Abstract
Hybridization to a PCR product derived from conserved betaine choline carnitine transporter (BCCT) sequences led to the identification of a 3.4-kb Sinorhizobium meliloti DNA segment encoding a protein (BetS) that displays significant sequence identities to the choline transporter BetT of Escherichia coli (34%) and to the glycine betaine transporter OpuD of Bacillus subtilis (30%). Although the BetS protein shows a common structure with BCCT systems, it possesses an unusually long hydrophilic C-terminal extension (169 amino acids). After heterologous expression of betS in E. coli mutant strain MKH13, which lacks choline, glycine betaine, and proline transport systems, both glycine betaine and proline betaine uptake were restored, but only in cells grown at high osmolarity or subjected to a sudden osmotic upshock. Competition experiments demonstrated that choline, ectoine, carnitine, and proline were not effective competitors for BetS-mediated betaine transport. Kinetic analysis revealed that BetS has a high affinity for betaines, with Kms of 16 ± 2 μM and 56 ± 6 μM for glycine betaine and proline betaine, respectively, in cells grown in minimal medium with 0.3 M NaCl. BetS activity appears to be Na+ driven. In an S. meliloti betS mutant, glycine betaine and proline betaine uptake was reduced by about 60%, suggesting that BetS represents a major component of the overall betaine uptake activities in response to salt stress. β-Galactosidase activities of a betS-lacZ strain grown in various conditions showed that betS is constitutively expressed. Osmotic upshock experiments performed with wild-type and betS mutant cells, treated or not with chloramphenicol, indicated that BetS-mediated betaine uptake is the consequence of immediate activation of existing proteins by high osmolarity, most likely through posttranslational activation. Growth experiments underscored the crucial role of BetS as an emerging system involved in the rapid acquisition of betaines by S. meliloti subjected to osmotic upshock.
Osmoregulation is a fundamental phenomenon developed by bacteria, fungi, plants, and animals to overcome osmotic stress. The most widely distributed strategy of response to hyperosmotic stress is the accumulation of compatible solutes, which protects the cells and allows growth. One of the most effective compatible solutes widely used by bacteria is glycine betaine, the N-trimethyl derivative of glycine (8, 27), which can be accumulated intracellularly at high concentration through either synthesis, uptake, or both.
Transporters for glycine betaine have been extensively investigated at the molecular level in the gram-negative enteric bacteria Escherichia coli and Salmonella enterica serovar Typhimurium and in the gram-positive soil bacteria Bacillus subtilis and Corynebacterium glutamicum. In E. coli and serovar Typhimurium, two transport systems, ProP and ProU, are primarily responsible for the uptake of glycine betaine. ProP, a secondary transporter, functions as an H+ symporter and is regulated mainly at the activity level (5, 31). ProU is a binding protein-dependent transporter, a member of the ABC superfamily, and is regulated at the level of both transcription and activity (6, 28).
In B. subtilis, three high-affinity effective glycine betaine transporters have been characterized so far. Two systems, OpuA and OpuC, are members of the ABC superfamily of transporters (19, 21, 22), and one, OpuD, is a secondary transporter (20). OpuA and OpuC present identity to the periplasmic binding protein ProU transporter from E. coli, but as a gram-positive bacterium, B. subtilis lacks the periplasm, and the binding proteins are anchored in the cytoplasm membrane to prevent their loss in the surrounding medium (22). Whereas OpuC can transport a wide variety of compatible solutes such as glycine betaine, choline, ectoine, and carnitine, OpuA and OpuD exhibit a restricted substrate specificity for glycine betaine.
With respect to osmotic adaptation, C. glutamicum is another well-studied gram-positive soil bacterium. In this organism, two secondary carriers for the uptake of glycine betaine have been characterized: the high-affinity, Na+-coupled glycine betaine uptake system BetP, and EctP, which prefers ectoine to glycine betaine (10, 36, 38). Both systems are regulated by the external osmolarity on the level of activity. BetP and EctP are closely related to each other and to other prokaryotic carriers for compatible solutes, such as the glycine betaine transporter OpuD from B. subtilis, the choline transporter BetT and the carnitine transporter CaiT from E. coli, the glycine betaine transporter BetL from Listeria monocytogenes (49), and the putative BetP proteins from Mycobacterium tuberculosis (39) and from Haemophilus influenzae (14). These proteins constitute a small family of structurally and functionally related systems, all belonging to the betaine choline carnitine transporters (BCCT) or trimethylammonium transporters (20).
Previous works have demonstrated the crucial role of glycine betaine for osmotic stress resistance in the gram-negative soil bacterium Sinorhizobium meliloti, the alfalfa (Medicago sativa L.)-symbiotic species, and have led to investigations of glycine betaine synthesis and transport (25, 50). The biosynthetic pathway, the enzymatic oxidation of choline or choline-O-sulfate to glycine betaine, has been well characterized at the molecular level (34, 43) and involves four genes, betICBA, organized in one operon. In addition to the genes encoding a presumed regulatory protein (betI), the betaine aldehyde dehydrogenase (betB), and the choline dehydrogenase (betA), enzymes also found in E. coli (24), S. meliloti, unlike other bacteria, possesses an additional gene (betC) which encodes a choline sulfatase catalyzing the conversion of choline-O-sulfate and, to a lesser extent, phosphorylcholine into choline (34).
The genes encoding transport systems for glycine betaine in S. meliloti are largely unexplored, although it has been demonstrated previously that glycine betaine transport activity is strongly stimulated in cells grown at high osmolarity (2) and that a glycine betaine-binding protein exists in the periplasm of such cells (26). In addition to glycine betaine, S. meliloti uses proline betaine, also known as stachydrine or dimethylproline, as an osmoprotectant (17). This quaternary ammonium derivative of proline occurs widely in Medicago species (42) and has been identified as an inducer of S. meliloti nodulation genes (40), although the proline betaine transport system has not been characterized. Nevertheless, recently we have described an ATP-binding cassette histidine transporter (Hut), also involved in high-affinity proline and proline betaine uptake and low-affinity glycine betaine transport (3). Expression analysis of the hut operon revealed induction by histidine but not by osmotic stress, suggesting that this uptake system has a catabolic role rather than being involved in osmoprotection.
Here, we report the molecular characterization and disruption of betS, a gene which plays an important role in high-affinity Na+-coupled glycine betaine and proline betaine transport in S. meliloti. Furthermore, we show that betS is constitutively expressed, whereas BetS activity depends on posttranslational activation by high osmolarity and is most likely the emergency system transporting betaines for immediate osmotic protection.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and β-galactosidase assays.
