Abstract
In Bacillus subtilis, maturation of 5S rRNA is catalyzed by an enzyme called RNase M5. We searched for potential mRNA substrates for RNase M5 by gene array technology, based on the premise that most endonucleolytic cleavages have an effect on the stability of RNA and hence on steady-state levels of expression. Only a handful of genes had significantly altered expression in rnmV mutants compared to wild-type strains that could subsequently be confirmed by Northern blotting. The effect of RNase M5 on the expression of the best candidates, the odhAB and sucCD operons, is indirect, by a mechanism we do not yet understand. We show that an effect of RNase M5 on the expression of the remaining candidate, ctsR, is due to the failure to process the 5S rRNA contained in the rrnW lying directly upstream. We thus conclude that RNase M5 has very few or possibly no mRNA substrates in B. subtilis.
In Bacillus subtilis, 5S rRNA processing is performed by an enzyme known as RNase M5 (19). This enzyme cleaves the 5S rRNA precursor on both sides of a double-stranded stem, extending from the binding site of ribosomal protein L5, to yield mature 5S rRNA in one step. A cofactor is required for this reaction and has been identified as ribosomal protein L18 (20). L18 binds 5S rRNA and in doing so is thought to present the substrate to RNase M5 in the correct conformation for cleavage, rather than playing a direct role in the processing reaction itself (15). L5, which binds to the 5S processing stalk, inhibits the cleavage reaction at high concentrations (20). Recently, quantities of RNase M5 sufficient to obtain an N-terminal amino acid sequence and thus identify its gene were purified (4). The gene was named rnmV and shown to be highly conserved among low-G+C gram-positive organisms. The rnmV gene is not essential for B. subtilis viability, despite the fact that strains lacking a functional copy of this gene make no mature 5S rRNA whatsoever. 5S rRNA is found as precursor species of different lengths (depending on which of the 10 rRNA operons it originated from) in both ribosomes and polysomes in such strains without a major effect on growth rate, suggesting that 5S rRNA maturation is dispensable for ribosome function, at least in B. subtilis.
In Escherichia coli, 5S rRNA processing is carried out by RNase E (6), an essential enzyme (2) that also plays a major role in mRNA degradation (14) and processing of 16S rRNA (12) and tRNA (11). RNase E is thought to catalyze the rate-limiting initial cleavage of most RNAs, and thus, the half-life of most mRNAs and hence their steady-state levels is increased significantly in RNase E mutants (rne) (14). We were curious to know whether, like RNase E, RNase M5 also had other substrates in the cell besides the 5S rRNA precursor. By analogy to the effect of the rne mutation on steady-state mRNA levels in E. coli, we chose to search for variations in steady-state mRNA levels of individual genes in B. subtilis strains carrying an inactivated RNase M5 gene, using gene array technology. The expression of only a limited number of genes was reproducibly affected by inactivation of the rnmV gene and most of these effects were quite small. An effect of RNase M5 on the expression of three out of five candidate genes or operons examined was confirmed by Northern blotting. For two of these, odhAB and sucCD, the effect of RNase M5 appears to be indirect, and for the third, ctsR/clpC, the effect is due to lack of processing of 5S rRNA immediately upstream. This suggests that the number of mRNA substrates of RNase M5 is very limited, if indeed any exist.
Search for mNA substrates of RNase M5 using gene arrays.
