Abstract
The Escherichia coli chromosome contains two distantly located genes, gadA and gadB, which encode biochemically undistinguishable isoforms of glutamic acid decarboxylase (Gad). The Gad reaction contributes to pH homeostasis by consuming intracellular H+ and producing γ-aminobutyric acid. This compound is exported via the protein product of the gadC gene, which is cotranscribed with gadB. Here we demonstrate that transcription of both gadA and gadBC is positively controlled by gadX, a gene downstream of gadA, encoding a transcriptional regulator belonging to the AraC/XylS family. The gadX promoter encompasses the 67-bp region preceding the gadX transcription start site and contains both RpoD and RpoS putative recognition sites. Transcription of gadX occurs in neutral rich medium upon entry into the stationary phase and is increased at acidic pH, paralleling the expression profile of the gad structural genes. However, PT5lacO-controlled gadX expression in neutral rich medium results in upregulation of target genes even in exponential phase, i.e., when the gad system is normally repressed. Autoregulation of the whole gad system is inferred by the positive effect of GadX on the gadA promoter and gadAX cotranscription. Transcription of gadX is derepressed in an hns mutant and strongly reduced in both rpoS and hns rpoS mutants, consistent with the expression profile of gad structural genes in these genetic backgrounds. Gel shift and DNase I footprinting analyses with a MalE-GadX fusion protein demonstrate that GadX binds gadA and gadBC promoters at different sites and with different binding affinities.
The ability to survive in an acidic environment is essential for successful colonization of the mammalian host by both commensal and pathogenic enteric bacteria. These microorganisms are faced with an extremely acidic shock (pH <2.5) during their passage through the stomach and must counteract the deleterious effect of volatile fatty acids while living in the gut. Enteric bacteria have evolved a number of strategies enabling them to overcome the acidic stress, including, among others, the amino acid decarboxylase-based systems (14).
The glutamate decarboxylase (Gad) system has recently gained interest because of its major role in the acid resistance of enteric pathogens such as Escherichia coli, Shigella flexneri, and Listeria monocytogenes (6, 8, 9, 35). The E. coli chromosome contains two genes located 2,100 kb apart, gadA and gadB, encoding two biochemically undistinguishable forms of Gad, differing in five amino acid residues (4, 10).
The chemistry of the Gad-dependent pH neutralizing system is simplified by the folllowing intracellular reaction: glutamate−1 + H+ → γ-aminobutyrate + CO2. Following decarboxylation, one intracellular proton is consumed, and both products are released by the cell. Gas-liquid partition of CO2 is pH dependent, the gaseous state being favored at low pH. Moreover, γ-aminobutyrate (GABA; pI ≈ 7.0) is less acidic than glutamate (pI ≈ 3.1), thereby providing local buffering of the extracellular environment.
The logical sequence of these chemical events (decarboxylation followed by GABA export) reflects the physical association of the genes coding for the system components. In E. coli, the gadB gene constitutes a single transcriptional unit with the downstream gadC gene, encoding the putative glutamate/GABA antiporter (9). In a gadB-deficient background, the system activity is guaranteed by the second decarboxylase isoform, the gadA gene product (6).
Transcript analysis and enzyme activity measurements indicate that expression of gad genes is switched on at the stationary phase under oxidative growth conditions and responds positively to acidic and hyper- and hypo-osmotic shocks (9). The extent of gadA and gadBC expression can be differentiated depending on the culture conditions, and stationary phase and acid pH activate distinct regulatory circuits. The contribution of GadA and GadB to the total amount of cellular glutamate decarboxylase activity, as determined by using gadA and gadB mutants, reveals that cells grown in exponential phase under acidic conditions express more GadA than GadB, the latter isoform being more abundant in stationary phase at pH 7 (6).
The nucleoid protein H-NS is involved in the negative control of gadA and gadBC transcription during the exponential growth phase under oxidative conditions, while the alternative sigma factor RpoS is responsible for gad expression at the stationary phase but not during fermentative growth (6, 7, 9). It has previously been suggested that H-NS could directly bind gad promoters, thereby silencing transcription of gad genes during the exponential phase (9). Alternatively, H-NS could indirectly repress expression of gad genes by negatively acting on RpoS stability (3, 36). Accordingly, acid pH, which is the main stimulus for gad induction, also increases the cellular levels of RpoS during the exponential phase (16).
Evidence has recently emerged that the activation of the gad system is mediated by the GadX protein (17, 30, 34), a member of the AraC/XylS family of transcriptional regulators (15), encoded by the gadX gene (formerly designated yhiX), located downstream of gadA. GadX expression was positively correlated with both upregulation of gadA and gadBC genes and resistance to acidic pH (17). Shortly after, a role of GadX in the transcriptional control of the plasmid-encoded regulator (per) of enteropathogenic E. coli virulence genes was demonstrated (30), linking pH sensing with differential expression of genes involved in acid resistance and synthesis of virulence factors.