The bacterial strains and plasmids used in this study are listed in Table 1. E. coli strains were routinely grown in Luria-Bertani (LB) medium (47) at 37°C, and S. meliloti strains were routinely grown in LB supplemented with 2.5 mM MgSO4 and 2.5 mM CaCl2 (LBmc) at 30°C. When required, antibiotics were added at the final concentrations described previously (11, 43). For transport assays, E. coli cells were grown in M63 minimal medium supplemented with 0.2% glucose as the carbon source (7) or in M63 modified medium containing Na2HPO4 (40.8 mM) instead of K2HPO4. S. meliloti strains were grown in MCAA medium (50) for transport assays or in LAS minimum medium containing 10 mM sodium lactate and 10 mM sodium aspartate (2) for β-galactosidase assays. The osmotic strength of the media was increased by the addition of 0.3 or 0.5 M NaCl, 0.3 M KCl, or 0.48 M mannitol. β-Galactosidase activity was determined by the method of Miller (30) using overnight induced cultures at an optical density at 600 nm (OD600) of 0.4.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| E. coli | ||
| DH5α | F−supE44 ΔlacU169 (φ80lacZΔM15) hsdR17(rK− mK+) recA1 | 47 |
| MT616 | pro-82 thi-1 hsdR17 supE44 recA56 | 12 |
| MC4100 | F−araD139 Δ(argF-lac)U169 rpsL150 relA1 flb-5301 deoC1 ptsF25 rbsR Δbet Smr | 18 |
| MKH13 | MC4100 Δ(putPA)101 Δ(proP)2 Δ(proU)608 Smr | 18 |
| S. meliloti | ||
| 102F34 | Wild-type derivative, Rifr | 43 |
| GMI211 | Derivative of Rm2011, Smr Lac− | 33 |
| UNA315 | GMI211 betS::lacZ betS+ Kmr | This work |
| UNA316 | GMI211 betS::lacZ Kmr | This work |
| Plasmids | ||
| pRK600 | ColE1 replicon with RK2 transfer region, Cmr | 12 |
| pKOK5 | pSUP202 derivative; source of lacZ-Kmr cartridge, Apr Kmr | 23 |
| pUC119 | Sequencing vector, ColE1 oriV, Apr | 53 |
| pSUP202 | Replicon ColE1, Mob+ Tcr Apr Cmr | 48 |
| pLAFR3 | Cosmid cloning vector, Tcr | 15 |
| pBT47 | pLAFR3, S. meliloti 20-kb cosmid clone with betS gene, Tcr | This work |
| pBT58 | 3.4-kb SalI fragment from pBT47 with betS into pUC119 vector, Apr | This work |
| pSBI39 | 3.4-kb SalI fragment from pBT47 with betS into pSUP202 vector, Apr Cmr | This work |
| pSBA15 | pSBI39, betS::lacZ Kmr (insertion into BamHI) | This work |
| pSBA16 | pSBI39, betS::lacZ Kmr (insertion into BamHI), insertion in reverse orientation | This work |
Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Kmr, kanamycin resistance; Rifr, rifampin resistance; Smr, streptomycin resistance; Tcr, tetracycline resistance.
PCR amplification of the S. meliloti betS gene.
PCR mixtures contained 50 pmol of each degenerate primer; 200 ng of S. meliloti strain 102F34 genomic DNA; a 200 μM concentration each of dATP, dTTP, dGTP, and dCTP; 1× Taq polymerase buffer (Appligene, Illkirch, France); and 1 U of Taq DNA polymerase (Appligene) in a final volume of 50 μl. Reaction mixtures were cycled automatically using a Biometra thermocycler (T gradient model; Biometra GmbH, Göttingen, Germany) through temperature and time cycles as follows: denaturation, 95°C for 1 min; annealing, variable from 46 to 56°C for 1 min; and extension, 72°C for 1 min. The denaturation time of the first cycle was prolonged to 5 min to ensure a single-stranded template for the PCR, and the final extension time was increased to 10 min to ensure completion of strand synthesis. Twenty microliters of reaction mixture was analyzed by electrophoresis on 1.5% agarose gels. The sequences of the two degenerate primers used were 5′-TTY GCN GGN ATN GG-3′ (BT1) and 5′-CCA CCA NGC CCA RWA NA-3′ (BT3R).
DNA manipulations, sequencing, and plasmid and mutant constructions.
Restriction analysis, ligation, transformation, plasmid DNA extraction, and Southern hybridization were performed by standard methods (47). PCR-purified fragments were subcloned in the pGEM-T cloning vector (Promega, Charbonnières, France). DNA probes were labeled using the Prime-a-Gene random priming system (Promega) and [α-32P]dCTP (purchased from Amersham Corp., Little Chalfont, United Kingdom). Total DNA from S. meliloti was isolated as described previously (29). The genomic library of S. meliloti 2011, made up of an Sau3A partial DNA digest cloned into pLAFR3, was kindly provided by D. Kahn (Laboratoire CNRS-INRA, Castanet-Tolosan, France). Screening of the genomic library was performed by colony hybridization using the 700-bp PCR product (Fig. 1) as the probe, according to standard procedures (47).
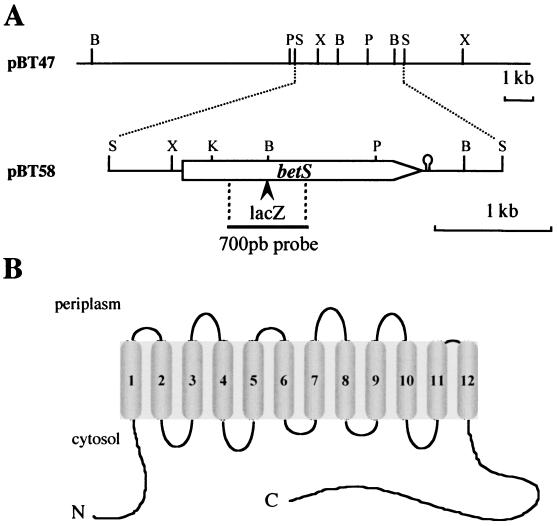
FIG. 1.
(A) Physical and genetic maps of the S. meliloti betS region. The betS gene is represented by an open arrow. The positions of both the lacZ fusion and the 700-bp probe are indicated below. The hairpin indicates the position of a stem-loop element able to form a secondary structure. Restriction sites: B, BamHI; P, PstI; S, SalI; K, KpnI; and X, XhoI. (B) Putative secondary structure of BetS.
The 3.4-kb SalI fragment from the identified cosmid (pBT47) which contained the betS gene was transferred into the sequencing vector pUC119 and suicide vector pSUP202 to yield pBT58 and pSBI39, respectively. Derivatives of pSBI39 were obtained after partial digestion with BamHI and ligation with a 4.7-kb BamHI fragment carrying a lacZ-Kmr cartridge from pKOK5 (23). The location and orientation of the cartridge at the correct BamHI site in betS were verified by restriction analysis. pSBA15 contains a transcriptional lacZ fusion in betS, and pSBA16 carries the lacZ coding sequence in the opposite orientation of betS.
In order to construct recombinant betS-lacZ fusion and mutant strains, these plasmids were transferred by triparental mating, using E. coli strain MT616(pRK600) as the helper, into S. meliloti GMI211 (12). A single recombinant clone, selected as Smr, Nmr, and Cmr and containing pSBA15, was named UNA315, while a double recombinant clone, selected as Smr, Nmr, and Cms and containing pSBA16, was designated UNA316. The location of the cartridge at the correct BamHI site in betS was verified by Southern hybridization. The nucleotide sequence of both strands of pBT58 SalI fragment was established using the fluorescent ABI dye-labeled deoxy terminator method of MWG Biotech (Edersberg, Germany). The DNA and derived protein sequences were analyzed by using ClustalW (51) and Blast (1) software programs.
Transport assays.