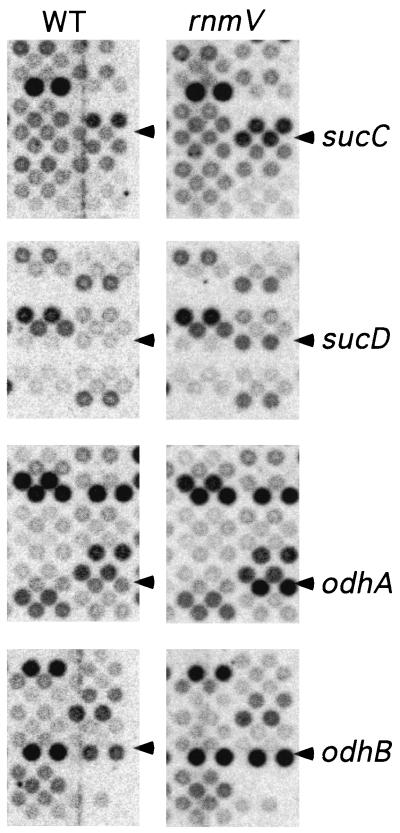
Bulk mRNA is stabilized in RNase E mutants in E. coli (14), suggesting that the majority of mRNAs are substrates for RNase E cleavage. We wondered whether RNase M5, like RNase E, had substrates other than the 5S rRNA precursor that we could detect by altered mRNA stability and hence steady-state levels of expression. We compared the levels of expression of the 4,107 putative B. subtilis open reading frames arrayed on commercial membranes (Sigma-Genosys) in wild-type (B. subtilis W168) and rnmV mutant (strain SSB312, described previously [4]) cells, using 33P-labeled cDNA synthesized from 1 μg of total RNA as a probe. Statistical analysis of the signals generated by five batches of probe synthesized from two independent RNA preparations revealed that the expression of only a small number of genes was affected by RNase M5 inactivation. Two methods were used to calculate ratios of wild-type to mutant expression. In the first, the ratio was calculated for each of the five experiments performed and then the ratios were averaged; in the second, the expression levels were averaged over the five experiments before the ratio was calculated. Using the first method, 36 genes were identified whose expression varied by at least a factor of 2.0 and where the ratio of the standard deviation to the average was arbitrarily cut off at 0.7 or better (Table 1). When the second method of calculation was used, i.e., the expression levels were averaged before the ratio was calculated, only six candidate genes were deemed statistically significant (Table 1). The expression of only four genes, odhA, odhB, sucC, and sucD, could be considered significantly affected by both methods of calculation. An example of the signal observed for these four genes is given in Fig. 1. Interestingly, these genes form two operons, odhAB and sucCD, that encode the E1/E2 and α/β subunits of 2-oxoglutarate dehydrogenase and succinyl-coenzyme A synthetase, respectively, enzymes which catalyze consecutive steps in the tricarboxylic acid cycle.
TABLE 1.
Genes whose expression is affected by the rnmV mutation
| Effect on mRNA expression | Genea | WT/rnmV or rnmV/WTb
|
SD/avg | Gene product or function | |
|---|---|---|---|---|---|
| Avg | SD | ||||
| Decreased | groES | 2.65 | 1.40 | 0.53 | Class I heat shock protein (chaperonin) |
| sodA | 2.62 | 1.21 | 0.46 | Superoxide dismutase | |
| ppiB | 2.38 | 1.44 | 0.60 | Peptidyl-prolyl isomerase | |
| hrcA | 2.36 | 1.31 | 0.56 | Transcriptional repressor of class I heat shock genes | |
| ypjD | 2.36 | 0.98 | 0.42 | Unknown; similar to unknown proteins | |
| alaS | 2.30 | 1.52 | 0.66 | Alanyl-tRNA synthetase | |
| clpP | 2.30 | 1.48 | 0.65 | ATP-dependent protease (class III heat shock protein) | |
| yddH | 2.21 | 0.84 | 0.38 | Unknown; similar to transposon protein | |
| prsA | 2.13 | 1.34 | 0.63 | Protein secretion (posttranslocation molecular chaperone) | |
| ecsA | 2.10 | 1.29 | 0.61 | ABC transporter (ATP-binding protein) | |
