Abstract
1. An electrophysiological analysis has been made of the storage and release of noradrenaline (NAd) in the sympathetic nerve terminals of the isolated vas deferens of the mouse. The amplitude of the excitatory junction potentials (e.j.p.s) recorded intracellularly in smooth muscle cells was taken as a measure of the NAd output per impulse from the terminals of sympathetic axons.
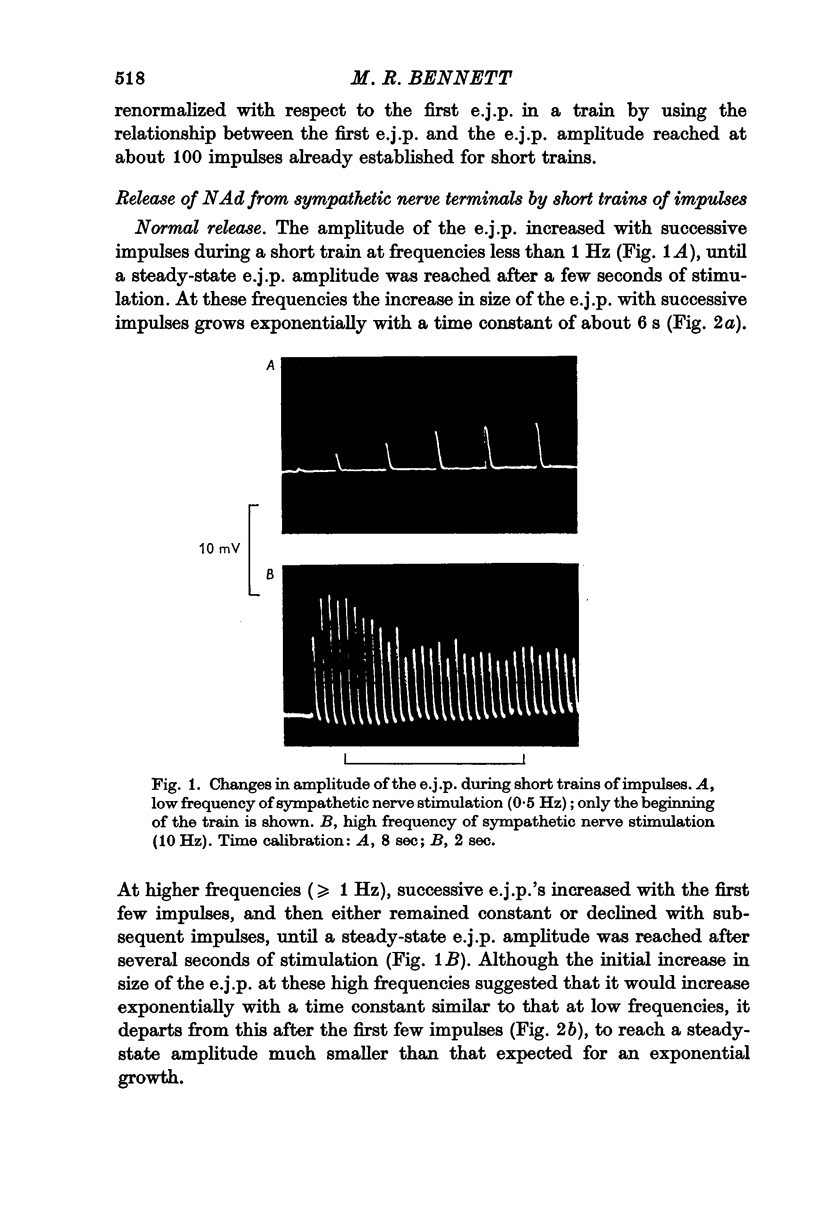
2. During short trains of impulses (< 100), the amplitude of the e.j.p. increased with successive impulses at the beginning of a train, and then either continued to increase until a steady-state amplitude was reached (frequencies < 1 Hz), or decreased until a depressed steady amplitude was reached (frequencies > 1 Hz).