In this report, we focus on gadX regulation and on the mechanism by which GadX positively controls the expression of the gadA and gadBC gene system. We provide evidence that GadX is a DNA-binding protein which recognizes the promoters of gadA and gadBC genes to a different extent and that GadX expression results in upregulation of target genes during exponential growth in rich neutral medium, i.e., when the gad system is normally repressed. The expression profile of gadX shares features with that of the gad structural genes, suggesting that GadX is a terminal component of the H-NS- and RpoS-dependent regulatory cascade responsible for gadA and gadBC transcriptional control.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
The bacterial strains and plasmids used in this study are listed in Table 1. Growth was monitored by optical density measurements at 600 nm (OD600). The media included Luria-Bertani (LB) (2), LBG (LB supplemented with 0.4% glucose and acidified to pH 5.0 with HCl), and LB-MES (LB buffered at pH 5.5 with 100 mM morpholinoethanesulfonic acid) (19). Ampicillin, kanamycin, and tetracycline were added at concentrations of 100, 25, and 12.5 μg/ml, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| ATCC 11246 | Wild type | American Type Culture Collection (27) |
| DDBGX | gadX::pJPgadX′ derivative of ATCC 11246 tet | This work |
| DH5αF′ | F′ recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 Δ(lacZYA-argF)U169 (φ80d lacZΔM15) | 2 |
| JM109 | (F′ traD36 proA+proB+lacIqlacZΔM15) recA1 endAI gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB) | 2 |
| S17.1 | pro thi hsdR recA RP4.2 (tet::Mu) (kan::Tn7) | 31 |
| MC4100 | F−araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB5301 deoC ptsF25 rbsR | 5 |
| RH90 | MC4100 rpoS359::Tn10 | 18 |
| YK4122 | trp+ derivative of YK1100 | 37 |
| YK4124 | trp+hns-2 derivative of YK1100 | 37 |
| YK4124rpoS | YK4124 rpoS359::Tn10 | 9 |
| Plasmids | ||
| pRS415 | Operon fusion vector; ColE1 replicon, lacZYA bla | 32 |
| pJP5608 | Mobilizable cloning vector for construction of single-crossover insertional mutants; R6K replicon, mob tet Tra− | 28 |
| pBluescript SK (pBs) | Multicopy phagemid vector; ColE1 replicon, lacZα bla | Stratagene |
| pBsgadX | 832-bp amplicon generated from pBsAX, encompassing the entire gadX coding sequence, ligated to BamHI and HindIII sites of pBs | This work |
| pT | pBs-derived T/A vector for cloning PCR products | 20 |
| pTgadX | 838-bp amplicon generated from pBsAX, encompassing entire gadX coding sequence, ligated to pT | This work |
| pMAL-c2 | Phagemid vector for protein fusion and expression; ColE1 replicon, Ptac malE::lacZα lacIqbla | New England Biolabs |
| pmalE::gadX | 852-bp fragment encompassing entire gadX coding sequence and part of the multicloning site of pBs ligated to the EcoRI-HindIII sites of pMAL-c2 under Ptac control | This work |
| pQE60 | Expression vector; ColE1 replicon, PT5-lacO RBSII | Qiagen |
| pQEgadX | 832-bp fragment encompassing the entire gadX coding sequence ligated to the BamHI-HindIII sites of pQE60 under PT5-lacO RBSII control | This work |
| pBsAX | 4.1-kb ClaI genomic fragment from E. coli ATCC 11246, encompassing gadA and gadX genes, cloned in pBs | 10 |
| pBsA | 2.8-kb ClaI-NcoI genomic fragment from E. coli ATCC 11246, encompassing gadA gene, cloned in pBs | This work |
| pBsX | 2.1-kb EcoRI-NcoI genomic fragment from pBsAX, encompassing gadX gene, cloned in pBs | This work |
| pBsB | 4.5-kb HindIII genomic fragment from E. coli ATCC 11246, encompassing gadB gene, cloned in pBs | 10 |
| pBsBC | 5.5-kb EcoRI-HindIII genomic fragment from E. coli ATCC 11246, encompassing gadBC operon, cloned in pBs | 9 |
| pJPgadX′ | 255-bp NcoI-HinfI fragment from pQEgadX cloned in pJP5608 | This work |
Construction of GadX expression systems.
The 838-bp DNA fragment encompassing the entire gadX gene was generated by PCR using Vent polymerase (New England Biolabs) from pBsAX with primers 5′-GGGGATCCATGCAATCACTACACGGGAATT-3′ and 5′-GGAAGCTTCTATAATCTTATTCCTTCCGCAGA-3′ (restriction sites are italicized; the gadX start and stop codons are underlined). After digestion with BamHI and HindIII, the amplicon was ligated to the corresponding restriction sites of pBlueScript (pBs), yielding pBsgadX. This construct was used to transform E. coli DH5αF′ competent cells and sequenced on both strands. The 832-bp BamHI-HindIII fragment from pBsgadX was then ligated to the corresponding sites of the expression vector pQE60, yielding pQEgadX. This construct was used to transform E. coli JM109 competent cells.
To generate the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible pmalE::gadX construct, the 838-bp DNA fragment encompassing the entire gadX gene was amplified by PCR using Taq polymerase (Perkin Elmer) from pBsAX with the primers 5′-GGCATATGCAATCACTACATGGGA-3′ and 5′-CCGGATCCCTATAATCTTATTCCTTCCG-3′ (the gadX start and stop codons are underlined). The amplicon was initially T/A ligated to the pBs-derived T-vector (20), yielding pTgadX, and sequenced on both strands to ensure that mispriming did not occur during amplification. Subsequently, the 852-bp EcoRI-HindIII fragment from pTgadX, containing the whole gadX open reading frame and part of the multicloning site of pBs, was ligated in frame with the malE gene into the corresponding sites of the expression vector pMAL-c2 (New England Biolabs), yielding pmalE::gadX. As a consequence of the cloning strategy, the pmalE::gadX fusion construct contained 18 additional nucleotides in the malE-gadX linker region, coding for an extra amino acid sequence (SEFDWH). E. coli laboratory strains DH5αF′ and JM109 were used for pmalE::gadX propagation and MalE-GadX expression, respectively.
Construction of gadX::lacZ transcriptional fusions and β-galactosidase assay.