| ybeF | 2.07 | 1.07 | 0.52 | Unknown; similar to unknown proteins | |
| panB | 2.07 | 1.30 | 0.63 | Ketopantoate hydroxymethyltransferase | |
| ctsR | 2.06 | 1.10 | 0.53 | Transcriptional repressor of class III stress genes | |
| yfmL* | 1.29 | 0.17 | 0.13 | Unknown; similar to RNA helicase | |
| Increased | sucC* | 5.82 | 3.83 | 0.66 | Succinyl-CoA synthetase (beta subunit) |
| odhA* | 4.69 | 2.84 | 0.61 | 2-Oxoglutarate dehydrogenase (E1 subunit) | |
| sucD* | 3.62 | 1.88 | 0.52 | Succinyl-CoA synthetase (alpha subunit) | |
| odhB* | 3.55 | 1.50 | 0.42 | 2-Oxoglutarate dehydrogenase (E2 subunit) | |
| yorL | 2.75 | 1.33 | 0.48 | Unknown; similar to DNA polymerase III (alpha subunit) | |
| yoqU | 2.74 | 1.91 | 0.70 | Unknown | |
| yosE | 2.46 | 1.43 | 0.58 | Unknown | |
| yneS | 2.44 | 1.67 | 0.68 | Unknown; similar to unknown proteins | |
| yqel | 2.37 | 1.60 | 0.67 | Unknown; similar to dihydrodipicolinate reductase | |
| yoaJ | 2.36 | 1.55 | 0.65 | Unknown; similar to extracellular endoglucanase precursor | |
| rplT | 2.26 | 1.49 | 0.66 | Ribosomal protein L20 | |
| ylnE | 2.26 | 1.50 | 0.66 | Unknown; similar to unknown proteins | |
| rpmJ | 2.23 | 1.32 | 0.59 | Ribosomal protein L36 (ribosomal protein B) | |
| ykqA | 2.18 | 1.49 | 0.68 | Unknown; similar to unknown proteins (also ylxU. yzaB) | |
| rplV | 2.16 | 1.12 | 0.52 | Ribosomal protein L22 (BL17) | |
| yorP | 2.11 | 0.92 | 0.44 | Unknown | |
| bfmBAA | 2.09 | 0.83 | 0.40 | 2-Oxoisovalerate dehydrogenase (alpha subunit) | |
| infA | 2.08 | 1.33 | 0.64 | Initiation factor IF-I | |
| treP | 2.07 | 0.86 | 0.42 | Trehalose-specific enzyme IIBC (PTDS) component | |
| yobB | 2.02 | 1.06 | 0.52 | Unknown | |
| rpsM | 2.01 | 1.01 | 0.50 | Ribosomal protein S13 | |
| hisB | 2.01 | 1.34 | 0.67 | Imidazoleglycerol-phosphate dehydratase | |
| yorM | 2.00 | 1.16 | 0.58 | Unknown; similar to unknown proteins | |
| ptsG* | 1.94 | 0.62 | 0.32 | Glucose-specific enzyme IIABC component | |
Asterisks indicate genes identified as potential RNase MS substrates by the second method of ratio calculation (see the text); bold indicates genes identified as potential substrates by both methods.
WT/rnmV for decreased expression and rnmV/WT for increased expression.
FIG. 1.
Alteration of odhA, odhB, sucC, and sucD expression detected by gene array analysis. Labeled cDNAs were prepared with the set of labeling primers available from Sigma-Genosys. Membranes were hybridized according to the manufacturer's instructions, exposed to PhosphorImager (Molecular Dynamics) screens overnight, and quantified with ArrayVision (Molecular Dynamics) software. Spot intensities were normalized to the average spot intensity for each membrane. The experiment was repeated five times, and membranes were permuted with each experiment to avoid artifacts due to the membrane manufacturing process. Pairs of spots corresponding to these genes in wild-type (WT) and mutant (rnmV) RNA samples are marked with arrowheads.
Northern blots of candidate RNAs.
