Abstract
1. In unanaesthetized monkeys acclimated to primate chairs, 101 isolated sites in the hypothalamus and mesencephalon were perfused at a rate of 30-50 μl./min by means of push—pull cannulae. The perfusate, which contained an anticholinesterase, was assayed for acetylcholine (ACh) activity on the guinea-pig ileum in the presence of neostigmine.
2. The body temperature of each animal was monitored continuously during an experiment by colonic and brain thermistors. To alter the ambient temperature by 15-20° C, either a stream of warm air was passed over the monkey's trunk or containers of ice were placed in its chair chamber to cool the same region.
3. Assays of the effluent revealed that the release of ACh varied according to the ambient temperature as follows: elevated only during cooling; elevated only during warming; elevated by both thermal stimuli; suppressed only by cooling; suppressed only by warming; suppressed by both thermal stimuli; elevated during cooling but suppressed by warming; and elevated by warming and suppressed by cooling.
4. A composite anatomical `mapping' of all perfusion sites revealed that in response to either peripheral cooling or warming, the output of ACh varied at only 36% of all sites anterior to the mid-hypothalamic plane, but at 65% of those loci caudal to this coronal plane.
5. In the anterior, preoptic area, cooling enhanced the output of ACh at 88% of the active releasing sites, whereas warming reduced the release of ACh at 80% of these perfusion loci. Posterior to this region, ACh release was elevated by cooling at about half of the active releasing sites, but lowered by warming at nearly every active perfusion locus. Within the mesencephalon, the ratio of the temperature-induced change in ACh release was similar but in an opposite direction, since the level of ACh in the effluent collected from two out of three sites was augmented by cooling, but diminished by warming.
6. These results provide additional evidence for the neurochemical model of Myers & Yaksh (1969), which suggests that a cholinergic pathway originating in the anterior, preoptic region transmits efferent signals for heat production. Further, within the posterior hypothalamic area as well as in the mesencephalon of the monkey, the characteristics of the ACh releasing sites reflect a function delegated primarily to heat gain, although evidence of a cholinergic pathway for the heat loss system is also presented.
Full text
PDF



















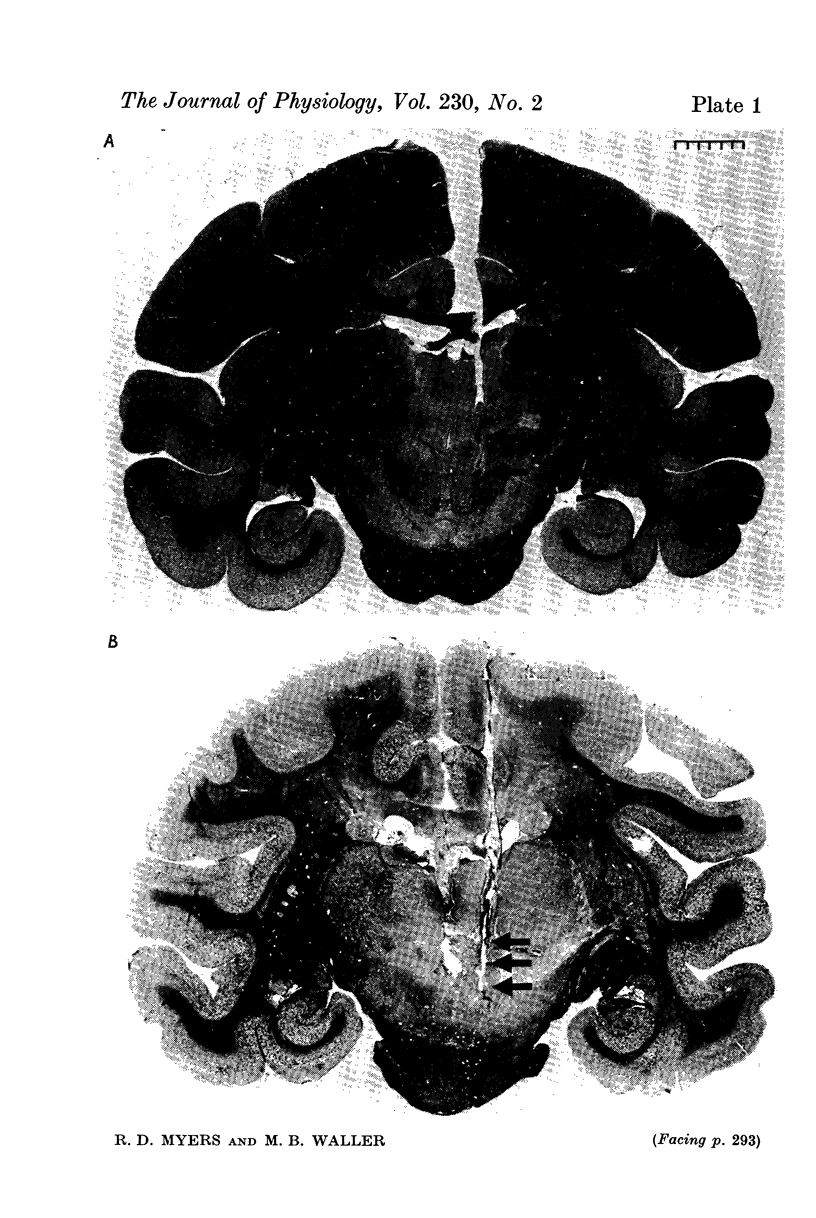

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avery D. D. Hyperthermia induced by direct injections of carbachol in the anterior hypothalamus. Neuropharmacology. 1970 Mar;9(2):175–178. doi: 10.1016/0028-3908(70)90061-4. [DOI] [PubMed] [Google Scholar]