Radioactive [methyl-14C]glycine betaine was prepared from [methyl-14C]choline (2.04 GBq/mmol; Amersham Corp.) as previously described (35). [U-14C]proline betaine (4.6 GBq/mmol) was obtained from the Commissariat à l'Energie Atomique (Gif-sur-Yvette, France). E. coli cells were harvested at an OD420 of 0.8 to 1.0 and washed twice in the medium used for the culture. All assays were carried out at 37°C with 1 ml of the cell suspension and radioactive substrates (100,000 dpm). Uptake was determined by rapid filtration through GF/F glass microfiber filters (Whatman) rinsed with 3 ml of the corresponding medium. The radioactivity remaining on the filters was determined with a liquid scintillation spectrometer (model LS6000SC; Beckman Instruments, Villepinte, France). For kinetics studies with E. coli cells, the radioactive substrates were used at final concentrations of 1 to 100 μM. For competition experiments, unlabeled choline, carnitine, ectoine, histidine, proline, and proline betaine were added at a final concentration of 100 μM or 1 mM to a 10 μM [methyl-14C]glycine betaine solution. Competition uptake assays were run on a 5-min incubation period before filtration.
Transport assays with S. meliloti cells were realized in the same conditions as described for E. coli except that [methyl-14C]glycine betaine and [U-14C]proline betaine were used at 40 μM. Upshock effects on betaine transport activities in S. meliloti were measured from cells grown in low-osmolarity MCAA medium to the late exponential phase (OD420 of 0.8). The culture was then divided into two parts, and one was treated with 100 μg of chloramphenicol per ml. After 30 min, the chloramphenicol-treated culture and the untreated culture were subjected to a sudden osmotic upshock by adding NaCl to the growth medium at a final concentration of 0.3 M. Measurements of [methyl-14C]glycine betaine uptake were done at various times before and after the upshock.
Nucleotide sequence accession number.
The nucleotide sequence of the betS gene has been deposited in the GenBank database under accession number AF323271.
RESULTS
Identification and sequence analysis of the betS gene of S. meliloti.
A PCR strategy was used to isolate a trimethylammonium transporter in S. meliloti. Alignment of various amino acid sequences of BCCT transporters, such as the glycine betaine uptake systems OpuD of B. subtilis (accession number GI 1524397) (20), BetP of C. glutamicum (accession number GI 1325948) (36), and BetL of L. monocytogenes (accession number GI 11279740) (49), the choline transporter BetT of E. coli (accession number GI 1786506) (24), and the BetT-like protein of H. influenzae (accession number GI 6626252) (14), revealed blocks of well-conserved amino acid residues. Strongly conserved regions are found in the putative transmembrane α-helix 8 and the small adjacent cytoplasmic connecting loop.
Two stretches of amino acids, FAAGM/IG and F/YWAWW, were defined to synthesize degenerate primers called BT1 and BT3R (see Materials and Methods). PCR amplification using total genomic DNA of S. meliloti 102F34 as the template with these primers resulted in a 700-bp amplified fragment of the expected size. This fragment was subcloned in pGEM-T, amplified, and used as a probe to screen a genomic DNA library of S. meliloti 2011. Ten positive clones were detected, and one was analyzed further. It contained a recombinant cosmid, pBT47, carrying a 20-kb Sau3A insert. By restriction analysis and Southern hybridization, the region homologous to the PCR-amplified fragment was restricted to a 3.4-kb SalI fragment and subcloned in pBT58 (Fig. 1A). The nucleotide sequence of the 3,375-bp DNA fragment carried by pBT58 led to the identification of one major open reading frame (ORF) of 2,121 nucleotides which encoded a polypeptide of 706 residues with a calculated molecular mass of 77.6 kDa. This ORF, hereafter referred to as the betS gene, is preceded by a potential ribosome-binding site (GAGGA) adjacent to the putative ATG start codon. An inverted repeat sequence is present downstream of the stop codon and might serve as a transcription terminator. Analysis of the annotated genomic sequence of the S. meliloti genome (http://sequence.toulouse.inra.fr/meliloti.html) has shown that this nucleotide sequence is localized on the pSymb megaplasmid (smbY20333).
A search for related proteins revealed significant sequence identities with all BCCT transport systems involved in the uptake of trimethylammonium compounds. When considering BetS in its entire length, the highest identity scores were obtained with the BetT -like protein of H. influenzae (37%), BetT of E. coli (34%), OpuD of B. subtilis (30%), BetL of L. monocytogenes (28%), and BetP of C. glutamicum (25%). The hydrophobicity profile (DAS program; Stockholm University, Stockholm, Sweden) of BetS predicts 12 membrane-spanning segments (Fig. 1B), each with a length of approximately 20 amino acid residues and with both the N- and C-terminal extensions located in the cytoplasm. Considering the predicted structure and the identity scores mentioned previously, it is obvious that BetS of S. meliloti belongs to the BCCT transporter family.
It should be noticed that the smbY20333 sequence from the annotated genome of S. meliloti refers to a gene encoding a polypeptide of 621 amino acids, i.e., 85 residues shorter than BetS. Indeed, the ATG used to describe BetS is located 255 bp upstream from the ATG codon used for smbY20333. The putative protein encoded by smbY20333 lacks the first transmembrane helix and thus presents an N-terminal extension located in the periplasmic space, which is not a structure characteristic of BCCT transporters.
Interestingly, the BetS protein possesses an important cytoplasmic C-terminal end (169 amino acid residues). Among members of the BCCT family, a similar C-terminal extension is present only in the BetT proteins of E. coli and H. influenzae. This C-terminal end is much shorter (about 50 amino acids) in the EctP (38) and BetP (36) transporters of C. glutamicum. S. meliloti BetS shows a cytoplasmic N-terminal domain of 52 amino acids, and such a long extension is found only in the BetP protein of C. glutamicum.
Transport activities and specificity of BetS expressed in E. coli MKH13.
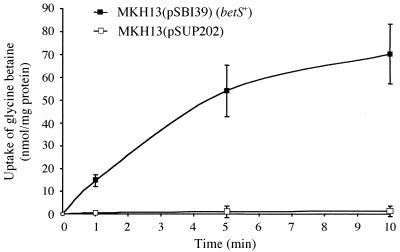
To investigate the function of BetS, the betS gene of S. meliloti was expressed in the MKH13 mutant strain of E. coli. In contrast to the E. coli parental strain, MC4100, the mutant MKH13, which cannot synthesize glycine betaine from its precursor, choline, and lacks the PutP, ProP, and ProU transport systems, is unable to grow on minimal medium of high osmolarity containing choline or glycine betaine as an osmoprotectant (18). Plasmid pSBI39, which contains the betS gene cloned in pSUP202, was transferred into MKH13. The presence of pSBI39, but not of pSUP202, restored the growth of MKH13 on high-osmolarity (0.5 M NaCl) minimal medium plates supplemented with 1 mM glycine betaine. As in MKH13, [14C]glycine betaine transport at high affinity (10 μM) could not be detected in strains MKH13(pSUP202) and MKH13(pSBI39) when grown in low-osmolarity minimal medium containing 1 mM glycine betaine or not (data not shown). However, in contrast to MKH13 and MKH13(pSUP202), [14C]glycine betaine was taken up by MKH13(pSBI39) when the cells were grown in high-osmolarity medium (0.3 M NaCl), supplemented or not with glycine betaine (Fig. 2). Therefore, the functional complementation observed in MKH13(pSBI39) demonstrates that betS encodes a glycine betaine transporter.
FIG. 2.