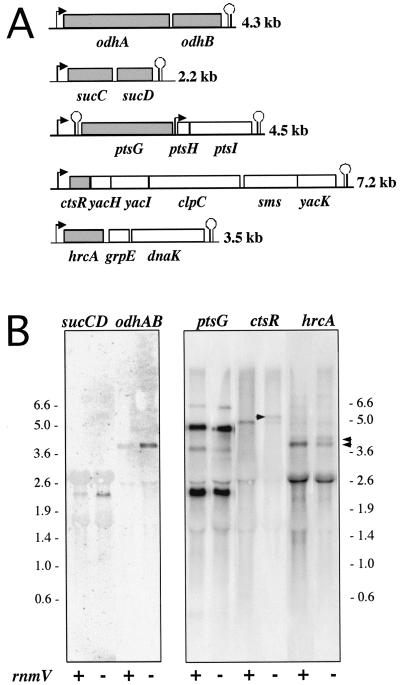
The handful of genes whose expression was altered in RNase M5-inactivated strains provided a starting point to look for other RNA substrates cleaved by this enzyme. We synthesized probes against five operons containing seven genes with altered expression by one method of ratio calculation or the other: the odhAB and sucCD operons, the dnaK operon (hrcA), the clpC operon (ctsR), and the ptsGHI operon (ptsG). The probes are depicted in Fig. 2A and were used to determine whether we could observe similar differences in gene expression by Northern blot analysis (Fig. 2B). RNA was isolated from late-log-phase (optical density at 600 nm = 1.0) B. subtilis cells growing in 2×YT-0.5% glucose medium by the glass-bead lysis method of Mayford and Weisblum (13). RNA (10 μg) was run on 1% agarose-formaldehyde gels and blotted overnight to Hybond N membranes (Amersham). A quantitative effect of the rnmV mutation on the expression of the odh and suc operons was confirmed by Northern blotting, with expression being reproducibly increased ninefold and fourfold, respectively, over wild-type levels. In contrast, while some minor alterations were observed in the intensity of individual bands detected by the other three probes, for example, two bands around 4 kb detected with the hrcA probe, these differences were generally difficult to reproduce.
FIG. 2.
Analysis of candidate RNase M5 substrates by Northern blotting. (A) PCR fragments used to synthesize probes. PCR-amplified DNA fragments (25 ng) were used to synthesized probes by the random-prime method of Feinberg and Vogelstein (5). Hybridization conditions were as described previously (21). The genes whose expression was deemed altered by gene array analysis are shaded in gray. (B) Northern blots. Probe names correspond to the gene designations for the shaded area of panel A. Bands mentioned in text are marked with arrowheads. rnmV + and −, wild-type and mutant RNA samples, respectively. Size standards are in kilobases.
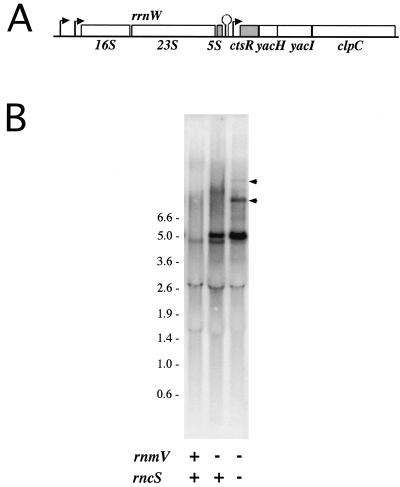
One qualitative difference was consistently observed with the ctsR probe, however: the appearance of a second band in the rnmV lane, about 0.3 kb larger than the band observed in the wild-type RNA sample, estimated at 4.7 kb. The ctsR gene lies just downstream of the rRNA operon rrnW at around 100 kb on the B. subtilis genome (Fig. 3A). B. subtilis has 10 such operons, each containing the 16S, 23S, and 5S rRNA genes transcribed in that order. A substrate for RNase M5 therefore lies immediately upstream of the ctsR gene. The 4.7-kb species we detected with the ctsR probe in the wild-type RNA sample was the size expected for a ctsR-yacH-yacI-clpC-containing fragment, although this species has apparently not been detected in other studies of this operon (9). The size of the second RNA species identified in the RNase M5 mutant was consistent with a