- Avery D. D. Intrahypothalamic adrenergic and cholinergic injection effects on temperature and ingestive behavior in the rat. Neuropharmacology. 1971 Nov;10(6):753–763. doi: 10.1016/0028-3908(71)90090-6. [DOI] [PubMed] [Google Scholar]
- Avery D. D. Thermoregulatory effects of intrahypothalamic injections of adrenergic and cholinergic substances at different environmental temperatures. J Physiol. 1972 Jan;220(2):257–266. doi: 10.1113/jphysiol.1972.sp009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee U., Burks T. F., Feldberg W. Effect on temperature of 5-hydroxytryptamine injected into the cerebral ventricles of cats. J Physiol. 1968 Mar;195(1):245–251. doi: 10.1113/jphysiol.1968.sp008456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman A. L., Carlisle H. J. Effect of intrahypothalamic infusion of acetylcholine on behavioural and physiological thermoregulation in the rat. Nature. 1969 Feb 8;221(5180):561–562. doi: 10.1038/221561a0. [DOI] [PubMed] [Google Scholar]
- Beckman A. L., Eisenman J. S. Microelectrophoresis of biogenic amines on hypothalamic thermosensitive cells. Science. 1970 Oct 16;170(3955):334–336. doi: 10.1126/science.170.3955.334. [DOI] [PubMed] [Google Scholar]
- Beleslin D. B., Myers R. D. The release of acetylcholine and 5-hydroxytryptamine from the mesencephalon of the unanesthetized rhesus monkey. Brain Res. 1970 Oct 28;23(3):437–442. doi: 10.1016/0006-8993(70)90071-5. [DOI] [PubMed] [Google Scholar]
- Benzinger T. H. Heat regulation: homeostasis of central temperature in man. Physiol Rev. 1969 Oct;49(4):671–759. doi: 10.1152/physrev.1969.49.4.671. [DOI] [PubMed] [Google Scholar]
- Bligh J., Cottle W. H., Maskrey M. Influence of ambient temperature on the thermoregulatory responses to 5-hydroxytryptamine, noradrenaline and acetylcholine injected into the lateral cerebral ventricles of sheep, goats and rabbits. J Physiol. 1971 Jan;212(2):377–392. doi: 10.1113/jphysiol.1971.sp009330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh J., Maskrey M. A possible role of acetylcholine in the central control of body temperature in sheep. J Physiol. 1969 Jul;203(1):55P–57P. [PubMed] [Google Scholar]
- Bligh J. The thermosensitivity of the hypothalamus and thermoregulation in mammals. Biol Rev Camb Philos Soc. 1966 Aug;41(3):317–368. doi: 10.1111/j.1469-185x.1966.tb01496.x. [DOI] [PubMed] [Google Scholar]