BetS-mediated glycine betaine uptake in E. coli MKH13. Cells were grown in M63 medium containing 0.3 M NaCl to mid-log phase and assayed for the uptake of [14C]glycine betaine at a final substrate concentration of 10 μM. Values are means from three independent experiments with standard errors as indicated.
Since the maximum uptake rate was observed when the bacteria were grown in the presence of 0.3 M NaCl without glycine betaine (data not shown), this condition was used to determine the Michaelis-Menten parameters of BetS in the heterologous background strain MKH13(pSBI39). The apparent Km value of glycine betaine uptake was found to be 16 ± 2 μM, with a Vmax of 41 ± 5 nmol/min/mg of protein, indicating a high-affinity transport system. A similar Km value, 10 μM, was previously reported for glycine betaine uptake in wild-type S. meliloti cells grown in high-osmolarity medium (2).
Since BCCT transporters are involved in the uptake of choline, ectoine, and carnitine in addition to glycine betaine, the substrate specificity of BetS was analyzed by competition assays using strain MKH13(pSBI39) grown in the presence of 0.3 M NaCl. The uptake of 10 μM [14C]glycine betaine (Table 2) was greatly inhibited by the addition of unlabeled proline betaine, with 50 and 90% inhibition, with a 10- and 100-fold excess, respectively, of competitor. Measurements of [14C]proline betaine uptake in strain MKH13(pSBI39) grown in the presence of 0.3 M NaCl showed a maximum uptake rate of 75 ± 18 nmol/min/mg of protein and a Km of 56 ± 6 μM, whereas strain MKH13(pSUP202) did not show any transport. The addition of choline, ectoine, and carnitine had no effect on glycine betaine uptake activity despite a 100-fold excess of competitor. Proline and histidine, substrates of the Hut transporter which is also involved in glycine betaine uptake in S. meliloti (3), were not competitors. These results demonstrated that BetS is a high-affinity uptake system with a narrow specificity for glycine betaine and proline betaine.
TABLE 2.
Effects of various compounds on glycine betaine uptake in E. coli MKH13(pSBI39)a
| Competitor | % Inhibition of uptake with unlabeled competitor at:
|
|
|---|---|---|
| 100 μM | 1 mM | |
| Choline | −5 | 4 |
| Carnitine | 4 | 13 |
| Ectoine | −2 | 3 |
| Proline | 1 | 2 |
| Proline betaine | 47 | 89 |
| Histidine | −1 | 1 |
Cells were grown in M63 medium containing 0.3 M NaCl. The results are expressed as percent inhibition of glycine betaine uptake and are means of three independent experiments with variations of less than 5%. Uptake was realized with 10 μM [14C]glycine betaine. The control value without competitor was 6.4 nmol of glycine betaine transported per min per mg of protein.
Regulation of BetS activity in E. coli MKH13.
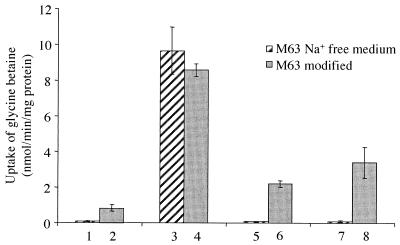
Since the transport activities of glycine betaine and proline betaine mediated by BetS were detectable in the E. coli MKH13 mutant complemented by pSBI39 only when NaCl was added to the medium, we wanted to determine whether BetS activity is coupled to the transport of sodium and subject to immediate activation by the osmolarity of the medium. Thus, E. coli MKH13(pSBI39) cells grown in low-osmolarity medium were subjected to sudden osmotic upshift by the addition of NaCl, KCl, or mannitol (Fig. 3). When the cells were cultivated at low osmolarity in M63 Na+-free medium, glycine betaine transport was barely detectable (less than 0.1 nmol/min/mg of protein), whereas addition of NaCl (0.3 M final concentration) strongly increased the uptake within 2 min following upshock (9.5 nmol/min/mg of protein). When the uptake was performed by the addition of KCl or mannitol used at concentrations osmotically equivalent to 0.3 M NaCl, no activation of uptake was observed (Fig. 3).
FIG. 3.
Influence of medium composition on BetS activity in E. coli MKH13(pSBI39). [14C]glycine betaine uptake was measured in cells grown in M63 medium with or without Na+, at low osmolarity (1 and 2), or subjected to osmotic upshock with 0.3 M NaCl (3 and 4), 0.3 M KCl (5 and 6), or 0.48 M mannitol (7 and 8). Measurements were done in the presence of 10 μM substrate 10 min after solute addition. Values are means from duplicates of three independent cultures with standard errors as indicated.
For further elucidation, the cells were grown at low osmolarity in M63 modified medium containing Na2HPO4 instead of K2HPO4 (40.8 mM Na+). In this medium, glycine betaine uptake in unstressed cells was clearly increased but still remained quite low. As observed previously, BetS was again strongly activated after upshock performed with NaCl. Interestingly, in contrast to what was found for cells grown in Na+-free medium, addition of KCl or mannitol also led to a strong enhancement in transport activity even though the stimulation was 4- and 2.6-fold less, respectively, than the increase observed in the presence of NaCl (Fig. 3). The difference may result from a specific effect of Na+ at high concentration, but can also be due to a weaker stress because of significant uptake of K+ or mannitol. Taken together, uptake measurements in cells grown in the presence or the absence of NaCl and upshock experiments carried out with NaCl, KCl, or mannitol indicate that Na+ is most likely the coupling ion for BetS activity and suggest that BetS is subjected to posttranslational activation by high osmolarity, since such rapid regulation cannot be the consequence of activated transcription.
Glycine betaine and proline betaine transport activities in S. meliloti.
To characterize the contribution of BetS to glycine betaine and proline betaine uptake in S. meliloti, a double recombinant GMI211 strain carrying a lacZ-Kmr cassette in the betS gene inserted in the opposite orientation (UNA316) was constructed as described in Materials and Methods and shown in Fig. 1A. Uptake of glycine betaine and proline betaine, used at a final concentration of 40 μM, was measured in both strains, wild type and mutant UNA316, grown overnight in MCAA medium in either the presence or the absence of 0.3 M NaCl. While the mutant strain was completely unaffected in glycine betaine uptake compared with the wild-type strain when the cells were grown at low osmolarity, at high osmolarity it showed a 60% decrease in uptake rate (Table 3). When glycine betaine was added to the growth medium, again a strong reduction of uptake was observed only in the mutant salt-stressed cells. In addition, the presence of the substrate stimulated the uptake in cells grown at low osmolarity by two- to threefold, suggesting the presence of a glycine betaine-induced transporter(s) in both strains. Such stimulation was not observed in cells grown at high osmolarity.
TABLE 3.
Glycine betaine and proline betaine uptake activities in S. meliloti wild-type (GMI211) and betS mutant (UNA316) strainsa
| Growth conditions | Uptake activity (nmol/min/mg of protein)
|
|||
|---|---|---|---|---|
| Glycine betaine
|
Proline betaine
|
|||
| GMI211 | UNA316 | GMI211 | UNA316 | |
| Control | 3.9 ± 0.1 | 3.6 ± 0.6 | 1.1 ± 0.2 | 1.0 ± 0.1 |
| + Glycine betaine (1 mM) | 10.5 ± 0.9 | 10.6 ± 0.8 | 2.0 ± 0.3 | 1.9 ± 0.2 |
| + NaCl (0.3 M) | 18.4 ± 1.4 | 7.0 ± 1.0 | 7.2 ± 0.4 | 3.0 ± 0.5 |
| + NaCl (0.3 M) + glycine betaine (1 mM) | 20.0 ± 4.0 | 7.7 ± 0.6 | 7.8 ± 0.9 | 3.6 ± 0.5 |
Cells were grown for 16 h in low-osmolarity MCAA medium (control) or in MCAA medium supplemented as indicated. Uptake measurements were done at a final substrate concentration of 40 μM. Values are means from duplicates of three independent cultures, and standard deviations are also shown.