Three transcriptional fusions were generated for the gadX gene by cloning into the vector pRS415 (32) PCR-generated DNA fragments obtained using pBsAX as the template and spanning from positions −222, −67, and +18 to position +121, relative to the gadX transcription start site. Primers used for PCR amplification were 21 to 23 bases in length and designed to allow directional cloning at the EcoRI and BamHI sites of pRS415. PCR amplification (25 cycles) was carried out using Vent polymerase as follows: 95°C for 1 min, 52°C for 1 min, and 74°C for 1 min. The amplicons were sequenced on both strands. β-Galactosidase activity was measured according to Miller (24) and expressed as follows: 1,000 × [(OD420 − 1.75 × OD550)/(OD600 culture × reaction time × volume)].
Purification of nucleic acids, blotting analysis, and primer extension.
RNA was isolated from cells grown under different conditions at specified optical densities, using a modified hot phenol extraction method (9). The RNA concentration was estimated by measuring the optical density at 260 and 280 nm in 0.1 N NaOH. Electrophoretic conditions, blotting, and labeling of probes were carried out essentially as previously described (9). The gadA/B probe was a 1.4-kb PCR-generated DNA fragment corresponding to the entire coding sequence of gadB. Membrane hybridization and washing were carried out as previously described (9). The gadX probe was the 832-bp fragment obtained from BamHI-HindIII digestion of plasmid pBsgadX. When using the gadX probe, membrane prehybridization was as follows: 42°C in 50% formamide-6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-5× Denhardt's solution-0.5% sodium dodecyl sulfate (SDS)-100 μg of denatured herring sperm DNA ml−1. After 20 h of hybridization, the membranes were washed at room temperature twice in 2× SSC-0.05% SDS for a total of 30 min and twice at higher stringency in 1× SSC-0.1% SDS for a total of 20 min. Northern blot filters were exposed to Kodak X-Omat UV autoradiographic films for a period varying from a few hours to 10 days, depending on the strain under investigation.
Primer extension analysis was performed with a 21-mer complementary to the region spanning from nucleotides +101 to +121 relative to the gadX transcription start point, and using RNA extracted from E. coli ATCC 11246 grown to the stationary phase in LB-MES, pH 5.5. The entire procedure was essentially as previously described (9).
Genetic procedures.
For construction of the gadX knockout, a 255-bp NcoI-HinfI fragment, obtained from pTgadX and encompassing codons 21 to 105 of gadX, was treated with Klenow polymerase to fill recessed ends and blunt-end ligated in the multicloning site of the 6.2-kb mobilizable suicide vector pJP5608 (28). This construct, designated pJPgadX′, was conjugated from E. coli S17.1 (λpir) to wild-type E. coli ATCC 11246. Exconjugants were selected for the tetracycline resistance marker carried by the suicide plasmid. Nine exconjugants were obtained, all carrying site-specific insertion of the suicide construct into gadX, as confirmed by Southern blot hybridization analysis of the ClaI-digested chromosomal DNA (9).
Purification of MalE-GadX fusion protein.
Overnight cultures of E. coli JM109(pmalE::gadX) were diluted 100-fold in 125 ml of fresh LB medium containing 0.2% glucose and 100 μg of ampicillin per ml and incubated at 37°C with aeration. When the culture density reached an OD600 of ≈0.5, the expression of MalE-GadX was induced by addition of 0.3 mM IPTG, and incubation was continued for an additional 4 h at 37°C. Cells were harvested by centrifugation at 4,100 × g in a Sorvall GSA rotor at 4°C for 30 min and resuspended in 10 ml of T200 buffer (20 mM Tris-HCl [pH 7.5], 2 mM EDTA, and 200 mM NaCl). Resuspended cells were disrupted by sonication on ice and centrifuged at 8,000 × g in a Sorvall SS-34 rotor at 4°C for 20 min to remove cell debris. The supernatant was loaded at a flow rate of 15 ml/h onto a 5-ml column of amylose resin (New England Biolabs) preequilibrated in T200 buffer at 4°C. After loading, the column was washed with T200 buffer at the same flow rate until the protein content of the flowthrough returned to baseline (60 ml of T200 buffer).
Bound proteins were eluted with 15 ml of T200 buffer containing 10 mM maltose. Of the 12 fractions (1 ml each) collected, four (3 to 6) contained the highest concentration of MalE-GadX, as judged by SDS-polyacrylamide gel electrophoresis (PAGE) analysis. These fractions were pooled, dialyzed against T100 buffer (20 mM Tris-HCl [pH 7.5], 2 mM EDTA, and 100 mM NaCl) and loaded at a flow rate of 15 ml/h onto a 5-ml heparin-agarose column preequilibrated at 4°C in T100 buffer. After loading, the column was washed with T100 buffer at the same flow rate until the protein content of the flowthrough returned to baseline (45 ml of T100 buffer). The bound MalE-GadX protein was eluted with a 40-ml linear gradient from 100 to 600 mM NaCl in T buffer (20 mM Tris-HCl [pH 7.5], 2 mM EDTA) and collected in 20 fractions of 2 ml each. MalE-GadX-containing fractions were pooled, concentrated by passage through a Centricon-30 ultrafiltration unit (Millipore), and stored at 4°C.
Electrophoretic mobility shift assay and DNase I footprinting.