fragment extending from the 3′ end of the 23S rRNA gene to the end of clpC, i.e., 5S-ctsR-yacH-yacI-clpC, suggesting that in the absence of RNase M5, rrnW and ctsR are cotranscribed, despite the presence of a transcription terminator between the two operons. To confirm this hypothesis, we compared Northern blots of total RNA isolated from wild-type, rnmV, and rnmV rncS strains hybridized with the ctsR probe. The rncS gene encodes RNase III, an enzyme which cleaves the full-length 30S rRNA transcripts on each side of two long double-stranded helical regions to liberate 16S and 23S precursor rRNAs. As in E. coli, this cleavage reaction can be bypassed by other enzymes in vivo, so that while some 30S precursor does accumulate, the majority of rRNA is still found as mature 16S and 23S species (8). We reasoned that if rrnW and ctsR were indeed cotranscribed, we should detect RNAs corresponding to 23S-5S-ctsR-yacH-yacI-clpC and 16S-23S-5S-ctsR-yacH-yacI-clpC in the rnmV rncS double mutant. Two new bands of the expected sizes, 8.0 and 10 kb, respectively, were detected with the ctsR probe in the Northern blot shown in Fig. 3B, confirming the cotranscription of rrnW and ctsR in these strains and suggesting that correct processing of 5S rRNA by RNase M5 is somehow important in preventing read-through of the transcription terminator at the end of the rrnW operon. In the absence of this cleavage, RNA polymerase appears to read through the transcription terminator into the downstream operon, albeit at a very low level relative to total rrn transcription (data not shown). The effect of RNase M5 on transcription termination seems to be specific to rrnW. No increase in band size was detected on Northern blots of total RNA isolated from the rnmV mutant compared to the wild type when probed for the genes downstream of rrnA (csfB) and rrnG (ybaR) (data not shown).
FIG. 3.
Evidence that rrnW and ctsR are cotranscribed in rRNA processing mutants. (A) Region of the B. subtilis chromosome around ctsR. (B) Northern blot of RNA samples isolated from wild-type, rnmV, and rncS rnmV strains, probed with the ctsR probe. The rncS rnmV mutant, BG337, was made by transformation of BG322, a spontaneous extragenic revertant of the ΔrncS strain BE600 (8), with SSB312 chromosomal DNA and selection for erythromycin resistance. + and −, wild-type and mutant RNA samples, respectively. Bands mentioned in the text are indicated by arrowheads. Size standards are in kilobases.
The effect of RNase M5 on odh and suc operon expression appears to be indirect.
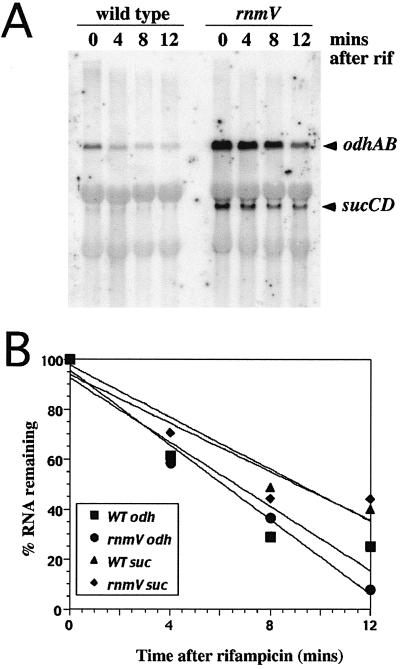
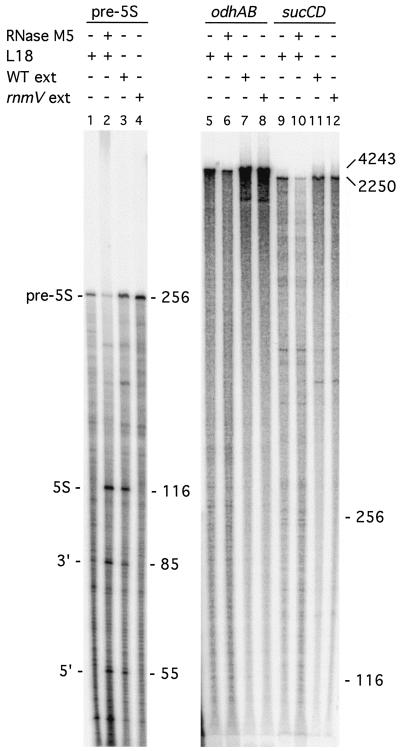