When uptake of proline betaine (40 μM) was measured with cells grown as previously, the results obtained with the wild-type and the mutant strains were in good agreement with the corresponding results obtained with glycine betaine (Table 3). If proline betaine uptake activity appeared about three- to fourfold lower than glycine betaine uptake activity, depending on the growth, it is important to note that 40 μM substrate represents 2.5-fold the Km value for glycine betaine but less than the Km value for proline betaine. Given that the strong reduction of betaine uptake in the mutant strain was observed only in cells grown at high osmolarity, we assume that the BetS transporter represents an important component in the overall glycine betaine and proline betaine uptake activities in response to salt stress in S. meliloti.
Osmotic regulation of BetS in S. meliloti.
To analyze the expression of betS in S. meliloti, a transcriptional betS-lacZ fusion was constructed, resulting in strain UNA315 (see Materials and Methods). The β-galactosidase activity of UNA315 was measured in cells grown in LAS minimal medium and compared to the basal level of β-galactosidase activity of UNA316. In strain UNA315, the betS-lacZ fusion was strongly expressed in cells grown in minimal medium (Table 4). Addition of NaCl (0.3 M), glycine betaine (1 mM), or a combination of both did not significantly induce betS expression. These results indicate that the betS gene is constitutively expressed and exclude significant transcriptional induction by either salt stress or glycine betaine. Consequently, in S. meliloti, the activation of BetS-mediated glycine betaine transport by osmotic stress is most likely due to a posttranscriptional regulation, as previously observed in the complemented E. coli MKH13 strain.
TABLE 4.
β-Galactosidase activities of S. meliloti GMI211 carrying a betS-lacZ fusion integrated into the chromosome (strain UNA315)a
| Growth conditions | β-Galactosidase activity (Miller units) |
|---|---|
| Control | 1,155 ± 76 |
| + NaCl (0.3 M) | 1,425 ± 51 |
| + Glycine betaine (1 mM) | 1,180 ± 7 |
| + NaCl (0.3 M) + glycine betaine (1 mM) | 1,472 ± 23 |
Cells were grown overnight in low-osmolarity LAS minimal medium (control). Values are means from duplicates of two independent cultures, and standard deviations are also shown.
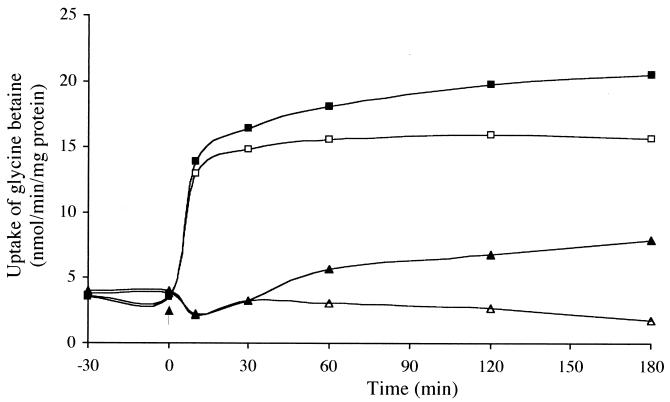
Since betS is not regulated at the expression level, it was important to establish whether BetS activity, which is observed only at high osmolarity, depends on osmotic activation of existing BetS proteins. Thus, glycine betaine uptake was measured in exponentially growing wild-type GMI211 and betS mutant UNA316 cells, treated or not with chloramphenicol, and subjected to a sudden osmotic upshock by adding NaCl (final concentration, 0.3 M). As shown in Fig. 4, glycine betaine uptake by GMI211 was immediately stimulated upon osmotic upshock (from 4 to 15 nmol/min/mg of protein). A strong stimulation was observed in less than 2 min, but maximal activation required approximately 8 to 10 min. Then, the rate of uptake continued to rise slowly over a period of 180 min to reach approximately 20 nmol/min/mg of protein. Chloramphenicol-treated cells showed a similar response to the upshock. However, the slow further increase observed previously could no longer be detected, and the rate of transport remained constant during 180 min. In contrast to what was observed with the wild type, glycine betaine uptake in the betS mutant was slightly decreased for about 10 min after the osmotic upshock and then slowly increased as in the wild type. In chloramphenicol-treated cells, this increase was completely abolished. Almost similar results were obtained with proline betaine (80 μM) as the substrate (data not shown).
FIG. 4.
Effect of NaCl upshock on glycine betaine uptake activity in S. meliloti GMI211 (▪, □) and betS mutant (▴, ▵) strains. Cells were grown in MCAA low-osmolarity medium to mid-log phase and subjected to a sudden upshock (indicated by the arrow) by adding NaCl at a final concentration of 0.3 M. Uptake measurements of [14C]glycine betaine were done at a final concentration of 40 μM using cells treated (□, ▵) or not (▪, ▴) with chloramphenicol (100 μg/ml). Values are means from duplicates of three independent cultures, with standard errors of less than 5%.
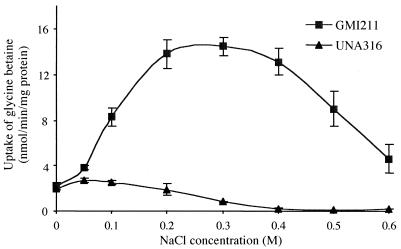
From all of these experiments, we conclude the following: (i) upon osmotic upshock, the strong increase in glycine betaine and proline betaine uptake via the BetS transporter is the consequence of the activation of existing BetS proteins, probably through conformational changes; (ii) besides BetS, at least one other uptake system for both betaines is operative, and its expression is most likely transcriptionally induced by osmotic stress. For further elucidation of the role of osmolarity on BetS activation, glycine betaine transport was measured in GMI211 and UNA316 following osmotic upshock obtained by the addition of increased NaCl concentrations (Fig. 5). Clearly, the maximal activation of BetS-mediated glycine betaine transport was obtained with 0.3 M NaCl. At higher concentrations, the rate of transport decreased, probably because of inhibitory effects of the high ion concentration.
FIG. 5.
Dependence of glycine betaine uptake in S. meliloti wild-type and betS mutant strains on increases in medium osmolarity imposed by NaCl. Cells were grown in low-osmolarity MCAA medium and then subjected to osmotic upshock by addition of NaCl. The initial uptake rates were determined at 40 μM [14C]glycine betaine, 10 min after upshock, and plotted against the NaCl concentration added to the medium. Data are means from duplicates of three separate experiments; standard deviations are also shown.
Contribution of BetS to osmoprotection by glycine betaine and proline betaine in S. meliloti.