- FELDBERG W., MYERS R. D. CHANGES IN TEMPERATURE PRODUCED BY MICRO-INJECTIONS OF AMINES INTO THE ANTERIOR HYPOTHALAMUS OF CATS. J Physiol. 1965 Mar;177:239–245. doi: 10.1113/jphysiol.1965.sp007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W. Synthesis of acetylcholine by tissue of the central nervous system. J Physiol. 1945 Mar 28;103(4):367–402. doi: 10.1113/jphysiol.1945.sp004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDY J. D., HELLON R. F., SUTHERLAND K. TEMPERATURE-SENSITIVE NEURONES IN THE DOG'S HYPOTHALAMUS. J Physiol. 1964 Dec;175:242–253. doi: 10.1113/jphysiol.1964.sp007515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDY J. D. Physiology of temperature regulation. Physiol Rev. 1961 Jul;41:521–606. doi: 10.1152/physrev.1961.41.3.521. [DOI] [PubMed] [Google Scholar]
- Hall G. H., Myers R. D. Hypothermia produced by nicotine perfused through the cerabral ventricles of the unanaesthetized monkey. Neuropharmacology. 1971 Jul;10(4):391–398. doi: 10.1016/0028-3908(71)90067-0. [DOI] [PubMed] [Google Scholar]
- Hall G. H., Myers R. D. Temperature changes produced by nicotine injected into the hypothalamus of the conscious monkey. Brain Res. 1972 Feb 25;37(2):241–251. doi: 10.1016/0006-8993(72)90669-5. [DOI] [PubMed] [Google Scholar]
- Hellon R. F. Thermal stimulation of hypothalamic neurones in unanaesthetized rabbits. J Physiol. 1967 Nov;193(2):381–395. doi: 10.1113/jphysiol.1967.sp008364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLUVER H., BARRERA E. A method for the combined staining of cells and fibers in the nervous system. J Neuropathol Exp Neurol. 1953 Oct;12(4):400–403. doi: 10.1097/00005072-195312040-00008. [DOI] [PubMed] [Google Scholar]
- Lomax P., Jenden D. J. Hypothermia following systematic and intracerebral injection of oxotremorine in the rat. Int J Neuropharmacol. 1966 Sep;5(5):353–359. doi: 10.1016/0028-3908(66)90013-x. [DOI] [PubMed] [Google Scholar]
- MCLENNAN H. THE RELEASE OF ACETYLCHOLINE AND OF 3-HYDROXYTYRAMINE FROM THE CAUDATE NUCLEUS. J Physiol. 1964 Oct;174:152–156. doi: 10.1113/jphysiol.1964.sp007478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. D. An improved push-pull cannula system for perfusing an isolated region of the brain. Physiol Behav. 1970 Feb;5(2):243–246. doi: 10.1016/0031-9384(70)90073-9. [DOI] [PubMed] [Google Scholar]
- Myers R. D., Beleslin D. B. Changes in serotonin release in hypothalamus during cooling or warming of the monkey. Am J Physiol. 1971 Jun;220(6):1746–1754. doi: 10.1152/ajplegacy.1971.220.6.1746. [DOI] [PubMed] [Google Scholar]
- Myers R. D., Beleslin D. B. The spontaneous release of 5-hydroxytryptamine and acetylcholine within the diencephalon of the unanaesthetized rhesus monkey. Exp Brain Res. 1970;11(5):539–552. doi: 10.1007/BF00233974. [DOI] [PubMed] [Google Scholar]
- Myers R. D. Chemical mechanisms in the hypothalamus mediating eating and drinking in the monkey. Ann N Y Acad Sci. 1969 May 15;157(2):918–933. doi: 10.1111/j.1749-6632.1969.tb12928.x. [DOI] [PubMed] [Google Scholar]