DNA mobility shift assays were conducted using the gadA, gadBC, and gadC promoter (P) regions encompassing positions −176 to +77 (PgadA), −173 to +77 (PgadBC), and −120 to +142 (PgadC), relative to the transcription start sites of the individual genes (9). PgadA and PgadBC were transferred from the corresponding promoter-probe constructs in pRS415 (9) into the EcoRI and BamHI sites of pBs to yield pBsPgadA and pBsPgadBC, respectively. PgadC was excised by PstI-ClaI digestion of pBsB (10; this work) and cloned into the corresponding sites of pBs, yielding pBsPgadC. PgadA and PgadBC fragments were generated by EcoRI-BamHI digestion from pBsPgadA and pBsPgadBC, respectively, while PgadC was obtained by PstI-SalI digestion from pBsPgadC. This latter digestion was necessary to make possible terminal labeling of the PgadC fragment. The 5′ protruding ends of PgadA, PgadBC, and PgadC fragments were filled in with Klenow DNA polymerase (Roche) and [α-32P]dATP, according to standard protocols (2).
Radiolabeled DNA fragments (10 fmol) were incubated with increasing concentrations of MalE-GadX (from 0 to 10 pmol) at 22°C for 30 min in 20 μl of binding buffer (10 mM Tris-HCl [pH 8.0], 50 mM KCl, 0.5 mM EDTA, 6% glycerol, 200 μg of bovine serum albumin [BSA] per ml, and 100 ng of poly[dI-dC] per μl). Samples were loaded onto 5% nondenaturing acrylamide gels in 0.5× TAE (Tris-acetate-EDTA) buffer. Gels were run at room temperature in 0.5× TAE buffer for a total of 4 h at 10 V/cm and immediately exposed to X-ray films.
For DNase I footprinting experiments, 5 ng of PCR-generated PgadA and PgadB (9) were incubated for 20 min at 25°C with the indicated amounts of purified MalE-GadX in 30 μl of a mixture containing 40 mM HEPES (pH 8.0), 100 mM KCl, 10 mM magnesium acetate, and 0.5 mM dithiothreitol (DTT). After DNase I treatment, the partial digestion products were ethanol precipitated and subjected to 30 cycles of asymmetric PCR using the 5′-32P-end labeled primers, essentially as previously described (12). Primers were gadAfrw (5′-GGGAATTCATCGCCCGAACAGGAATG-3′) and gadBfrw (5′-GGGAATTCAATAACAACACAACACAC-3′ for the gadA and gadB promoter regions, respectively, and gadABrev (5′-GGGGATCCCGTGAATCGAGTAGTTC-3′) for both. The PCR-generated fragments were separated on a 7% sequencing gel.
Immunoblot analysis and Gad activity assay.
Aliquots of 2.5 μg of protein from the soluble fraction of cell lysates were used for Western blot analysis. Samples were run on SDS-10% PAGE and directly electroblotted onto polyvinylidene difluoride membranes (Immobilon-P, Millipore). Both GadA and GadB were detected with affinity-purified anti-GadA rabbit polyclonal antibodies (9) and horseradish peroxidase-labeled secondary antibody provided with the BM chemiluminescence Western blotting kit (Roche).
Glutamate decarboxylase activity assays and GABA measurements were performed as previously described (9).
RESULTS
Identification of a regulatory gene of the gad system downstream of gadA.
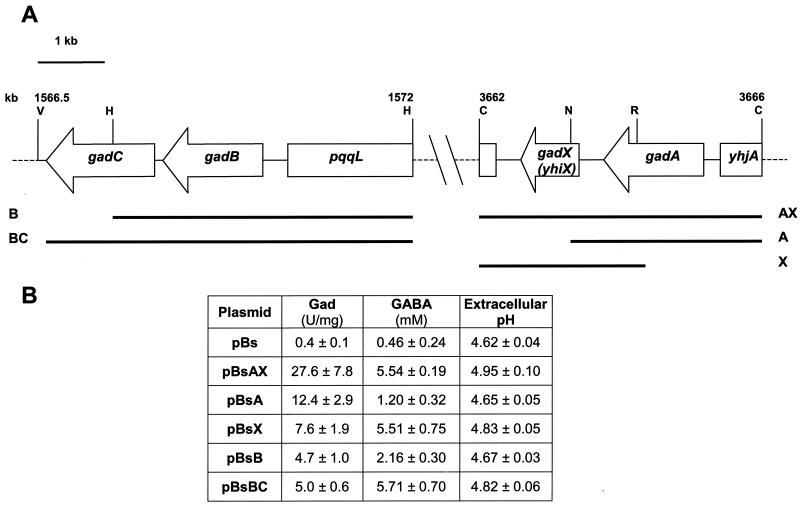
The physical organization of the E. coli ATCC 11246 genomic fragments previously used to clone the entire gadBC operon and the gadA gene (10) is shown in Fig. 1A. By homology with the E. coli K-12 genome sequence (4), the 4.1-kb ClaI genomic fragment encompassing the gadA gene was later recognized to include the entire gadX (yhiX) gene as well. The gadX gene of E. coli ATCC 11246 is predicted to encode a protein of 274 amino acids with an estimated molecular mass of 31,565 Da and a pI of 8.94.
FIG. 1.
(A) Chromosomal organization of the gad gene system in E. coli. Black bars identify the chromosomal regions of E. coli ATCC 11246 cloned in the pBluscript (pBs) vector and containing the specified genes: A (gadA), B (gadB), BC (gadB and gadC), AX (gadA and gadX), and X (gadX). Numbering (kilobases) is relative to the E. coli K-12 map (4). Restriction sites used for cloning the individual chromosomal fragments are indicated: V, EcoRV; H, HindIII; C, ClaI; N, NcoI; R, EcoRI. (B) Intracellular Gad activity, GABA levels, and pHs of the spent medium are given for E. coli JM109 carrying the individual constructs. The reported values are relative to those in 24-h cultures in LBG medium (pH 5.0) and represent the means (± standard deviations) of at least five determinations.