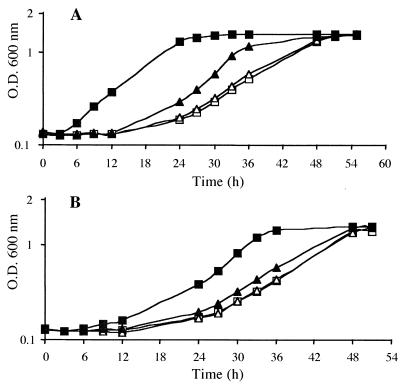
To assess the contribution of the BetS transporter to osmoprotection by glycine betaine and proline betaine, GMI211 and UNA316 were grown in MCAA medium under high osmolarity (0.5 M NaCl) in the absence or presence of betaine (1 mM). As already known, (i) growth of both strains was strongly impaired in the absence of osmoprotectant, (ii) addition of 1 mM glycine betaine or 1 mM proline betaine strongly alleviated growth inhibition in GMI211, and (iii) glycine betaine was more efficient than proline betaine in protecting S. meliloti against osmotic stress (Fig. 6). During the exponential growth phase, the generation time obtained for salt-stressed cells grown in the presence of betaines was not significantly different between GMI211 and UNA316. However, UNA316 showed a much longer delay in its response to osmotic stress: for example, in the presence of glycine betaine, the lag phase for GMI211 was about 3 h, compared to 12 h for UNA316 (Fig. 6A). Similar results were obtained when proline betaine replaced glycine betaine (Fig. 6B). Hence, the BetS transporter of S. meliloti is absolutely required for a rapid adaptation to osmotic stress, through uptake of glycine betaine or proline betaine.
FIG. 6.
Growth of S. meliloti GMI211 wild-type (▪, □) and betS mutant (▴, ▵) strains. Cells were grown in MCAA medium with 0.5 M NaCl in the presence (solid symbols) or not (open symbols) of 1 mM glycine betaine (A) or 1 mM proline betaine (B). Values are means from duplicates of three independent cultures, with variations of less than 3%.
DISCUSSION
Glycine betaine and proline betaine accumulation in salt-stressed S. meliloti allows the cells to grow at high osmolarity, both betaines being very potent osmoprotectants (17, 25). These betaines are mainly plant-derived compounds which may reach soil bacteria through root exudation or plant decomposition (41). In addition, because proline betaine occurs widely in Medicago species (42) and not in other genera of the Leguminosae (54), this betaine, which is also released from germinating Medicago seeds (42), may be ecologically important for root colonization of alfalfa by S. meliloti.
In this study, we report the identification and characterization of BetS, a major S. meliloti transporter for glycine betaine and proline betaine, whose inactivation results in loss of protection by both betaines after an osmotic upshock. BetS shows significant identity with various BCCT transporters (BetT, OpuD, BetL, and BetP) and, from a computer-assisted analysis, possesses 12 transmembrane domains, with both the N- and C-terminal ends located in the cytoplasm, a structural feature common in secondary transporters (46). In addition, a highly conserved amino acid sequence within helix 8 contains, at residue 357, the BCCT signature, (GSDN)-WT-(LIVM)-X-(FY)-WXWW. With respect to betaine uptake in S. meliloti, only an ATP-binding cassette histidine transporter (Hut system), also involved in glycine betaine and proline betaine, has been characterized previously (3). However, this system, which is induced by histidine and not by high osmolarity, most likely has a catabolic role (nitrogen assimilation) rather than being involved in osmoprotection. Hence, the BetS system characterized here is the first osmoregulated BCCT identified in S. meliloti and furthermore the only bacterial BCCT showing high affinity for proline betaine. The uptake of this betaine is mediated through the ABC system ProU in E. coli (18) and through the ProU homologues OpuA and OpuC in B. subtilis (4), even though their affinities for proline betaine have not been determined so far.
Upon expression in a betaine uptake-deficient E. coli mutant grown at elevated osmolarity, BetS displays high affinity towards glycine betaine (Km value of 16 μM) and proline betaine (Km value of 56 μM), allowing S. meliloti to scavenge both betaines from the rhizosphere or the soil. BetS appeared to be driven via cotransport with Na+, another feature common to this subclass of symporters. In addition, BetS failed to transport other trimethylammonium or related molecules, such as choline, carnitine, and ectoine, which proves a narrow specificity of BetS towards glycine betaine and proline betaine. A restricted specificity towards one or two molecules is also relevant to the BCCT transporters: OpuD, BetL, and BetP are highly specific for glycine betaine, BetT is a choline carrier, and EctT, a new BCCT recently characterized in Virgibacillus pantothenticus, is mainly involved in ectoine and hydroxyectoine uptake (A. Kuhlmann and E. Bremer, personal communication). Given the competition among soil bacteria for osmoprotectants, it is tempting to argue that S. meliloti is particularly well adapted for using proline betaine exuded in the rhizosphere of alfalfa.
A prominent finding of this work is the regulation of BetS. Upon betS expression in E. coli, whereas the carrier was completely inactive in cells grown at low osmolarity, its activity was strongly increased in cells subjected to salt stress. In S. meliloti, a transcriptional betS-lacZ fusion is constitutively expressed, but osmotic stress was required for BetS activity. More conclusively, a sudden osmotic upshock strongly activated BetS within minutes following stress application in the presence or the absence of a protein synthesis inhibitor. Hence, BetS is positively regulated by osmotic stress at the posttranslational level, most probably by conformational changes, as observed for BetP in C. glutamicum (36). The level of BetS activation was dependent on the intensity of the stress, and maximal induction was found at an NaCl concentration of 0.3 M (Fig. 5). The pattern of BetS activation is similar to that observed for BetP in C. glutamicum, which, however, presents optimal activity at 0.6 M NaCl (36), probably reflecting the higher turgor pressure in gram-positive bacteria compared to gram-negative bacteria.
Some osmoprotectant carriers, including both ABC transporters and ion symporters, have been shown to be upregulated at the posttranslational level by elevation of medium osmolarity (16, 37, 44). Because osmotic shifts elicit dramatic changes in bacterial cell structure, a model of direct sensing of the osmolarity of the environment by monitoring alteration in membrane tensions has been proposed to account for the activation of various osmoregulated transporters. Among them, the activation mechanisms of the H+-symporter ProP of E. coli, the Na+-dependent BCCT transporter BetP of C. glutamicum, and the ABC OpuA transporter of Lactococcus lactis have been analyzed in great details (37, 44, 45, 52). All three proteins have been purified, and the osmoregulated responses of the activities have been examined in proteoliposomes, indicating that osmoregulation of these transporters is mediated by changes in membrane properties, with the lipids playing an essential role. These carriers act as both osmoregulators and osmosensors for the cells.
In both ProP and BetP transporters, the positively charged C-terminal extension seems to be involved in sensing and/or transducing osmotic changes to the domain of the protein responsible for the translocation of the substrate (37). Interestingly, BetS contains a much longer stretch of internal hydrophilic residues (169 amino acids) in its C-terminal part than ProP and BetP. This extension is identical in length to that of the choline BetT transporter of E. coli. The BetS and BetT C-terminal regions show 23% identity but do not show similarity to the C-terminal domain of BetP. In addition, they do not contain any coiled-coil sequence, as in ProP (9, 32). Further studies are necessary to fully elucidate the role of the C-terminal end of BetS in the activation and the stability of the active conformation of the protein.
In view of the osmotic upshock results and growth experiments, S. meliloti BetS is clearly a major betaine transporter used for rapid adaptation to sudden increases in osmolarity. However, BetS can be distinguished from other osmoprotectant emergency transporters, such as the EctP system from C. glutamicum, by its narrow specificity. In contrast to EctP, which accepts glycine betaine, ectoine, proline, and probably all known compatible solutes, in C. glutamicum (38), BetS can only transport glycine betaine and proline betaine.
