Abstract
1. Distensibility characteristics of the isolated, perfused rabbit ear artery were measured in the presence and absence of different concentrations of adrenaline.
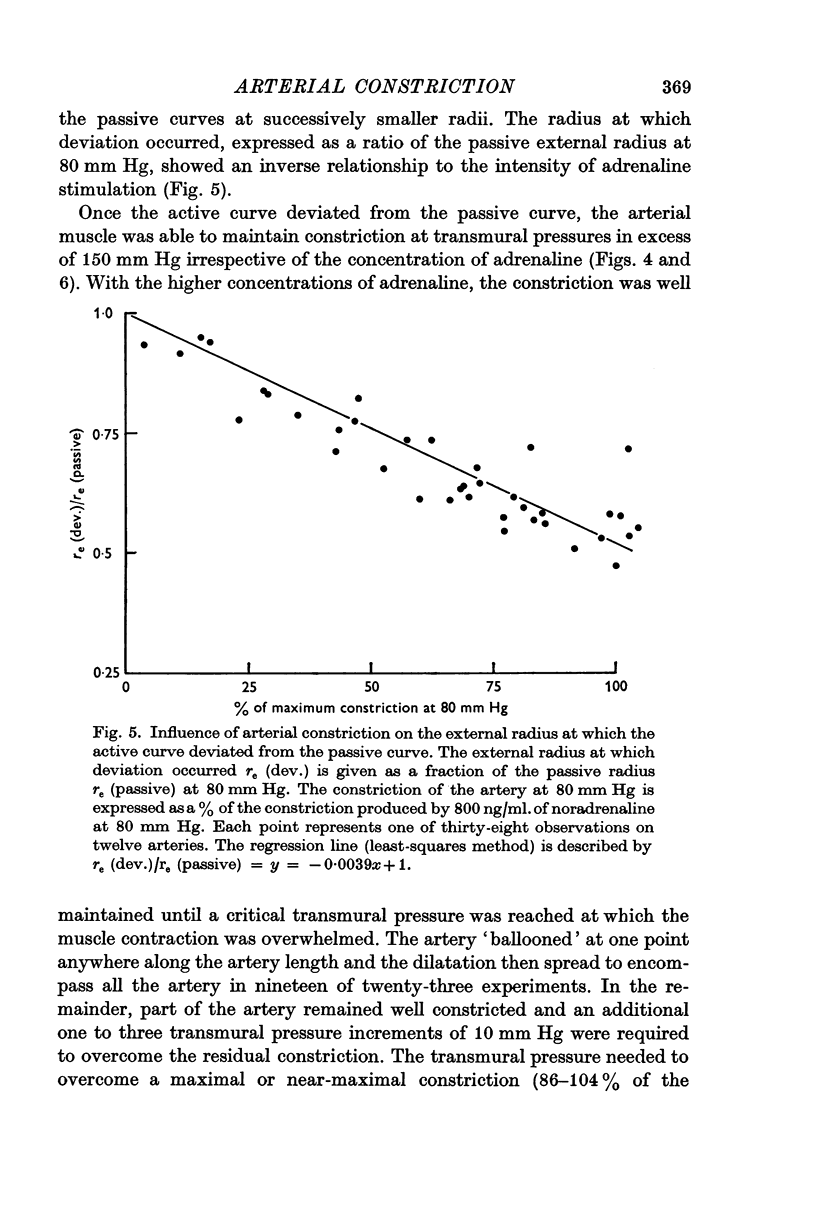
2. The major effect of varying the adrenaline concentration was to vary the radius at which active tension first developed. This radius was inversely related to the adrenaline concentration.
