Abstract
1. Spontaneous histamine release from isolated mast cells was found to be independent of calcium in the concentration range up to 1 m-mole/l. Phosphatidyl serine did not change the effect of calcium on spontaneous release.
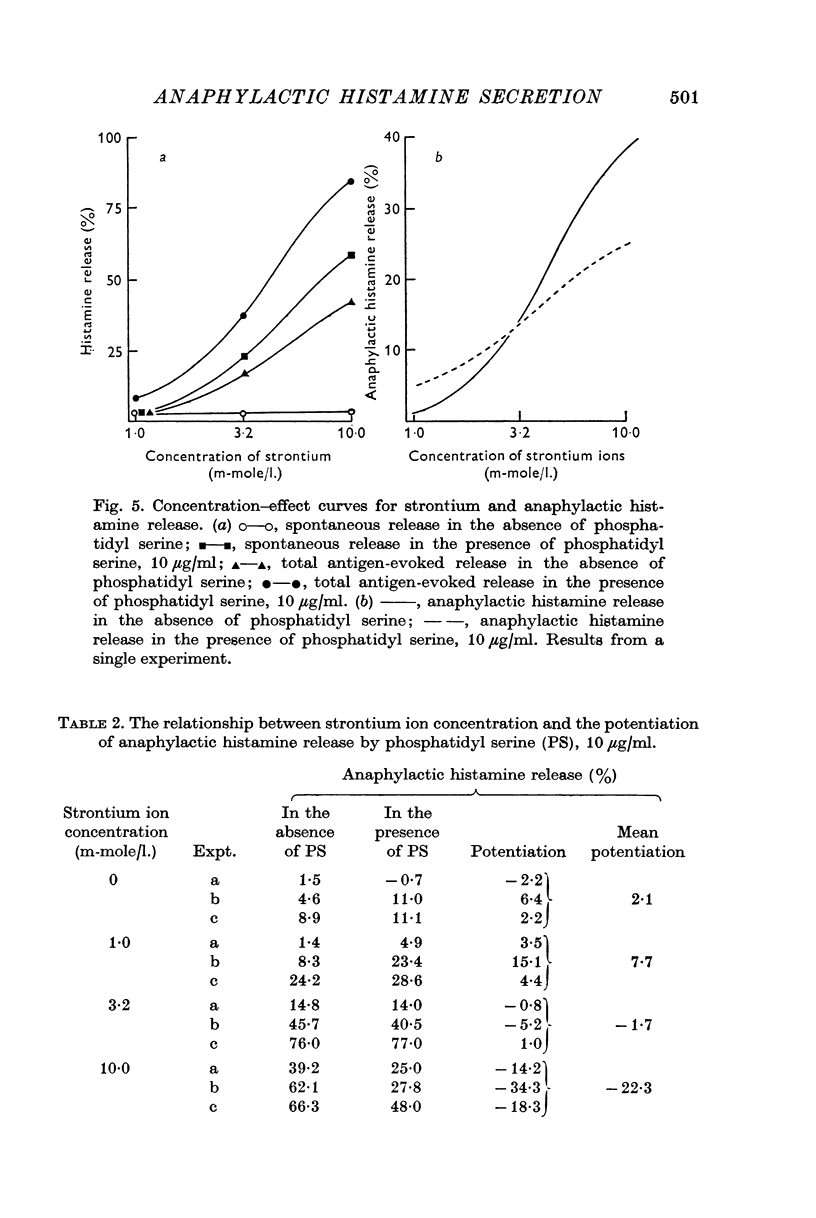
2. Spontaneous histamine release was found to vary with the strontium ion concentration. Graded increase in the release occurred as the concentration of strontium was raised from 1 to 10 m-mole/l. Phosphatidyl serine potentiated this action of strontium; the potentiation showed a graded increase as the phosphatidyl serine concentration was raised from 1 to 100 μg/ml.
3. The activation of anaphylactic histamine release by calcium was potentiated by phosphatidyl serine; the degree of potentiation showed a graded increase as the calcium concentration was raised from 0·1 to 1·0 m-mole/l.
4. The activation of anaphylactic histamine release by strontium showed little, if any, potentiation by phosphatidyl serine.
5. The response of the mast cells, in terms of anaphylactic histamine release, to calcium, in the presence of optimal concentrations of phosphatidyl serine, was found to be similar to that observed in the presence of strontium alone.