BLASTP analysis (1) disclosed a substantial similarity between the GadX protein and a number of transcriptional regulators belonging to the AraC/XylS family. The GadX variants from E. coli K-12 (4) and E. coli serotypes O157:H7 (29) and O126:H6 (30) were aligned with GadX from ATCC 11246. A total of 24 amino acid substitutions, mostly conservative, were detected, none of which occurred within the two helix-turn-helix motifs that represent the DNA-binding signature for AraC/XylS family proteins (data not shown).
Preliminary evidence for the role of gadX in the positive regulation of the gadA and gadBC system was provided by transactivation experiments conducted in E. coli JM109. This K-12 strain expresses very low glutamate decarboxylase levels (10) even under inducing conditions, i.e., after 24 h of growth in LBG medium, pH 5.0. Figure 1B shows the glutamate decarboxylase (Gad) activity, the concentration of released GABA, and the pH of the spent medium in E. coli JM109 carrying the cloned genomic fragments (Fig. 1A). The multicopy pBsAX plasmid (gadA-gadX) directed extremely high Gad and GABA release activities compared with the control vector (pBs). Excision from plasmid pBsAX of the NcoI-ClaI region downstream of gadA yielded plasmid pBsA. E. coli JM109(pBsA) expressed 2.2-fold less decarboxylase activity than pBsAX transformants and showed an even more pronounced reduction of the amount of released GABA (4.6-fold decrease). On the other hand, deletion of the whole gadA gene, as in plasmid pBsX, caused a substantial decrease in the decarboxylase activity (3.6-fold) without affecting the amount of exported GABA, which equaled that observed for E. coli transformants carrying pBsAX. More interestingly, the GABA export activity in the presence of the multicopy gadX gene (pBsAX and pBsX) equaled that observed for the multicopy decarboxylase/antiporter gadBC system (pBsBC). Gad activity and extracellular pH were similar in pBsX and pBsBC transformants, indicating that the gadX gene is involved in transactivation of the gad system.
gadX gene product is a transcriptional activator of gad structural genes.
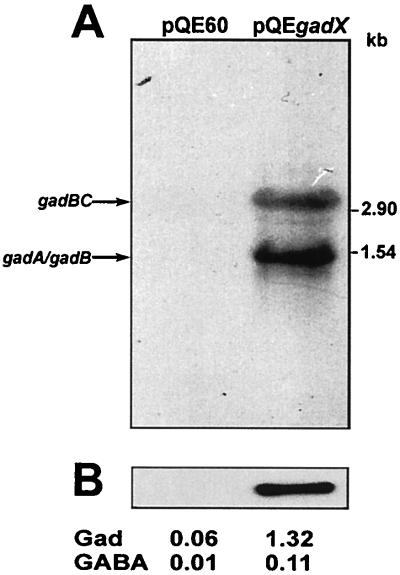
To correlate GadX expression with the upregulation of gad structural genes, we expressed the gadX gene under the control of the heterologous T5-lacO promoter-operator element and monitored transactivation of gad structural genes under conditions in which the gad genes are not expressed, i.e., during exponential growth in LB medium at pH 7.4. Expression of the gad system was undetectable in exponential cultures of E. coli JM109 carrying the control vector pQE60, while substantial gadA and gadBC transcription and increased glutamate decarboxylase and GABA export activities were observed when the gadX gene was provided in trans under the control of the exogenous promoter, as in pQEgadX (Fig. 2A and B). Due to leakage of the T5-lacO hybrid promoter, expression of the gad system was detectable even without induction, while addition of 0.1 mM IPTG was detrimental, probably due to a toxic effect resulting from excessive expression of the GadX activator protein.
FIG. 2.
GadX-dependent activation of gad genes. (A) Northern hybridization analysis of gad mRNAs extracted from E. coli JM109 carrying the pQE60 vector and the expression construct pQEgadX. Cells were grown at 37°C in neutral LB medium to the mid-exponential phase (OD600 ≈ 0.8). Aliquots of 10 μg of total RNA were electrophoresed, transferred onto nylon filters, and hybridized with the gadA/B probe. Sizes of RNA standards are given on the right. (B) Immunoblot analysis of GadA and GadB expression in whole bacterial lysates (2.5 μg of protein) probed with anti-GadA/B polyclonal antibodies. The decarboxylase activity (Gad, in units per milligram) in the cell lysates and the GABA levels (millimolar) in the growth medium are given for each condition.
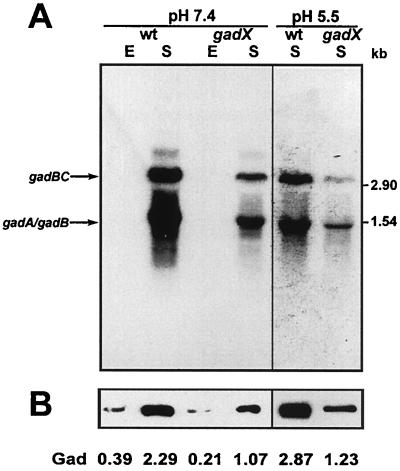
Evidence for the involvement of gadX in the positive control of gad genes in E. coli ATCC 11246 was provided by the analysis of gadA and gadBC expression in a gadX-defective background. For this purpose, a gadX site-specific mutant was generated by inserting the suicide plasmid pJPgadX′ within the gadX coding sequence of E. coli ATCC 11246. The gadX mutant, designated DDBGX, was compared with the parental strain for expression of gad structural genes under different growth conditions. During the stationary phase at neutral pH, gadA and gadBC transcripts were approximately fivefold less abundant in DDBGX (gadX) compared with the wild-type strain (Fig. 3A, left panel). A more pronounced reduction of transcript levels (eightfold) was observed under inducing conditions, i.e., during the stationary phase in acidic (pH 5.5) buffered LB-MES medium (Fig. 3A, right panel). This effect was also detectable, though to a lesser extent, at the level of protein expression and activity, as shown by immunoblot analysis of GadA/B expression in crude cell lysates and by direct measurements of glutamate decarboxylase activity in E. coli ATCC 11246 and in the isogenic gadX mutant (Fig. 3B). Thus, while the gadX gene product is dispensable for basal gadA and gadBC transcription, its presence is required for maximum expression of the gad gene system in E. coli ATCC 11246.