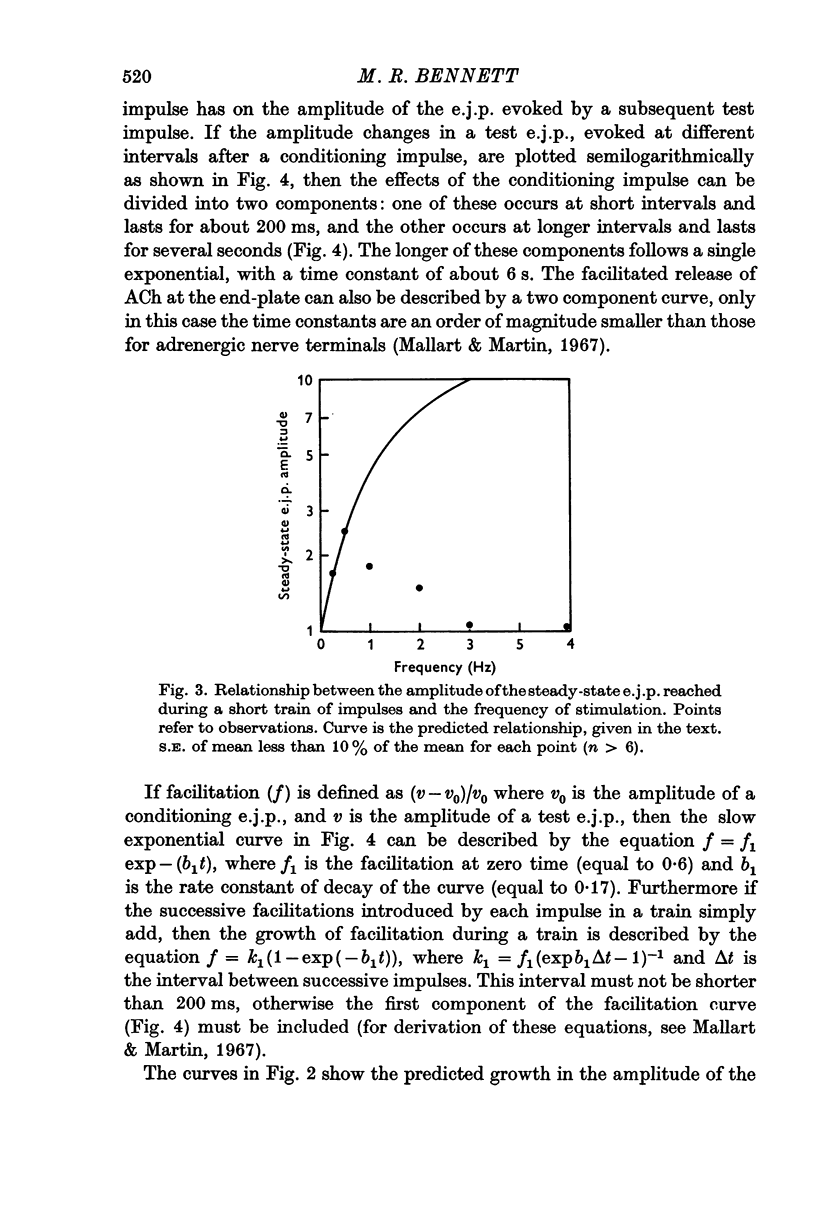
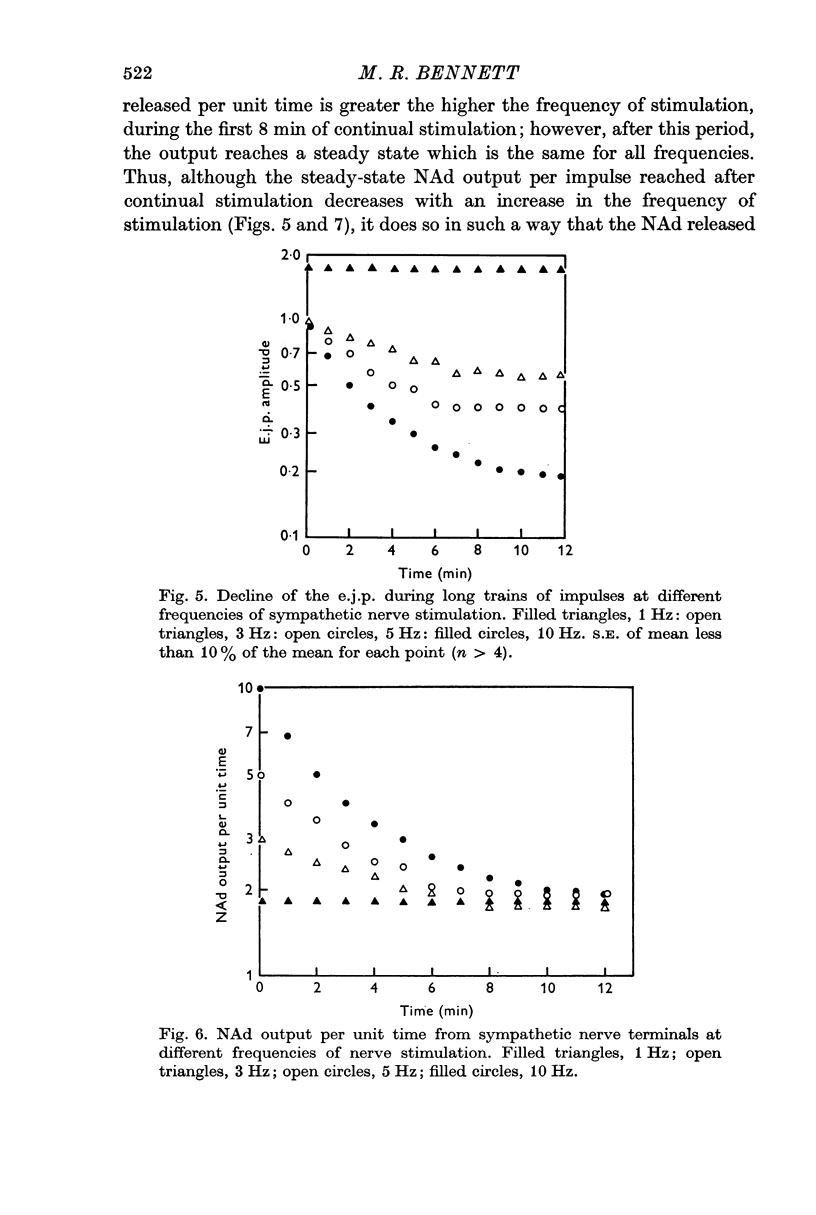
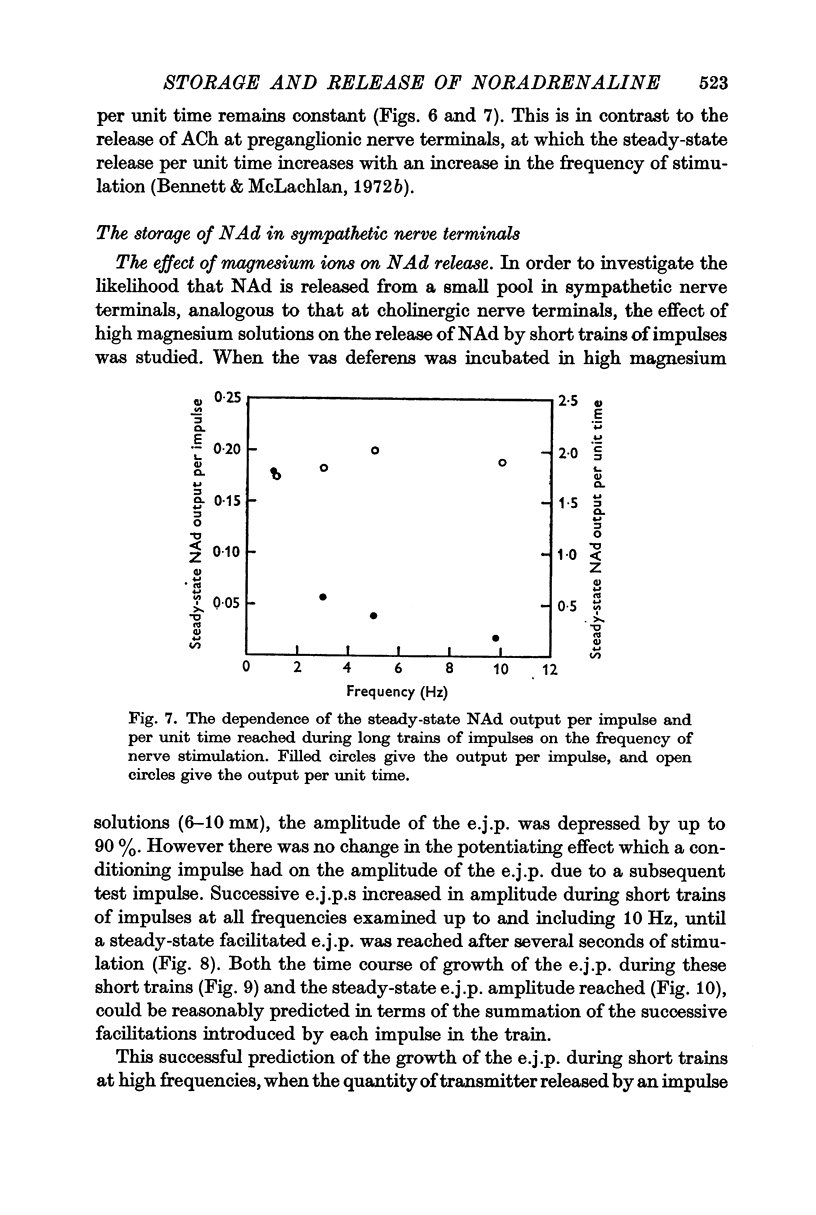
3. During trains of impulses lasting for several minutes, the amplitude of the e.j.p. continually declined (frequencies > 1 Hz) until a steady-state amplitude was reached after 8 min of stimulation. This steady-state amplitude is smaller, the higher the frequency of stimulation.