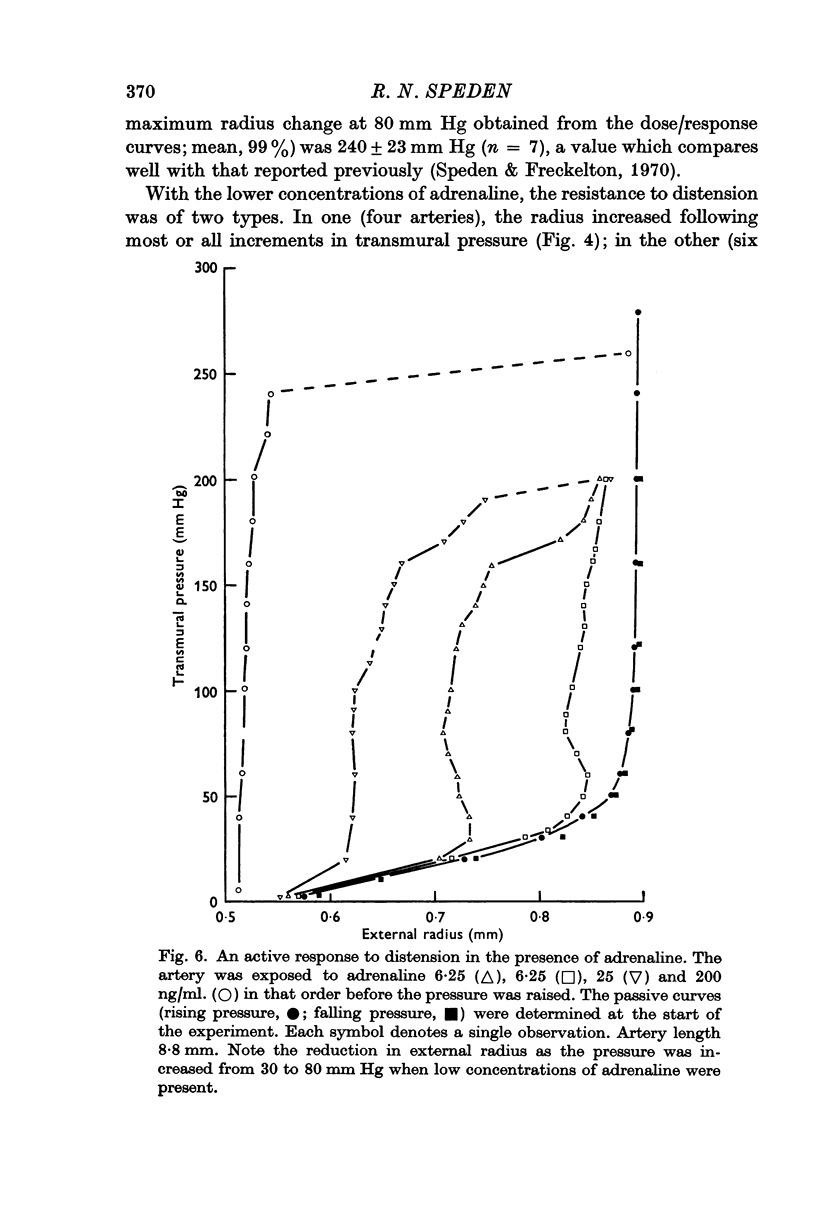
3. Increases in radius of the constricted artery produced by pressures rising from 40 to 150 mm Hg were small relative to the increase in wall stress. Distension was opposed largely by increases in active tension. With some arteries (60%) an increase in pressure between 30 and 80 mm Hg was associated with a decrease in radius when low concentrations of adrenaline were present.