The premise for the use of gene array technology to screen for potential RNase M5 substrates was that cleavage of substrate mRNAs would have a measurable effect on their half-life and thus steady-state levels. We therefore wished to know whether the half-life of the odh and suc mRNAs was increased to account for the effect of RNase M5 on their expression. Total RNA was isolated from both the rnmV mutant and its wild-type parental strain at various times after rifampin addition and probed with the odhAB and sucCD probes on Northern blots (Fig. 4A). Remarkably, the half-life of neither the odhAB (6 min) nor sucCD transcripts (>9 min) was significantly altered in the rnmV mutant (Fig. 4B), suggesting that the effect of the RNase M5 mutation is indirect and occurs at the transcriptional rather than posttranscriptional level. This idea is supported by the fact that, in the Northern blot shown in Fig. 2B, probed for the odh and suc operons, no bands were seen that might correspond to maturation products of RNase M5. This was the case even when the experiments were repeated in a polynucleotide phosphorylase-negative background in an attempt to slow down general mRNA degradation (data not shown). We nonetheless decided to verify that RNase M5 could cleave neither of these transcripts directly in vitro. Radiolabeled odhAB and sucCD transcripts were synthesized by T7 RNA polymerase in vitro and incubated with either RNase M5 alone (data not shown) or RNase M5 and its cofactor in the 5S rRNA processing reaction, ribosomal protein L18. No cleavage of these RNAs was detected (Fig. 5, lanes 6 and 10) under conditions where 5S rRNA precursor is converted to mature 5S rRNA (lane 2). To eliminate the possibility that a different cofactor might be required for the cleavage of either of these RNAs, we compared the abilities of wild-type and rnmV mutant extracts to cleave the odh and suc transcripts in vitro. Wild-type extracts correctly cleave 5S rRNA precursor (Fig. 5, lane 3), whereas rnmV mutant extracts do not (lane 4). Under the same conditions, no difference between the band pattern generated by the two extracts was observed for the odh (Fig. 5, lanes 7 and 8) and suc mRNAs (lanes 11 and 12). The gel shown is a 3% polyacrylamide gel, which resolves fragments in the 200- to 1,000-base range; identical results were obtained when the samples were migrated on 1% agarose-formaldehyde gels or 5 or 20% polyacrylamide gels (data not shown), which covered the full range of possible fragment sizes. We take this as confirmation that the effect of RNase M5 on odhAB and sucCD expression is indirect. The odhAB operon has been previously shown to be repressed by both glucose and entry into stationary phase, mainly at the transcriptional level (18). It is possible that, somehow, RNase M5 indirectly affects this control pathway.
FIG. 4.
Comparison of odhAB and sucCD mRNA half-lives in wild-type and rnmV mutant cells. (A) RNA samples were isolated after addition of rifampin (rif) to cultures and probed with the odhAB and sucCD probes described for Fig. 2. (B) Graph of data from panel A, showing the percent RNA remaining after rifampin addition. Results are averages of two experiments.
FIG. 5.