FIG. 3.
Effect of gadX mutation on the expression of gad structural genes and on glutamic acid decarboxylase and GABA export activities. (A) Northern blot analysis of total RNA extracted from E. coli strains ATCC 11246 (wild type, wt) and DDBGX (gadX) grown at 37°C in neutral LB medium (pH 7.4) or in mildly acidic LB-MES medium (pH 5.5). E, exponential-phase cultures (OD600 ≈ 0.5); S, stationary-phase cultures (OD600 ≈ 2.0). Aliquots of 10 μg of total RNA were electrophoresed, transferred onto nylon filters, and hybridized with the gadA/B probe (left panel, 1-day exposure; right panel, 6-h exposure). Sizes of RNA standards are given on the right. (B) Immunoblot analysis of GadA and GadB expression in whole bacterial lysates (2.5 μg of protein) probed with anti-GadA/B polyclonal antibodies. The decarboxylase activity (Gad, in units per milligram) in the cell lysate is given for each condition.
Mapping and characterization of gadX promoter region.
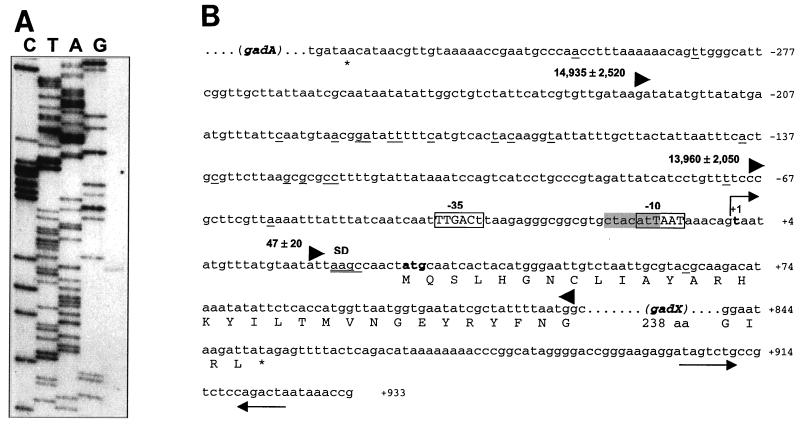
As a premise to the investigation of gadX transcriptional control, primer extension analysis was conducted using total RNA extracted from E. coli ATCC 11246 grown to the stationary phase in LB-MES at pH 5.5. Results demonstrate that transcription of gadX originates from the T residue located 29 nucleotides upstream of the ATG start codon (Fig. 4). The −35 and −10 hexamers (TTGACT-N19-ATTAAT) match 5 of 6 and 4 of 6 nucleotides, respectively, of the recognition site for RpoD-dependent RNA polymerase. The −10 hexamer overlaps the sequence 5′-CTACATT-3′, which matches 5 of 7 nucleotides of the RpoS consensus and is preceded by a potentially bent DNA region, as expected for RpoS-dependent promoters (11).
FIG. 4.
Promoter mapping and location of the transcription start point of gadX. (A) Mapping of the 5′ end of the gadX transcript by primer extension. RNA was extracted from stationary-phase cells grown at pH 5.5 and retrotranscribed after priming with a 5′-end-labeled oligonucleotide. Lanes C, T, A, and G are sequencing ladders of pBsAX with the same oligonucleotide used for the primer extension reaction. Sequencing reactions were run in parallel with the cDNA transcript (right lane) to determine exactly the 5′ end of the gadX message. (B) Sequence analysis of the gadX promoter region in E. coli ATCC 11246. The bent arrow indicates the transcriptional start site at the residue in bold, defined as +1. Differences from the E. coli RpoD consensus sequences for the −10 and −35 promoter elements are shown as lowercase letters within the boxed regions. The RpoS consensus is shaded in gray. Nucleotide sequence variations between ATCC 11246 and the K-12 strain MG1655 (4) are underlined. The potential Shine-Dalgarno (SD) sequence is double underlined. The triangles define the 5′-to-3′ boundaries of the DNA fragments tested for the ability to direct lacZ expression in pRS415 (32). The number preceding each forward-pointing triangle is the β-galactosidase activity value (in Miller units) expressed by exponential-phase cultures of E. coli MC4100 carrying the cloned promoter fragment. Sequence numbering is relative to the gadX transcription start point.
Evidence for the existence of an indigenous gadX promoter was also inferred by the analysis of reporter gene activity directed by gadX::lacZ transcriptional fusions in the promoter probe vector pRS415 (32). Strong promoter activity was observed for the DNA fragment encompassing nucleotides −67 to +121 relative to the gadX transcription start point. This region is likely to encompass the minimal gadX promoter element, since inclusion of the additional 155-nucleotide upstream sequence (−222 to +121 construct) did not significantly alter the reporter gene expression, while an 85-nucleotide deletion (+18 to +121 construct) totally abrogated promoter activity (Fig. 4B).
Transcription of gadX gene is activated during stationary phase and responds to acidic shock.