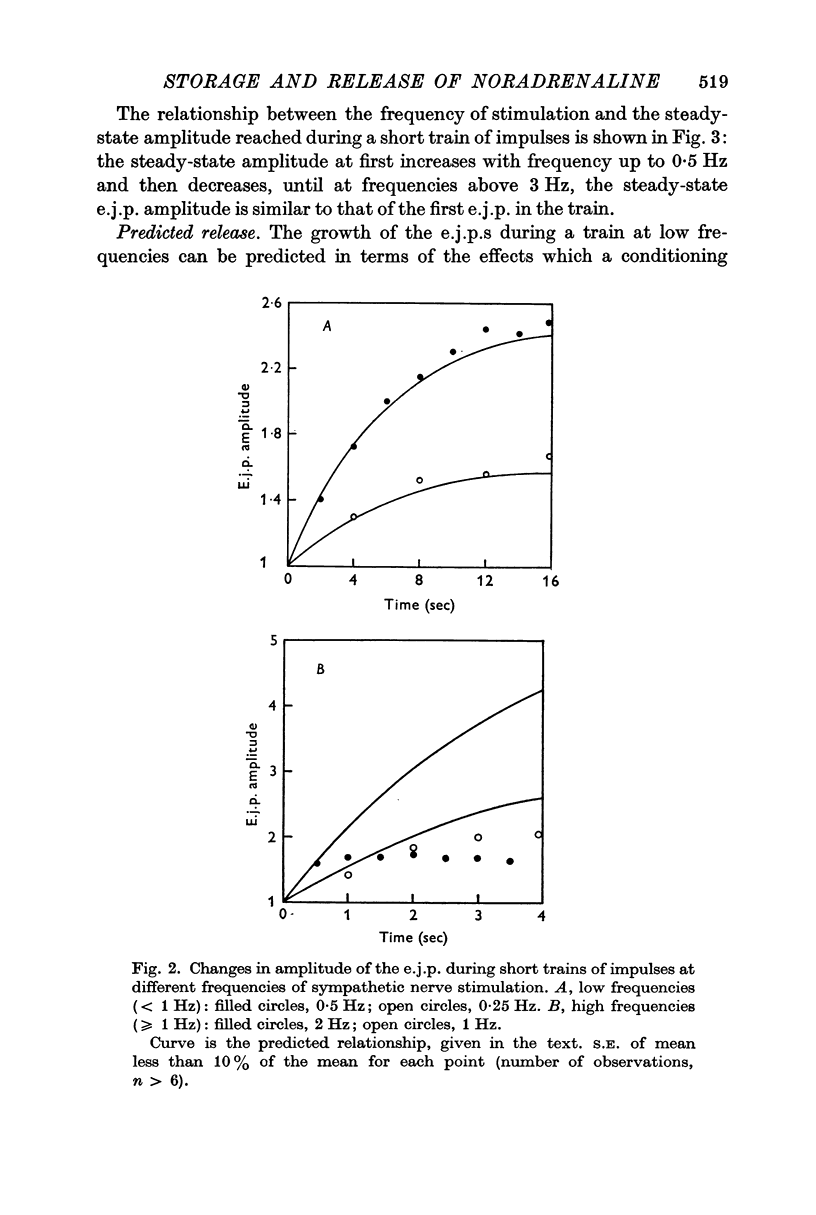
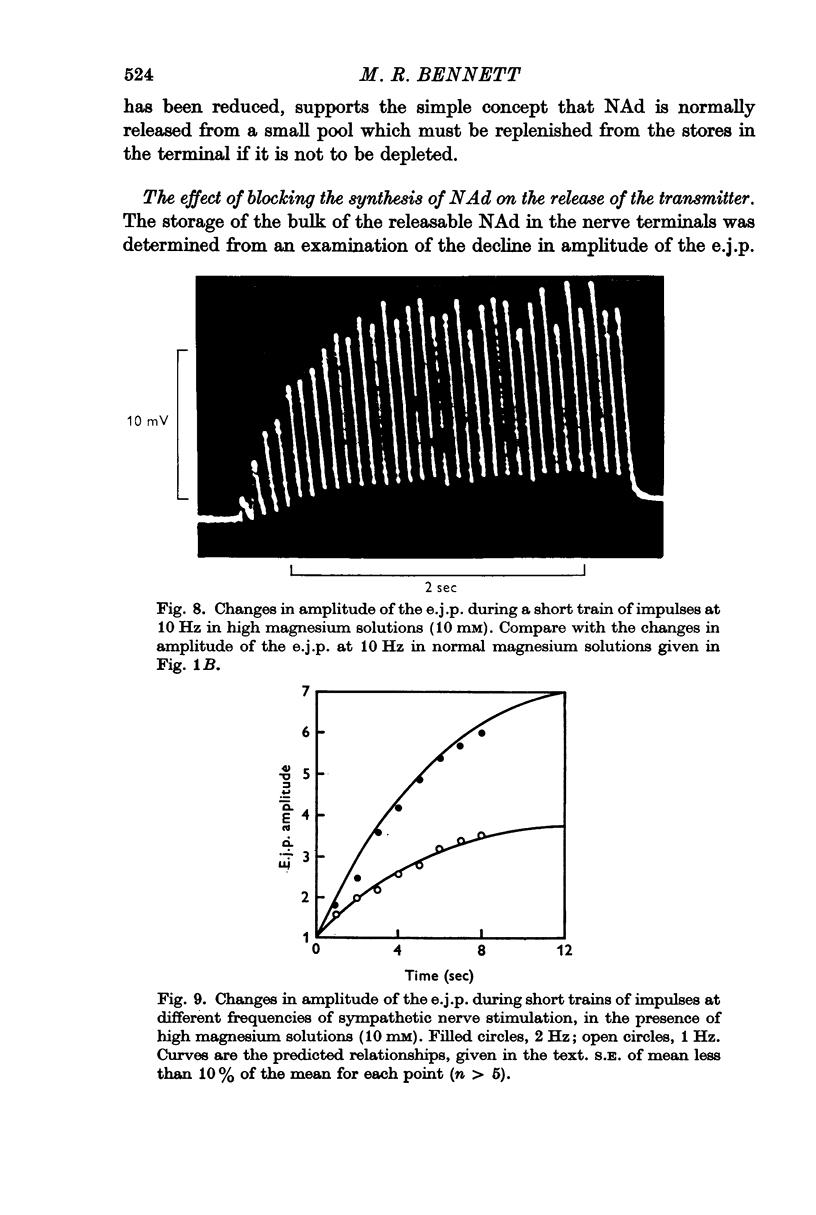
4. During short trains of impulses in the presence of high magnesium solutions, the amplitude of successive e.j.p.s increased until a steady