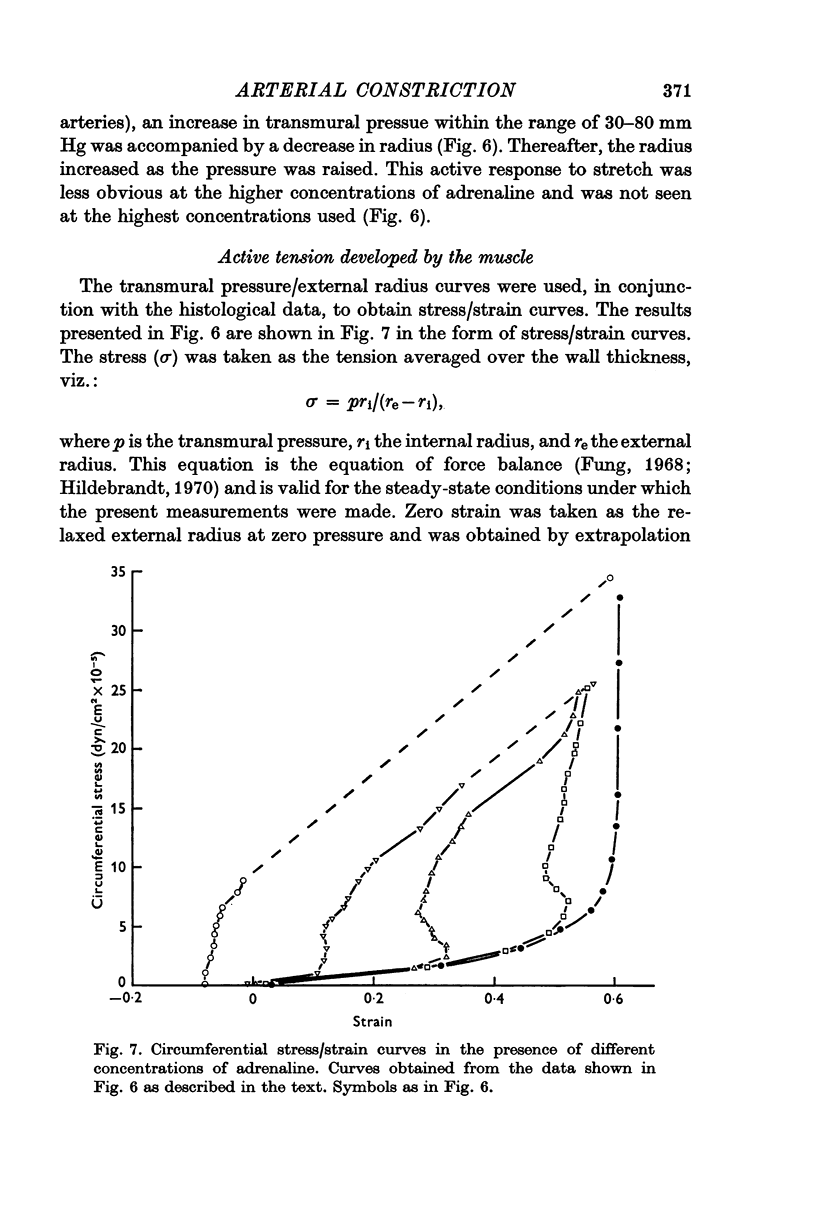
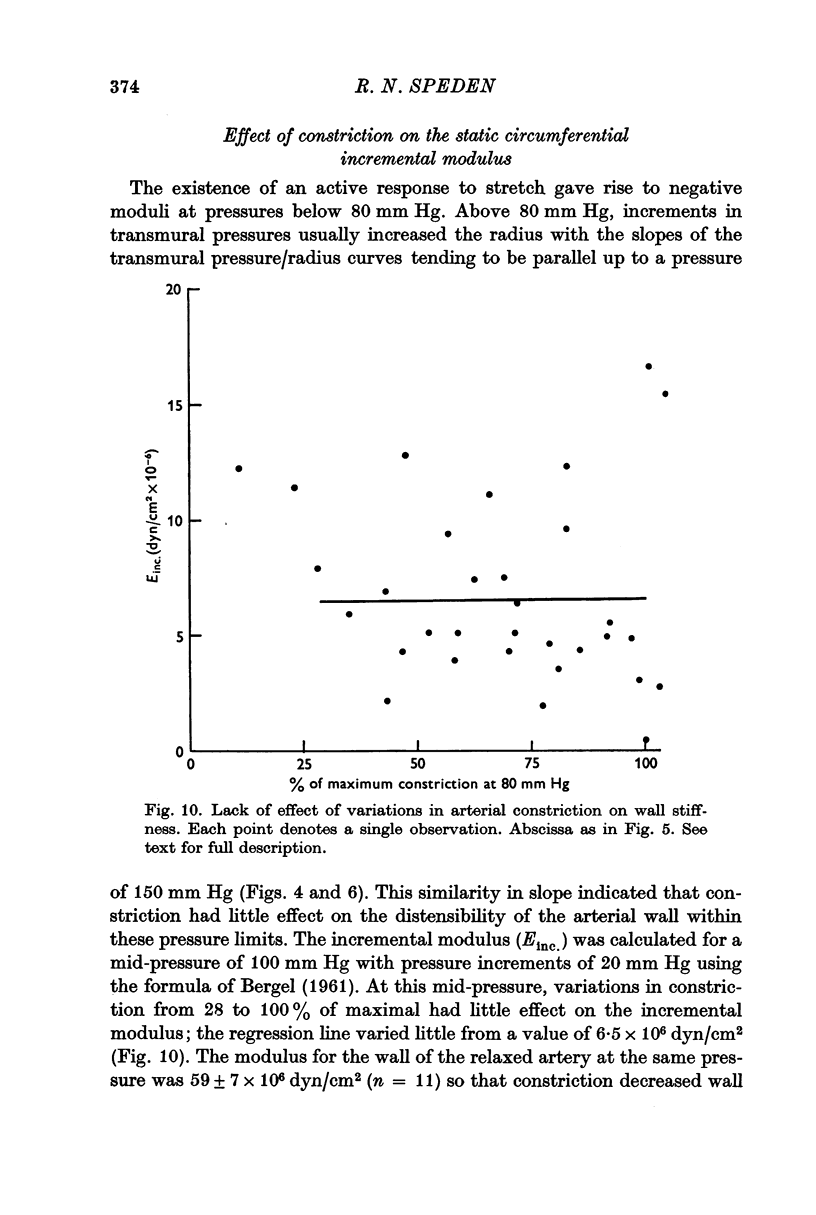
4. The ability of the constricted ear artery to resist distension at transmural pressures of 100 mm Hg was uninfluenced by the adrenaline concentration provided constriction exceeded 28% of maximal. The static, incremental, circumferential modulus of the artery wall varied little from a value of 6·5 × 106 dyn/cm2.