6. These observations are discussed in terms of the concepts of affinity and efficacy of the ions at their receptor sites.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIRKS R., HUXLEY H. E., KATZ B. The fine structure of the neuromuscular junction of the frog. J Physiol. 1960 Jan;150:134–144. doi: 10.1113/jphysiol.1960.sp006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A. M., Low E., Ishijimi M. Effect of phosphatidyl serine decarboxylase on neural excitation. Nat New Biol. 1972 Oct 4;239(92):150–151. doi: 10.1038/newbio239150a0. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The effect of magnesium on the activity of motor nerve endings. J Physiol. 1954 Jun 28;124(3):553–559. doi: 10.1113/jphysiol.1954.sp005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. CALCIUM MOVEMENT IN THE NEUROHYPOPHYSIS OF THE RAT AND ITS RELATION TO THE RELEASE OF VASOPRESSIN. J Physiol. 1964 Jul;172:19–30. doi: 10.1113/jphysiol.1964.sp007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster L. J., Copenhaver J. H., Jr Phosphatidyl serine requirement of (Na+-K+)-activated adenosine triphosphatase from rat kidney and brain. Biochim Biophys Acta. 1967 Apr 4;137(2):406–408. doi: 10.1016/0005-2760(67)90120-8. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. Effect of calcium on dextran-induced histamine release from isolated mast cells. Br J Pharmacol. 1972 Dec;46(4):767–769. doi: 10.1111/j.1476-5381.1972.tb06902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. The role of the alkaline earth ions in anaphylactic histamine secretion. J Physiol. 1972 Aug;224(3):753–769. doi: 10.1113/jphysiol.1972.sp009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C. The mechanism of spontaneous histamine release from mast cells. J Physiol. 1973 Feb;229(1):8P–9P. [PubMed] [Google Scholar]
- Goth A., Adams H. R., Knoohuizen M. Phosphatidylserine: selective enhancer of histamine release. Science. 1971 Sep 10;173(4001):1034–1035. doi: 10.1126/science.173.4001.1034. [DOI] [PubMed] [Google Scholar]
- Hauser H., Dawson R. M. The binding of calcium at lipid-water interfaces. Eur J Biochem. 1967 Mar;1(1):61–69. doi: 10.1007/978-3-662-25813-2_11. [DOI] [PubMed] [Google Scholar]
- MONGAR J. L., SCHILD H. O. The effect of calcium and pH on the anaphylactic reaction. J Physiol. 1958 Feb 17;140(2):272–284. doi: 10.1113/jphysiol.1958.sp005933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOTA I. THE MECHANISM OF ANAPHYLAXIS. I. PRODUCTION AND BIOLOGICAL PROPERTIES OF 'MAST CELL SENSITIZING' ANTIBODY. Immunology. 1964 Nov;7:681–699. [PMC free article] [PubMed] [Google Scholar]
- Mongar J. L., Svec P. The effect of phospholipids on anaphylactic histamine release. Br J Pharmacol. 1972 Dec;46(4):741–752. doi: 10.1111/j.1476-5381.1972.tb06899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., Bangham A. D. Biophysical properties of phospholipids. II. Permeability of phosphatidylserine liquid crystals to univalent ions. Biochim Biophys Acta. 1966 Sep 5;126(1):185–188. doi: 10.1016/0926-6585(66)90053-7. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Miller N. Phospholipid model membranes. I. Structural characteristics of hydrated liquid crystals. Biochim Biophys Acta. 1967 Sep 9;135(4):624–638. doi: 10.1016/0005-2736(67)90094-6. [DOI] [PubMed] [Google Scholar]
- ROJAS E., TOBIAS J. M. MEMBRANE MODEL: ASSOCIATION OF INORGANIC CATIONS WITH PHOSPHOLIPID MONOLAYERS. Biochim Biophys Acta. 1965 Mar 29;94:394–404. doi: 10.1016/0926-6585(65)90047-6. [DOI] [PubMed] [Google Scholar]
- WOODIN A. M., WIENEKE A. A. The accumulation of calcium by the polymorphonuclear leucocyte treated with staphylococcal leucocidin and its significance in the extrusion of protein. Biochem J. 1963 Jun;87:487–495. doi: 10.1042/bj0870487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler K. P., Whittam R. The involvement of phosphatidylserine in adenosine triphosphatase activity of the sodium pump. J Physiol. 1970 Apr;207(2):303–328. doi: 10.1113/jphysiol.1970.sp009063. [DOI] [PMC free article] [PubMed] [Google Scholar]