- Myers R. D., Chinn C. Evoked release of hypothalamic norepinephrine during thermoregulation in the cat. Am J Physiol. 1973 Feb;224(2):230–236. doi: 10.1152/ajplegacy.1973.224.2.230. [DOI] [PubMed] [Google Scholar]
- Myers R. D. Discussion of serotonin, norepinephrine, and fever. Adv Pharmacol. 1968;6(Pt A):318–321. doi: 10.1016/s1054-3589(08)61187-4. [DOI] [PubMed] [Google Scholar]
- Myers R. D. Hypothalamic mechanism of pyrogen action in the cat and monkey in: pyrogens and fever. Ciba Found Symp. 1971:131–153. [PubMed] [Google Scholar]
- Myers R. D., Sharpe L. G. Temperature in the monkey: transmitter factors released from the brain during thermoregulation. Science. 1968 Aug 9;161(3841):572–573. doi: 10.1126/science.161.3841.572. [DOI] [PubMed] [Google Scholar]
- Myers R. D., Veale W. L. Body temperature: possible ionic mechanism in the hypothalamus controlling the set point. Science. 1970 Oct 2;170(3953):95–97. doi: 10.1126/science.170.3953.95. [DOI] [PubMed] [Google Scholar]
- Myers R. D., Yaksh T. L. Control of body temperature in the unanaesthetized monkey by cholinergic and aminergic systems in the hypothalamus. J Physiol. 1969 Jun;202(2):483–500. doi: 10.1113/jphysiol.1969.sp008822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. D., Yaksh T. L. Thermoregulation around a new set-point' established in the monkey by altering the ratio of sodium to calcium ions within the hypothalamus. J Physiol. 1971 Nov;218(3):609–633. doi: 10.1113/jphysiol.1971.sp009636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutik S. L. Effect of temperature change of the preoptic region and skin on posterior hypothalamic neurons. J Physiol (Paris) 1971 May;63(3):368–370. [PubMed] [Google Scholar]
- PATON W. D. The action of morphine and related substances on contraction and on acetylcholine output of coaxially stimulated guinea-pig ileum. Br J Pharmacol Chemother. 1957 Mar;12(1):119–127. doi: 10.1111/j.1476-5381.1957.tb01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W., Tebecis A. K., York D. H. Acetylcholine release from the feline thalamus. J Pharm Pharmacol. 1968 Jun;20(6):476–478. doi: 10.1111/j.2042-7158.1968.tb09786.x. [DOI] [PubMed] [Google Scholar]
- Radouco-Thomas S. Interactions between calcium and membrane macromolecules. Int Z Klin Pharmakol Ther Toxikol. 1971 Dec;5(3):271–278. [PubMed] [Google Scholar]
- SATINOFF E. BEHAVIORAL THERMOREGULATION IN RESPONSE TO LOCAL COOLING OF THE RAT BRAIN. Am J Physiol. 1964 Jun;206:1389–1394. doi: 10.1152/ajplegacy.1964.206.6.1389. [DOI] [PubMed] [Google Scholar]
- Wit A., Wang S. C. Temperature-sensitive neurons in preoptic-anterior hypothalamic region: effects of increasing ambient temperature. Am J Physiol. 1968 Nov;215(5):1151–1159. doi: 10.1152/ajplegacy.1968.215.5.1151. [DOI] [PubMed] [Google Scholar]