As a specific activator of the gad gene system, gadX can be predicted to respond positively to stimuli which are known to induce expression of the gadA and gadBC genes. However, a gadX::lacZ promoter fusion from enteropathogenic E. coli was found not to be controlled by growth phase variation in LB medium, though it responded positively to acid induction (30).
To address the issue of gadX transcriptional control, total RNA was extracted from E. coli ATCC 11246 and MC4100 cells grown to exponential or stationary phase either in standard LB medium (pH 7.4) or in acid-buffered LB-MES medium (pH 5.5). Figure 5 shows that the gadX probe detected two mRNA species with apparent sizes of 1.0 and 2.7 kb. The size of the shorter transcript fits well with that predicted for a monocistronic gadX mRNA ending at the level of the stem-loop structure centered at position + 915 relative to the gadX transcription start point (Fig. 4B). The 2.7-kb mRNA species, accounting for nearly 10% of the total gadX-specific messages, is likely to be a dicistronic gadAX transcript originating from the gadA promoter, approximately 1.8 kb upstream of gadX. In fact, a similar-sized mRNA species was previously detected by autoradiography following hybridization with the gadA/B probe (9).
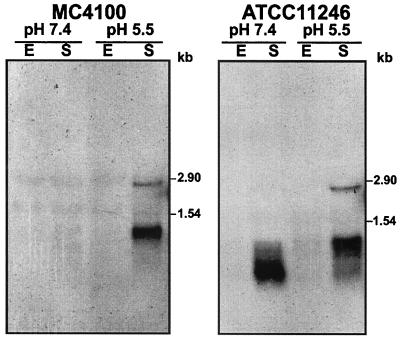
FIG. 5.
Analysis of gadX transcripts during the growth cycle and under acidic conditions. Total RNA was extracted from E. coli strains MC4100 (left) and ATCC 11246 (right) grown at 37°C in neutral LB medium (pH 7.4) or in mildly acidic LB-MES medium (pH 5.5). E, exponential-phase cultures (OD600 ≈ 0.5); S, stationary-phase cultures (OD600 ≈ 2.0). Aliquots of 10 μg of total RNA were electrophoresed, transferred onto nylon filters, and hybridized with the gadX probe. Sizes of RNA standards are given on the right of each panel.
Both gadX messages were detectable only after entry into the stationary phase, but their relative amounts varied depending on the acidity of the medium. In fact, gadX mRNAs were clearly detectable in stationary-phase cultures at pH 5.5, while only the shorter transcript was detected in stationary-phase cultures at pH 7.4 and appeared to undergo rapid degradation (Fig. 5). Thus, the transcription profile of gadX appears to be growth phase dependent and coordinated with that of the gadA and gadBC genes (9), though transcript levels differ quantitatively between the low Gad producer E. coli MC4100 and the overproducing wild-type strain ATCC 11246.
To address the issue of whether gadX is responsible for transcriptional activation at its own promoter (PgadX), the activity of PgadX::lacZ transcriptional fusions (nucleotides −222 to +121) was tested in E. coli carrying in trans the multicopy gadX gene under the control of its indigenous promoter. It was observed that PgadX was equally active both in the presence and in the absence of multicopy gadX, irrespective of the growth stage of the culture (data not shown). It can therefore be argued that GadX is not involved in activation of transcription arising from PgadX.
gadX gene is under transcriptional control of H-NS and RpoS.
The gadX gene has recently been identified as an H-NS-repressible gene (17), consistent with the previous observation that H-NS acts as a negative regulator of the gad structural genes during the exponential phase (9, 38). Moreover, we and others have also linked stationary-phase transcription of gadA and gadBC to the activity of the RpoS sigma factor (6, 9). Given that gadX expression is also stationary-phase dependent, we investigated the involvement of both H-NS and RpoS in the transcriptional control of gadX. The amount of gadX transcripts was therefore compared in wild-type E. coli K-12 and in hns, rpoS, and hns rpoS mutants.
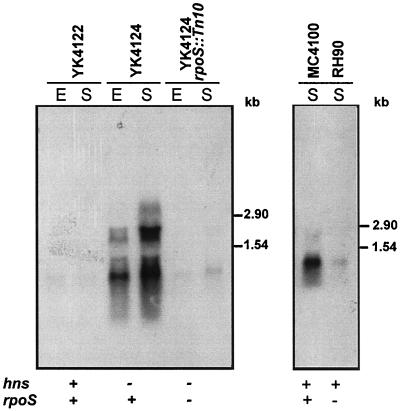
Figure 6 shows that repression of gadX is relieved in the hns-defective background, resulting in increased transcription of both gadX and gadAX mRNAs even during the exponential phase. This effect correlates with the upregulation of both the gadA and gadBC genes, whose transcription was found to occur during the exponential phase in the hns mutant (9). Moreover, RpoS primarily affects gadX expression, since the rpoS mutation strongly reduced gadX transcription irrespective of the hns background. Also in this case, the pattern of gadX regulation parallels that of the gadA and gadBC genes (9), indicating that H-NS and RpoS act as master regulators of the whole gad gene system.
FIG. 6.
Effect of hns and rpoS mutations on transcription of the gadX gene. Northern hybridization analysis of total RNA (10 μg in each lane) extracted from the wild-type E. coli strain YK4122 and from its hns (YK4124) and hns rpoS (YK4124 rpoS::Tn 10) mutants (left panel) and from wild-type E. coli strain MC4100 and its rpoS mutant (RH90) (right panel). Bacteria were grown at 37°C in neutral LB medium to the exponential (E) or stationary (S) phase. Nylon filters were hybridized with the gadX probe. Sizes of RNA standards are given on the right of each panel.
GadX binding to gadA and gadBC promoters produces multiple protein-DNA complexes.