RNase M5 does not cleave odhAB and sucCD mRNAs in vitro. (Right) 32P-labeled transcripts were synthesized by T7 RNA polymerase in vitro, using PCR-amplified templates with an integrated T7 promoter, and incubated with either 50 ng of purified RNase M5 (4) or 500 ng of whole-cell extracts (ext) from either wild-type (WT) or rnmV mutant cells. (Left) 5S rRNA precursor (pre-5S) is cleaved to yield mature 5S rRNA and a 5′ and 3′ fragment under the same conditions. The sizes of the different RNA species are given on the right of the autoradiogram. The autoradiogram on the left was produced from a 5% polyacrylamide gel to show the low-molecular-weight products of the 5S cleavage reaction. The autoradiogram on the right, with the higher-molecular-weight odh and suc transcripts, was produced from a 3% polyacrylamide gel. The experiment was done twice. Identical conclusions were reached when the same reactions were run on 1% agarose-formaldehyde gels and 5 or 20% polyacrylamide-urea gels.
Conclusions.
RNase E, RNase G, RNase III, and RNase P, the four main endoribonucleases involved in stable RNA processing, all have mRNA substrates in E. coli (1, 3, 10, 12, 14, 16, 17; for a review see reference 7). However, none of the few potential mRNA candidates for RNase M5 processing that we examined was cleaved directly by this endonuclease. Ribosomal protein L18, which binds directly to 5S rRNA, is an essential cofactor in the 5S rRNA maturation reaction. It is thought to act as an RNA chaperone, putting the 5S rRNA precursor in the correct conformation for cleavage. In this regard, it is perhaps not surprising that RNase M5 is apparently limited to this substrate. Nonetheless, we thought it possible that a different protein could play the role of L18 for another RNA, or that another RNA could adopt the correct conformation for cleavage by RNase M5 in the absence of an RNA chaperone. This was clearly not the case for the odhAB and sucCD mRNAs, since wild-type whole-cell extracts, which would presumably contain such “alternative” cofactors, cleave these RNAs with exactly the same specificity as rnmV mutant extracts. The dearth of other potential mRNA candidates for RNase M5 cleavage suggests that there is some feature of 5S rRNA bound to L18 that is unique in B. subtilis. It will be interesting to discover exactly what this feature is, by structural analysis of this complex.
Acknowledgments
We thank D. Bechhofer for providing us with the rnmV rncS double mutant BG327 and I. Guillouard and I. Martin-Verstraete, who helped with gene array analysis.
J.R. was supported by the ERASMUS program. This work was supported by funds from the CNRS (UPR 9073), MRE (contract 92C0315), Université Paris VII (contract DRED), and PRFMMIP from the Ministère de l'Education Nationale.
REFERENCES
- 1.Alifano, P., F. Rivellini, C. Piscitelli, C. M. Arraiano, C. B. Bruni, and M. S. Carlomagno. 1994. Ribonuclease E provides substrates for ribonuclease P-dependent processing of a polycistronic mRNA. Genes Dev. 8:3021-3031. [DOI] [PubMed] [Google Scholar]
- 2.Apirion, D., and A. B. Lassar. 1978. A conditional lethal mutant of Escherichia coli which affects processing of ribosomal RNA. J. Biol. Chem. 253:1738-1742. [PubMed] [Google Scholar]
- 3.Bardwell, J. C. A., P. Régnier, S.-M. Chen, Y. Nakamura, M. Grunberg-Manago, and D. Court. 1989. Autoregulation of RNAse III operon by mRNA processing. EMBO J. 8:3401-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Condon, C., D. Brechemier-Baey, B. Beltchev, M. Grunberg-Manago, and H. Putzer. 2001. Identification of the gene encoding the 5S ribosomal RNA maturase in Bacillus subtilis: mature 5S rRNA is dispensable for ribosome function. RNA 7:242-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feinberg, A. P., and B. Vogelstein. 1983. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal. Biochem. 132:6-13. [DOI] [PubMed] [Google Scholar]