Since the betS mutant retains 40% of the betaine transport capacity of the wild type under high osmolarity, S. meliloti uses at least one other uptake system, most probably transcriptionally induced and involved in long-term adaptation to high salt concentration. Furthermore, the measurements of glycine betaine uptake by cells grown at low osmolarity (Table 3) indicate that an additional betaine uptake activity inducible by the substrate is present in S. meliloti. Thus, in addition to BetS and to the Hut system, this bacterium may contain at least two other betaine carriers. A multiplicity of betaine uptake systems has been observed in various bacteria, such as B. subtilis (20). It is important to note that S. meliloti is unusually well equipped with transporters, particularly on the megaplasmid pSymb, as evidenced by the genome annotation (13), and this perfectly correlates with the extremely large spectrum of osmoprotectants and energy sources, including betaines, used by this bacterium.
Acknowledgments
This work was supported by the Centre National de la Recherche Scientifique and the Ministère de la Recherche. A.B. received a doctoral fellowship from the Ministère de la Recherche.
We are grateful to the colleagues cited in Table 1, who generously provided strains and the genomic bank of S. meliloti used in this study. We thank E. Bremer (Universität Marburg, Marburg, Germany) for helpful discussions and R. Krämer (Universität Cologne, Cologne, Germany) for the gift of unlabeled ectoine.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment research tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Bernard, T., J.-A. Pocard, B. Perroud, and D. Le Rudulier. 1986. Variations in the response of salt-stressed Rhizobium strains to betaines. Arch. Microbiol. 29:189-198. [Google Scholar]
- 3.Boncompagni, E., L. Dupont, T. Mignot, M. Østerås, A. Lambert, M.-C. Poggi, and D. Le Rudulier. 2000. Characterization of a Sinorhizobium meliloti ATP-binding cassette histidine transporter also involved in betaines and proline uptake. J. Bacteriol. 182:3717-3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bremer, E., and R. Krämer. 2000. Coping with osmotic challenges: osmoregulation through accumulation and release of compatible solutes in bacteria, p. 79-97. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. American Society for Microbiology, Washington, D.C.
- 5.Cairney, J., I. R. Booth, and C. F. Higgins. 1985. Salmonella typhimurium proP gene encodes a transport system for the osmoprotectant betaine. J. Bacteriol. 164:1218-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairney, J., I. R. Booth, and C. F. Higgins. 1985. Osmoregulation of gene expression in Salmonella typhimurium: proU encodes an osmotically induced betaine transport system. J. Bacteriol. 164:1224-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, G. N., and H. V. Rickenberg. 1956. Concentration spécifique réversible des aminoacides chez E. coli. Ann. Inst. Pasteur 91:693-720. [PubMed] [Google Scholar]
- 8.Csonka, L. N., and W. Epstein. 1996. Osmoregulation, p. 1210-1223. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 9.Culham, D. E., B. Tripet, K. I. Racher, R. T. Voegele, R. S. Hodges, and J. M. Wood. 2000. The role of the carboxyl terminal α-helical coiled-coil domain in osmosensing by transporter ProP of Escherichia coli. J. Mol. Recognit. 13:309-322. [DOI] [PubMed] [Google Scholar]
- 10.Farwick, M., R. M. Siewe, and R. Krämer. 1995. Glycine betaine uptake after hyperosmotic shift in Corynebacterium glutamicum. J. Bacteriol. 177:4690-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finan, T. M., E. K. Hartwieg, K. LeMieux, K. Bergman, G. C. Walker, and E. R. Signer. 1984. General transduction in Rhizobium meliloti. J. Bacteriol. 159:120-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finan, T. M., B. Kunkel, G. F. DeVos, and E. R. Signer. 1986. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J. Bacteriol. 167:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finan, T. M., S. Weidner, K. Wong, J. Buhrmester, P. Chain, F. J. Vorhölter, I. Hernandez-Lucas, A. Becker, A. Cowie, J. Gouzy, B. Golding, and A. Pühler. 2001. The complete sequence of the 1,683-kb pSymB megaplasmid from the N2-fixing endosymbiont Sinorhizobium meliloti. Proc. Natl. Acad. Sci. USA 98:9889-9894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleischmann, R. D., M. D. Adams, O. White, R. A. Clayton, E. F. Kirkness, A. R. Kerlavage, C. J. Bult, J. F. Tomb, B. A. Dougherty, J. M. Merrick, K. McKennie, G. Sutton, W. FitzHugh, C. Fields, J. D. Gocayne, J. Scott, R. Shirley, L.-I. Liu, A. Glodek, M. Kelley, J. F. Weidmann, T. Phillipps, T. Spriggs, E. Hedblom, M. D. Cotton, T. R. Utterback, M. C. Hanna, D. T. Nguyen, D. M. Saudek, R. C. Brandon, L. D. Fine, J. L. Fritchmann, J. L. Fuhrmann, N. S. M. Geoghagen, C. L. Gnehm, L. A. McDonald, K. V. Small, C. M. Fraser, H. O. Smith, and J. C. Venter. 1995. Whole random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496-512. [DOI] [PubMed] [Google Scholar]
- 15.Friedman, A. M., S. R. Long, W. S. E. Brown, W. J. Buikema, and F. M. Ausubel. 1982. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene 18:289-296. [DOI] [PubMed] [Google Scholar]
- 16.Glaasker, E., E. H. M. L. Heuberger, W. N. Konings, and B. Poolman. 1998. Mechanism of osmotic activation of the quaternary ammonium compound transporter (QacT) of Lactobacillus plantarum. J. Bacteriol. 180:5540-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gloux, K., and D. Le Rudulier. 1989. Transport et catabolism of proline betaine in salt-stressed Rhizobium meliloti. Arch. Microbiol. 151:143-148. [Google Scholar]
- 18.Haardt, M., B. Kempf, E. Faatz, and E. Bremer. 1995. The osmoprotectant proline betaine is a major substrate for binding-protein-dependent transport system ProU of Escherichia coli K-12. Mol. Gen. Genet. 246:783-786. [DOI] [PubMed] [Google Scholar]
- 19.Kappes, R. M., and E. Bremer. 1998. Responses of Bacillus subtilis to high osmolarity: uptake of carnitine, crotonobetaine, and γ-butyrobetaine via the ABC transport system OpuC. Microbiology 144:83-90. [DOI] [PubMed] [Google Scholar]
- 20.Kappes, R. M., B. Kempf, and E. Bremer. 1996. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of opuD. J. Bacteriol. 178:5071-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempf, B., and E. Bremer. 1995. OpuA, an osmotically regulated binding protein-dependent transport system for the osmoprotectant glycine betaine in Bacillus subtilis. J. Biol. Chem. 270:16701-16713. [DOI] [PubMed] [Google Scholar]
- 22.Kempf, B., J. Gade, and E. Bremer. 1997. Lipoprotein from the osmoregulated ABC transport system OpuA of Bacillus subtilis: purification of the glycine betaine binding protein and characterization of a functional lipidless mutant. J. Bacteriol. 179:6213-6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kokotek, W., and W. Lotz. 1989. Construction of a lacZ-kanamycin-resistance cassette, useful for site directed mutagenesis and as a promoter probe. Gene 84:467-471. [DOI] [PubMed] [Google Scholar]