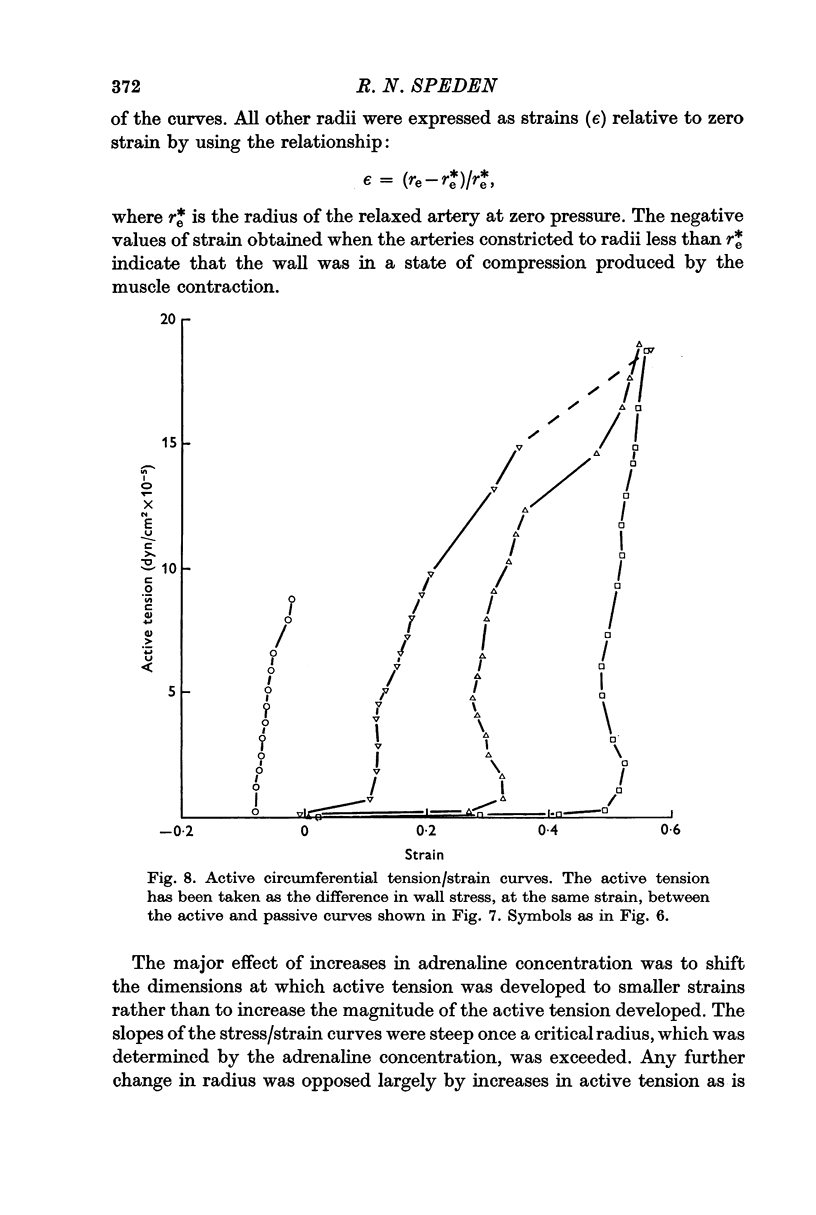
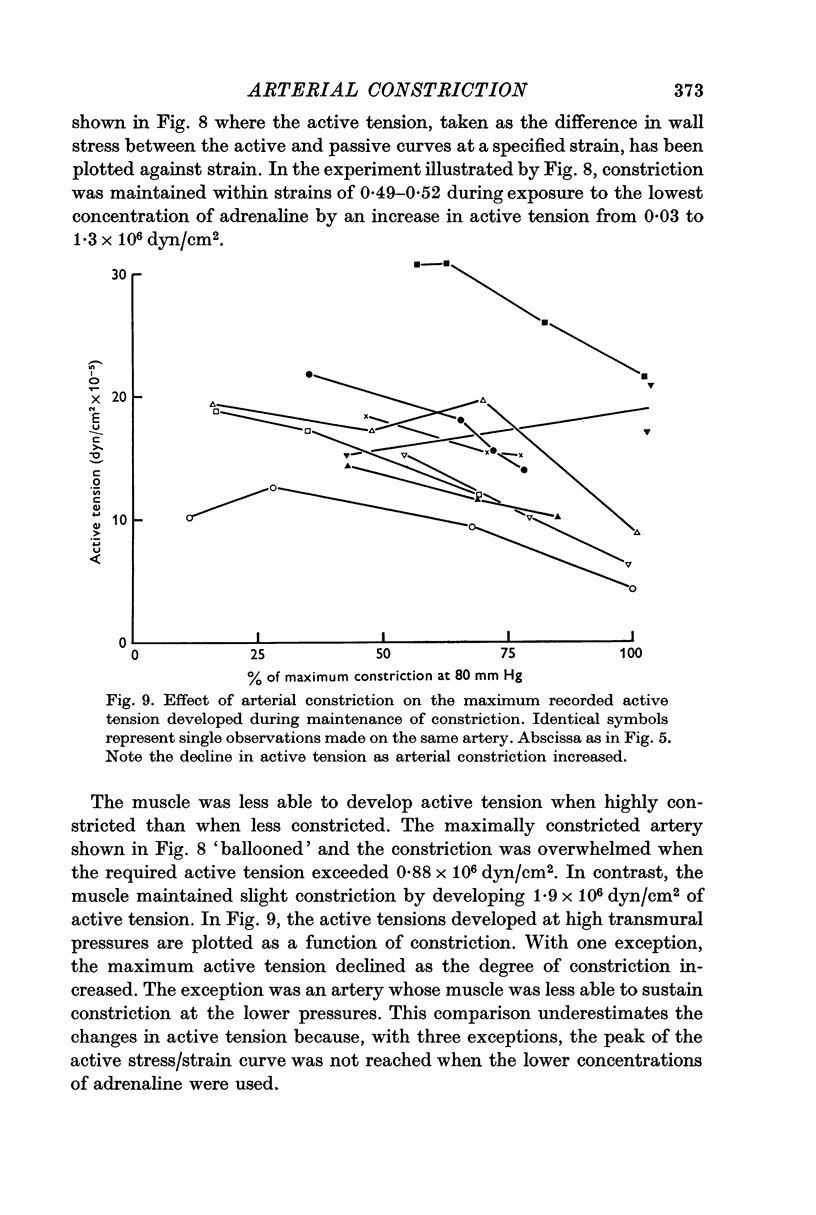
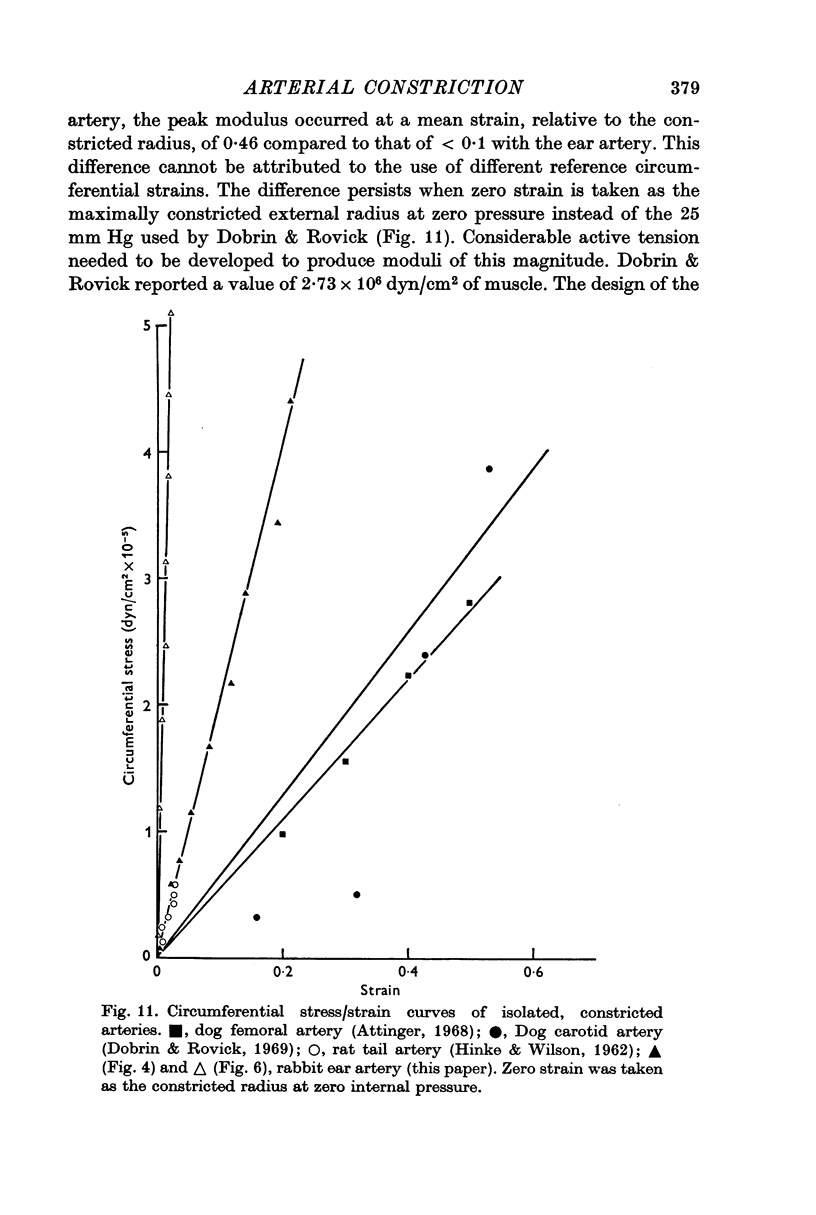
5. The maximum active tension required to maintain constriction was inversely related to the degree of constriction and hence to the adrenaline concentration. The modulus for fully or near-fully activated muscle was 18·5 × 106 dyn/cm2 of media.
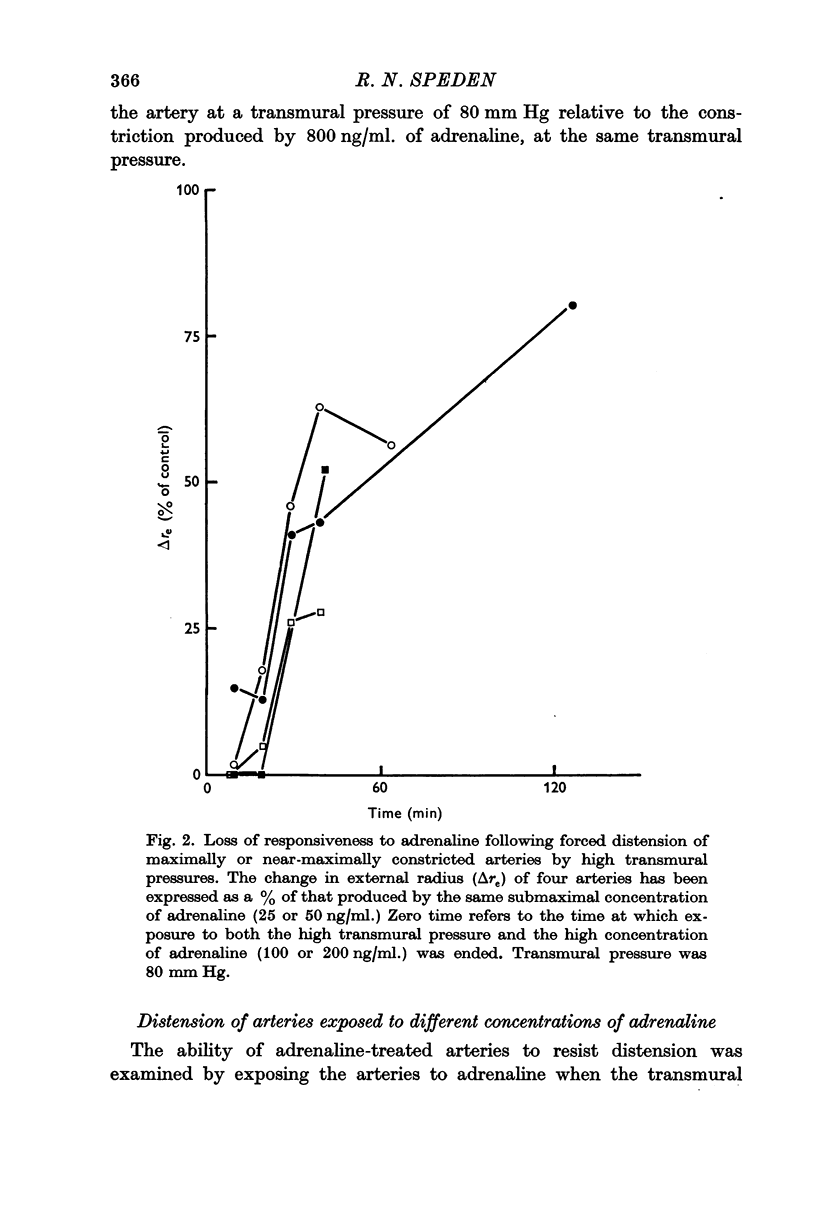
6. Muscle function deteriorated following exposure of constricted arteries to pressures sufficient to overwhelm the constriction.
7. These observations may be explained by a negative feedback system where the contractile elements are arranged in parallel with a length sensor element whose setting is determined by the concentration of adrenaline. The length sensor may be the cell membrane. It is concluded that a radius increase may be a primary stimulus for blood flow auto-regulation.