatases of prokaryotic organisms. A family portrait. FEMS Microbiol. Rev. 22:229-253. [DOI] [PubMed] [Google Scholar]
- 27.Shi, L., K. M. Bischoff, and P. J. Kennelly. 1999. The icfG gene cluster of Synechocystis sp. PCC6803 encodes an Rsb/Spo-like protein kinase, protein phosphatase, and two phosphoproteins. J. Bacteriol. 181:4761-4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shibuya, N., I. J. Goldstein, W. F. Broekaert, M. Nsimba-Lubaki, B. Peeters, and W. J. Peumans. 1987. The elderberry (Sambucus nigra L.) bark lectin recognizes the Nue5Ac(α2-6)Gal/GalNAc sequence. J. Biol. Chem. 262:1596-1601. [PubMed] [Google Scholar]
- 29.Shibuya, N., E. J. Goldstein, E. J. M. Van Damme, and W. J. Peumans. 1988. Binding properties of a mannose-specific lectin from the snowdrop (Galanthus nivalis) bulb. J. Biol. Chem. 263:728-734. [PubMed] [Google Scholar]
- 30.Skorko, R. 1984. Protein phosphorylation in the archaebacterium Sulfolobus acidocaldarius. Eur. J. Biochem. 145:617-622. [DOI] [PubMed] [Google Scholar]
- 31.Skorko, R. 1989. Polyphosphate as a source of phosphoryl group in protein modification in the archaebacterium Sulfolobus acidocaldarius. Biochimie 71:1089-1093. [DOI] [PubMed] [Google Scholar]
- 32.Smith, R. F., and K. Y. King. 1995. Identification of a eukaryote-like protein kinase gene in archaebacteria. Protein Sci. 4:126-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, S. C., B. McCartney, P. J. Kennelly, and M. Potts. 1997. Protein-tyrosine phosphorylation in the Archaea. J. Bacteriol. 179:2418-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solow, B., K. M. Bischoff, M. J. Zylka, and P. J. Kennelly. 1998. Identification of a hexosephosphate mutase and the α-subunit of succinyl-CoA synthetase in the extreme acidothermophile Sulfolobus solfataricus. Protein Sci. 7:105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spudich, E. A., and J. L. Spudich. 1981. Photosensitive phosphoproteins in halobacteria: regulatory coupling of transmembrane proton flux and protein dephosphorylation. J. Cell Biol. 91:895-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spudich, J. L., and W. Stoeckenius. 1980. Light-regulated retinal-dependent reversible phosphorylation of Halobacterium proteins. J. Biol. Chem. 255:5501-5503. [PubMed] [Google Scholar]
- 37.Wang, W.-C., and R. D. Cummings. 1988. The immobilized leukoagglutinin from seeds of Maackia amurensis binds with high affinity to complex-type and Asn-linked oligosaccharides containing terminal sialic acid-linked α-2,3 to penultimate galactose residues. J. Biol. Chem. 263:4576-4585. [PubMed] [Google Scholar]
- 38.Wu, J., N. Ohta, J.-L. Zhao, and A. Newton. 1999. A novel bacterial tyrosine kinase essential for cell division and differentiation. Proc. Natl. Acad. Sci. USA 96:13068-13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamashita, K., K. Totani, T. Okhura, S. Takasaki, I. J. Goldstein, and A. Kobata. 1987. Carbohydrate binding properties of complex-type oligosaccharides on immobilized Datura stramonium lectin. J. Biol. Chem. 262:1602-1607. [PubMed] [Google Scholar]
- 40.Yang, X., K. M. Kang, M. S. Brody, and C. W. Price. 1996. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 10:2265-2275. [DOI] [PubMed] [Google Scholar]