- 24.Lamark, T., I. Kaasen, M. W. Eshoo, P. Falkenberg, J. McDougall, and A. R. Strøm. 1991. DNA sequence and analysis of the bet genes encoding the osmoregulatory choline-glycine betaine pathway of Escherichia coli. Mol. Microbiol. 5:1049-1064. [DOI] [PubMed] [Google Scholar]
- 25.Le Rudulier, D., and T. Bernard. 1986. Salt tolerance in Rhizobium: a possible role for betaines. FEMS Microbiol. Rev. 39:67-72. [Google Scholar]
- 26.Le Rudulier, D., K. Gloux, and N. Riou. 1991. Identification of an osmotically induced periplasmic glycine betaine-binding protein from Rhizobium meliloti. Biochim. Biophys. Acta 1061:199-205. [DOI] [PubMed] [Google Scholar]
- 27.Le Rudulier, D., A. R. Strøm, A. M. Dandekar, L. T. Smith, and R. C. Valentine. 1984. Molecular biology of osmoregulation. Science 224:1064-1068. [DOI] [PubMed] [Google Scholar]
- 28.Lucht, J. M., and E. Bremer. 1994. Adaptation of Escherichia coli to high osmolarity environments: osmoregulation of the high-affinity glycine betaine transport system ProU. FEMS Microbiol. Rev. 14:3-20. [DOI] [PubMed] [Google Scholar]
- 29.Meade, H. M., S. R. Long, G. B. Ruvkun, S. E. Brown, and F. M. Ausubel. 1982. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 149:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Milner, J. L., S. Grothe, and J. M. Wood. 1988. Proline porter II is activated by a hyperosmotic shift in both whole cells and membrane vesicules of Escherichia coli K12. J. Biol. Chem. 263:14900-14905. [PubMed] [Google Scholar]
- 32.Milner, J. L., and J. M. Wood. 1989. Insertion proQ220::Tn5 alters regulation of proline porter II, a transporter of proline and glycine betaine in Escherichia coli. J. Bacteriol. 171:947-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niel, C., J. B. Guillaume, and M. Bechet. 1977. Mise en évidence de deux enzymes présentant une activité β-galactosidase chez Rhizobium meliloti. Can. J. Microbiol. 23:1178-1181. [PubMed] [Google Scholar]
- 34.Østerås, M., E. Boncompagni, N. Vincent, M. C. Poggi, and D. Le Rudulier. 1998. Presence of a gene encoding choline sulfatase in Sinorhizobium meliloti bet operon: choline-O-sulfate is metabolized into glycine betaine. Proc. Natl. Acad. Sci. USA 95:11394-11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perroud, B., and D. Le Rudulier. 1985. Glycine betaine transport in Escherichia coli: osmotic regulation. J. Bacteriol. 161:393-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peter, H., A. Burkovski, and R. Krämer. 1996. Isolation, characterisation, and expression of the Corynebacterium glutamicum betP gene, encoding the transport system for the compatible solute glycine betaine. J. Bacteriol. 178:5229-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peter, H., A. Burkovski, and R. Krämer. 1998. Osmo-sensing by N- and C-terminal extensions of the glycine betaine uptake system BetP of Corynebacterium glutamicum. J. Biol. Chem. 273:2567-2574. [DOI] [PubMed] [Google Scholar]
- 38.Peter, H., B. Weil, A. Burkovski, R. Krämer, and S. Morbach. 1998. Corynebacterium glutamicum is equipped with four secondary carriers for compatible solutes: identification, sequencing, and characterization of the proline/ectoine uptake system, ProP, and the ectoine/proline/glycine betaine carrier, EctP. J. Bacteriol. 180:6005-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Philipp, W. J., S. Poulet, K. Eiglmeier, L. Pascopella, V. Balasubramanian, B. Heym, S. Bergh, B. R. Bloom, W. R. Jacobs, and S. T. Cole. 1996. An integral map of the genome of the bacillus Mycobacterium tuberculosis H37Rv, and comparison with Mycobacterium leprae. Proc. Natl. Acad. Sci. USA 93:3132-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips, D. A., C. M. Joseph, and C. A. Maxwell. 1992. Trigonelline and stachydrine released from alfalfa seeds activate NodD2 protein in Rhizobium meliloti. Plant Physiol. 99:1526-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillips, D. A., and W. R. Streit. 1997. Applying plant-microbe signalling concepts to alfalfa: roles for secondary metabolites, p. 319-342. In B. D. McKersie and D. C. W. Brown (ed.), Biotechnology and the improvement of forage legumes. CAB International, Wallingford, England.
- 42.Phillips, D. A., J. Wery, C. M. Joseph, A. D. Jones, and L. R. Teuber. 1995. Release of flavonoids and betaines from seeds of seven Medicago species. Crop Sci. 35:805-808. [Google Scholar]
- 43.Pocard, J.-A., N. Vincent, E. Boncompagni, L. T. Smith, M. C. Poggi, and D. Le Rudulier. 1997. Molecular characterization of the bet genes encoding glycine betaine synthesis in Sinorhizobium meliloti 102F34. Microbiology 143:1369-1379. [DOI] [PubMed] [Google Scholar]
- 44.Racher, K. I., R. T. Voegele, E. V. Marshall, D. E. Culham, J. M. Wood, H. Jung, M. Bacon, M. T. Cairns, S. M. Ferguson, W. J. Liang, P. J. F. Henderson, G. White, and F. R. Hallett. 1999. Purification and reconstitution of an osmosensor: transporter ProP of Escherichia coli senses and responds to osmotic shifts. Biochemistry 38:1676-1684. [DOI] [PubMed] [Google Scholar]
- 45.Rübenhagen, R., H. Rönsch, H. Jung, R. Krämer, and S. Morbach. 2000. Osmosensor and osmoregulator properties of the betaine carrier BetP from Corynebacterium glutamicum in proteoliposomes. J. Biol. Chem. 275:735-741. [DOI] [PubMed] [Google Scholar]
- 46.Saier, M. H., Jr. 1994. Computer-aided analysis of transport protein sequences: gleaning evidence concerning function, structure, biogenesis, and evolution. Microbiol. Rev. 58:71-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 48.Simon, R., U. Priefer, and A. Pülher. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 49.Sleator, R. D., C. G. M. Gahan, T. Abee, and C. Hill. 1999. Identification and disruption of BetL, a secondary glycine betaine transport system linked to the salt tolerance of Listeria monocytogenes LO28. Appl. Environ. Microbiol. 65:2078-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith, L. T., J.-A. Pocard, T. Bernard, and D. Le Rudulier. 1988. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J. Bacteriol. 170:3142-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van der Heide, T., and B. Poolman. 2000. Osmoregulated ABC-transport system of Lactococcus lactis senses water stress via changes in the physical state of the membrane. Proc. Natl. Acad. Sci. USA 97:7102-7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vieira, J., and J. Messing. 1987. Production of single-stranded plasmid DNA. Methods Enzymol. 153:3-34. [DOI] [PubMed] [Google Scholar]
- 54.Wyn Jones, R. G., and R. Storey. 1981. Betaines, p. 171-204. In L. P. Paleg and D. Aspinall (ed.), The physiology and biochemistry of drought resistance in plants. Academic Press, Ltd., Sydney, Australia.