state was reached, no matter what the frequency of stimulation. This growth of the e.j.p. amplitude during a train could be quantitatively predicted in terms of the linear summation of the individual facilitatory effects introduced by each impulse in the train.
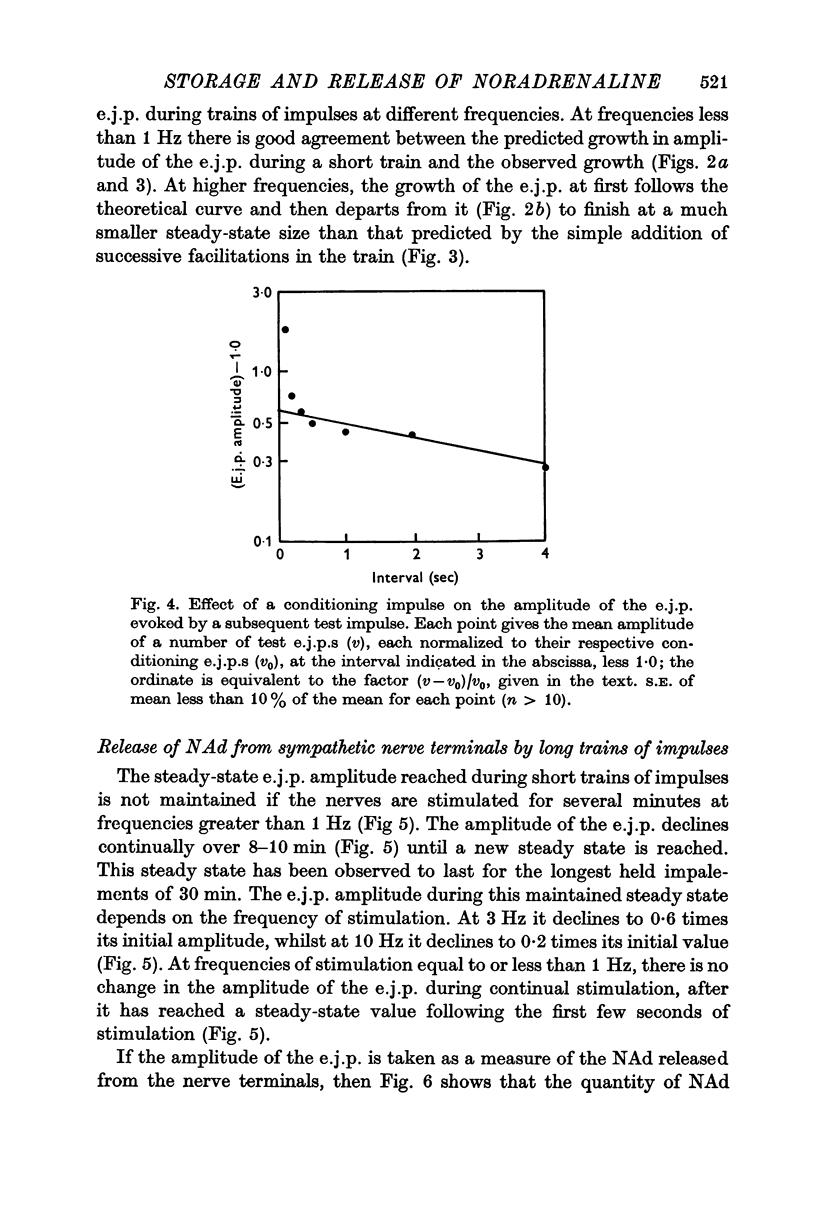
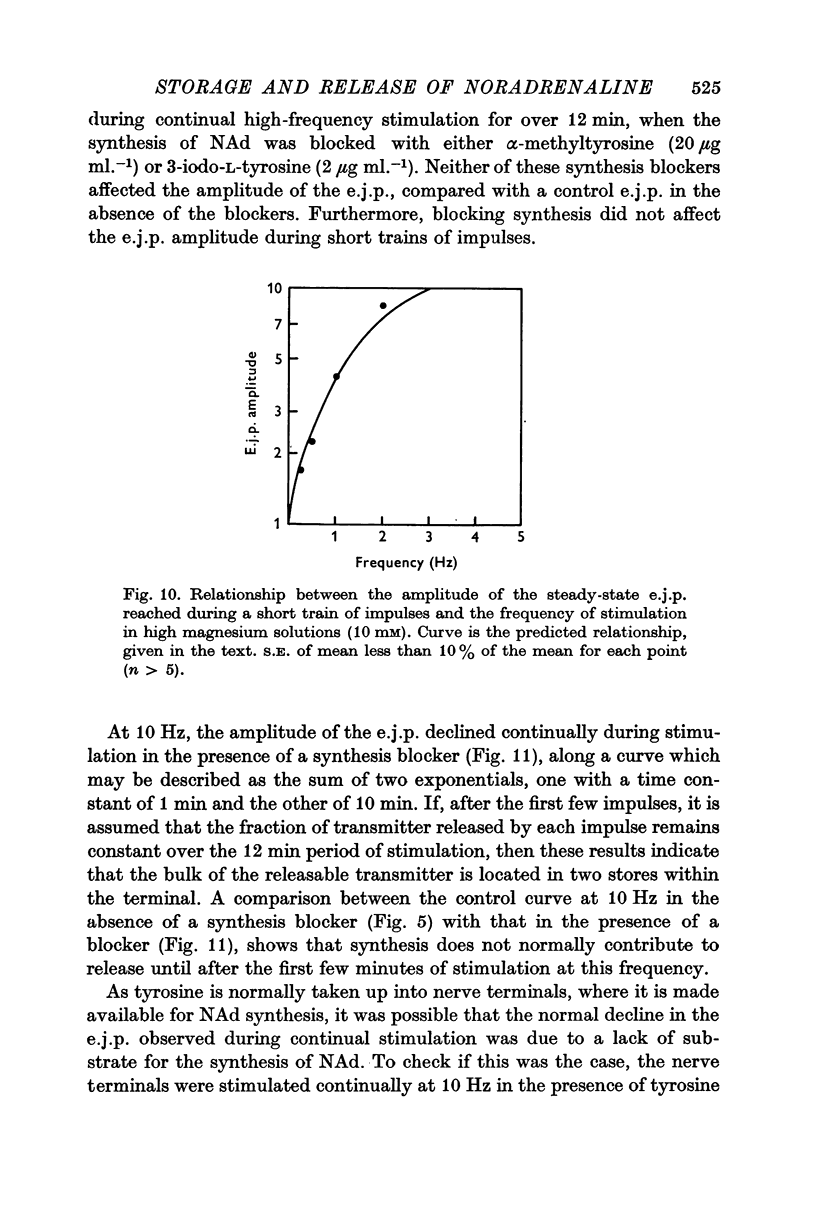
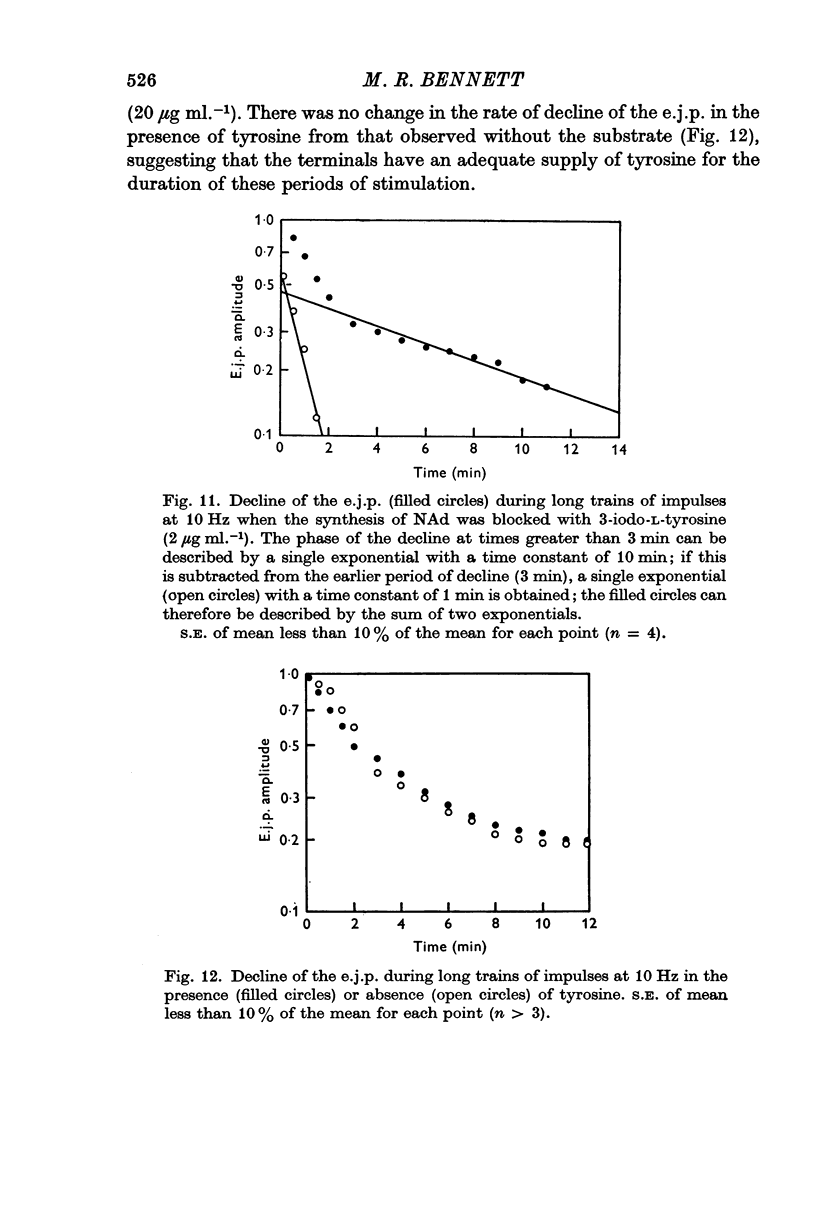
5. During trains of impulses lasting for several minutes, in the presence of a NAd synthesis blocker, the amplitude of the e.j.p. continually declined along a curve which could be described as the sum of two exponential components: one with a time constant of 1 min and the other of 10 min.