- 6.Ghora, B. K., and D. Apirion. 1978. Structural analysis and in vitro processing to p5 rRNA of a 9S RNA molecule isolated from an rne mutant of E. coli. Cell 15:1055-1066. [DOI] [PubMed] [Google Scholar]
- 7.Grunberg-Manago, M. 1999. Messenger RNA stability and its role in control of gene expression in bacteria and phages. Annu. Rev. Genet. 33:193-227. [DOI] [PubMed] [Google Scholar]
- 8.Herskowitz, M. A., and D. H. Bechhofer. 2000. Endoribonuclease RNase III is essential in Bacillus subtilis. Mol. Microbiol. 38:1027-1033. [DOI] [PubMed] [Google Scholar]
- 9.Kruger, E., T. Msadek, and M. Hecker. 1996. Alternate promoters direct stress-induced transcription of the Bacillus subtilis clpC operon. Mol. Microbiol. 20:713-723. [DOI] [PubMed] [Google Scholar]
- 10.Kuwano, M., M. Ono, H. Endo, K. Hori, K. Nakamura, Y. Hirota, and Y. Ohnishi. 1977. Gene affecting longevity of messenger RNA: a mutant of Escherichia coli with altered mRNA stability. Mol. Gen. Genet. 154:279-285. [DOI] [PubMed] [Google Scholar]
- 11.Li, Z., and M. P. Deutscher. 2002. RNase E plays an essential role in the maturation of Escherichia coli tRNA precursors. RNA 8:97-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, Z., S. Pandit, and M. P. Deutscher. 1999. RNase G (CafA protein) and RNase E are both required for the 5′ maturation of 16S ribosomal RNA. EMBO J. 18:2878-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayford, M., and B. Weisblum. 1989. Conformational alterations in the ermC transcript in vivo during induction. EMBO J. 8:4307-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ono, M., and M. Kuwano. 1979. A conditional lethal mutation in an E. coli strain with a longer chemical lifetime of messenger RNA. J. Mol. Biol. 129:343-357. [DOI] [PubMed] [Google Scholar]
- 15.Pace, B., D. A. Stahl, and N. R. Pace. 1984. The catalytic element of a ribosomal RNA-processing complex. J. Biol. Chem. 259:11454-11458. [PubMed] [Google Scholar]
- 16.Portier, C., L. Dondon, M. Grunberg-Manago, and P. Regnier. 1987. The first step in the functional inactivation of the Escherichia coli polynucleotide phosphorylase messenger is a ribonuclease III processing at the 5′ end. EMBO J. 6:2165-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regnier, P., and M. Grunberg-Manago. 1989. Cleavage by RNase III in the transcripts of the metY-nusA-infB operon of Escherichia coli releases the tRNA and initiates the decay of the downstream mRNA. J. Mol. Biol. 210:293-302. [DOI] [PubMed] [Google Scholar]
- 18.Resnekov, O., L. Melin, P. Carlsson, M. Mannerlov, A. von Gabain, and L. Hederstedt. 1992. Organization and regulation of the Bacillus subtilis odhAB operon, which encodes two of the subenzymes of the 2-oxoglutarate dehydrogenase complex. Mol. Gen. Genet. 234:285-296. [DOI] [PubMed] [Google Scholar]
- 19.Sogin, M. L., and N. R. Pace. 1974. In vitro maturation of precursors of 5S ribosomal RNA from Bacillus subtilis. Nature 252:598-600. [DOI] [PubMed] [Google Scholar]
- 20.Stahl, D. A., B. Pace, T. Marsh, and N. R. Pace. 1984. The ribonucleoprotein substrate for a ribosomal RNA-processing nuclease. J. Biol. Chem. 259:11448-11453. [PubMed] [Google Scholar]
- 21.Varshney, U., C. P. Lee, and U. L. RajBhandary. 1991. Direct analysis of aminoacylation levels of tRNAs in vivo. Application to studying recognition of Escherichia coli initiator tRNA mutants by glutaminyl-tRNA synthetase. J. Biol. Chem. 266:24712-24718. [PubMed] [Google Scholar]