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attinger F. M. Two-dimensional in-vitro studies of femoral arterial walls of the dog. Circ Res. 1968 Jun;22(6):829–840. doi: 10.1161/01.res.22.6.829. [DOI] [PubMed] [Google Scholar]
- BURTON A. C. On the physical equilibrium of small blood vessels. Am J Physiol. 1951 Feb;164(2):319–329. doi: 10.1152/ajplegacy.1951.164.2.319. [DOI] [PubMed] [Google Scholar]
- Bergel D. H. The static elastic properties of the arterial wall. J Physiol. 1961 May;156(3):445–457. doi: 10.1113/jphysiol.1961.sp006686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrin P. B., Rovick A. A. Influence of vascular smooth muscle on contractile mechanics and elasticity of arteries. Am J Physiol. 1969 Dec;217(6):1644–1651. doi: 10.1152/ajplegacy.1969.217.6.1644. [DOI] [PubMed] [Google Scholar]
- HINKE J. A., WILSON M. L. A study of elastic properties of a 550-microns artery in vitro. Am J Physiol. 1962 Dec;203:1153–1160. doi: 10.1152/ajplegacy.1962.203.6.1153. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. Extension of small-strain theory to finite deformation of cylindrical vessels by internal over-pressure. Angiologica. 1970;7(5):257–272. doi: 10.1159/000157841. [DOI] [PubMed] [Google Scholar]
- Holman M. E. Electrophysiology of vascular smooth muscle. Ergeb Physiol. 1969;61:137–177. doi: 10.1007/BFb0111448. [DOI] [PubMed] [Google Scholar]
- Mekata F. Electrophysiological studies of the smooth muscle cell membrane of the rabbit common carotid artery. J Gen Physiol. 1971 Jun;57(6):738–751. doi: 10.1085/jgp.57.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPARKS H. V., Jr, BOHR D. F. Effect of stretch on passive tension and contractility of isolated vascular smooth muscle. Am J Physiol. 1962 May;202:835–840. doi: 10.1152/ajplegacy.1962.202.5.835. [DOI] [PubMed] [Google Scholar]
- Speden R. N. Adrenergic transmission in small arteries. Nature. 1967 Oct 21;216(5112):289–290. doi: 10.1038/216289a0. [DOI] [PubMed] [Google Scholar]
- Speden R. N. The effect of initial strip length on the noradrenaline-induced contraction of arterial strips. J Physiol. 1960 Nov;154(1):15–25. doi: 10.1113/jphysiol.1960.sp006561. [DOI] [PMC free article] [PubMed] [Google Scholar]