6. These results suggest that NAd is released from a small pool of transmitter in sympathetic nerve terminals, which is replenished from two stores, which are in turn replenished by the synthesis of new NAd.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod J. Noradrenaline: fate and control of its biosynthesis. Science. 1971 Aug 13;173(3997):598–606. doi: 10.1126/science.173.3997.598. [DOI] [PubMed] [Google Scholar]
- BROWN G. L., GILLESPIE J. S. The output of sympathetic transmitter from the spleen of the cat. J Physiol. 1957 Aug 29;138(1):81–102. doi: 10.1113/jphysiol.1957.sp005839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R. An electrophysiological analysis of the uptake of noradrenaline at sympathetic nerve terminals. J Physiol. 1973 Mar;229(2):533–546. doi: 10.1113/jphysiol.1973.sp010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M. An electrophysiological analysis of the storage of acetylcholine in preganglionic nerve terminals. J Physiol. 1972 Mar;221(3):657–668. doi: 10.1113/jphysiol.1972.sp009774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M. An electrophysiological analysis of the synthesis of acetylcholine in preganglionic nerve terminals. J Physiol. 1972 Mar;221(3):669–682. doi: 10.1113/jphysiol.1972.sp009775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol. 1970 Mar;206(3):629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B. N., Martin A. R. Estimates of probability of transmitter release at the mammalian neuromuscular junction. J Physiol. 1970 Nov;210(4):933–945. doi: 10.1113/jphysiol.1970.sp009250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D. E., Jones C. J., Linley P. A. Histochemical fluorescence studies on noradrenaline accumulation by Uptake 2 in the isolated rat heart. Br J Pharmacol. 1969 Sep;37(1):1–9. doi: 10.1111/j.1476-5381.1969.tb09515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The effect of magnesium on the activity of motor nerve endings. J Physiol. 1954 Jun 28;124(3):553–559. doi: 10.1113/jphysiol.1954.sp005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearnaley D. P., Geffen L. B. Effect of nerve stimulation on the noradrenaline content of the spleen. Proc R Soc Lond B Biol Sci. 1966 Dec 13;166(1004):303–315. doi: 10.1098/rspb.1966.0101. [DOI] [PubMed] [Google Scholar]
- Elmqvist D., Quastel D. M. A quantitative study of end-plate potentials in isolated human muscle. J Physiol. 1965 Jun;178(3):505–529. doi: 10.1113/jphysiol.1965.sp007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillenz M. Fine structure of noradrenaline storage vesicles in nerve terminals of the rat vas deferens. Philos Trans R Soc Lond B Biol Sci. 1971 Jun 17;261(839):319–323. doi: 10.1098/rstb.1971.0063. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Campbell G. R., Gillard S. M., Malmfors T., Cobb J. L., Burnstock G. Cellular studies of sympathetic denervation produced by 6-hydroxydopamine in the vas deferens. J Pharmacol Exp Ther. 1970 Jul;174(1):111–122. [PubMed] [Google Scholar]
- Grillo M. A. Electron microscopy of sympathetic tissues. Pharmacol Rev. 1966 Mar;18(1):387–399. [PubMed] [Google Scholar]
- Kopin I. J., Breese G. R., Krauss K. R., Weise V. K. Selective release of newly synthesized norepinephrine from the cat spleen during sympathetic nerve stimulation. J Pharmacol Exp Ther. 1968 Jun;161(2):271–278. [PubMed] [Google Scholar]
- LILEY A. W., NORTH K. A. An electrical investigation of effects of repetitive stimulation on mammalian neuromuscular junction. J Neurophysiol. 1953 Sep;16(5):509–527. doi: 10.1152/jn.1953.16.5.509. [DOI] [PubMed] [Google Scholar]
- Langer S. Z. The metabolism of (3H)noradrenaline released by electrical stimulation from the isolated nictitating membrane of the cat and from the vas deferens of the rat. J Physiol. 1970 Jul;208(3):515–546. doi: 10.1113/jphysiol.1970.sp009135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman S. L., Iversen L. L. The role of uptake2 in the extraneuronal metabolism of catecholamines in the isolated rat heart. Br J Pharmacol. 1969 Nov;37(3):638–649. doi: 10.1111/j.1476-5381.1969.tb08502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. The relation between quantum content and facilitation at the neuromuscular junction of the frog. J Physiol. 1968 Jun;196(3):593–604. doi: 10.1113/jphysiol.1968.sp008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTSUKA M., ENDO M., NONOMURA Y. Presynaptic nature of neuromuscular depression. Jpn J Physiol. 1962 Dec 15;12:573–584. doi: 10.2170/jjphysiol.12.573. [DOI] [PubMed] [Google Scholar]
- Stjärne L., Wennmalm A. Preferential secretion of newly formed noradrenaline in the perfused rabbit heart. Acta Physiol Scand. 1970 Nov;80(3):428–429. doi: 10.1111/j.1748-1716.1970.tb04808.x. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A. The long-lasting depression in neuromuscular transmission of frog. Jpn J Physiol. 1958 Jun 15;8(2):102–113. doi: 10.2170/jjphysiol.8.102. [DOI] [PubMed] [Google Scholar]
- Tranzer J. P., Thoenen H. An electron microscopic study of selective, acute degeneration of sympathetic nerve terminals after administration of 6-hydroxydopamine. Experientia. 1968 Feb 15;24(2):155–156. doi: 10.1007/BF02146956. [DOI] [PubMed] [Google Scholar]