As a member of the AraC protein family, GadX is expected to bind specific sequence motifs located within or in the proximity of the target promoters. On this assumption, we generated a recombinant derivative of GadX and tested the in vitro binding of this protein to the promoter region of the gadA, gadBC, and gadC genes. To simplify the purification protocol of GadX and to overcome problems deriving from the poor solubility of AraC/XylS family proteins (15), a soluble MalE-GadX fusion was used for in vitro studies. Chimeric MalE-GadX was found to activate the gad system in vivo, although to a much lesser extent than the wild-type GadX protein, as expressed from pQEgadX and pBsX.
The pmalE::gadX construct was generated by cloning the gadX coding sequence in frame with the 3′ terminus of the malE gene in the pMAL-c2 expression vector. In doing so, we expected the C-terminal portion of GadX not to be affected by physical hindrance of fused MalE, thereby retaining the helix-turn-helix domains competent for interaction with target DNA. The pmalE::gadX construct was introduced in E. coli JM109 and used to overexpress the fusion protein following IPTG induction. SDS-PAGE analysis of crude cell extracts revealed that a 73-kDa protein was expressed upon induction, its mass being consistent with the expected size for the MalE-GadX fusion protein (42 kDa for MalE and 31 kDa for GadX; data not shown).
Given that more than 90% of the fusion protein was recovered from the soluble fraction of the induced cell lysate, this was used as the starting material for affinity purification on an amylose column, followed by chromatography onto a heparin-agarose column to remove residual protein contaminants. The purification protocol yielded approximately 95% pure MalE-GadX, as judged by SDS-PAGE. An attempt was made to remove the MalE moiety from the fusion protein by factor Xa proteolysis, but the results so far achieved indicate that GadX tends to precipitate in aqueous solution after MalE cleavage (data not shown). This feature, which has previously been reported for a number of proteins of the AraC/XylS family (13, 26, 33), led us to use the soluble MalE-GadX fusion protein for preliminary in vitro DNA-binding studies.
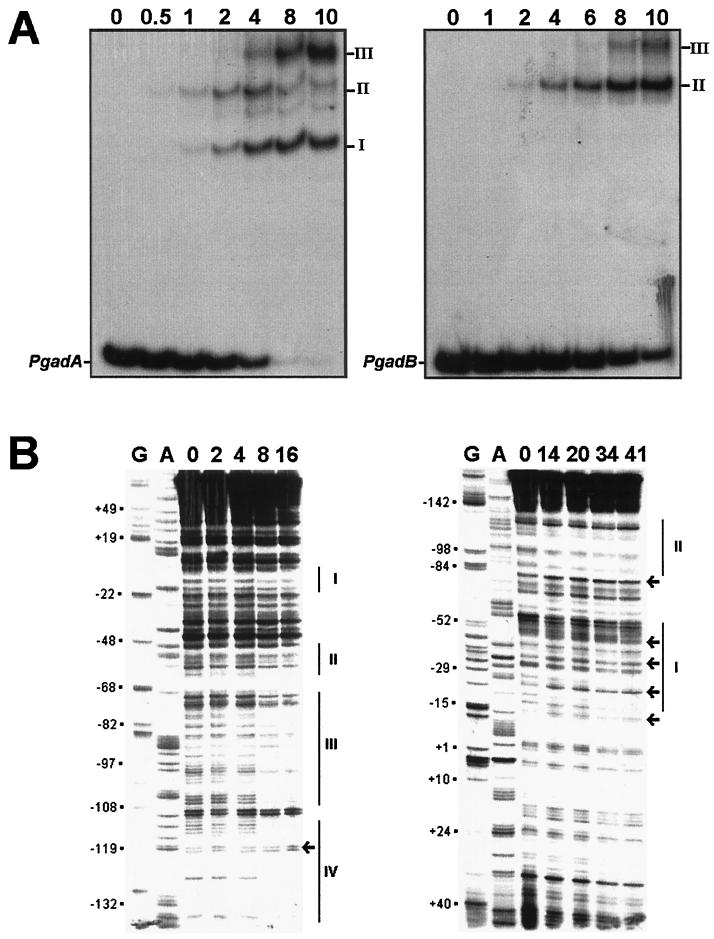
DNA probes encompassing the gadA, gadBC, and gadC promoter regions (see Materials and Methods) were used in protein-binding assays with increasing amounts of the MalE-GadX protein in the presence of a 500-fold molar excess of poly(dI-dC) as the competitor (Fig. 7A). Addition of up to 2 pmol of protein to the PgadA probe (10 fmol) produced two equally represented DNA-protein complexes with reduced mobility (forms I and II; Fig. 7A, left panel). At higher MalE-GadX concentrations, form II gradually decreased, giving way to a maximally shifted species (form III). A minor species of intermediate mobility between forms I and II also appeared. Then, at more than 8 pmol of MalE-GadX, the whole probe was shifted, prevalently generating forms I and III.
FIG. 7.
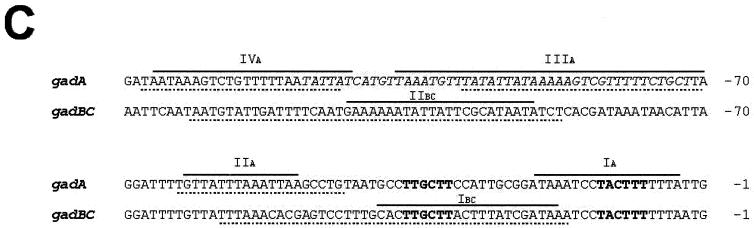
Identification of MalE-GadX binding sites in gadA and gadBC promoters. (A) Gel retardation assays of in vitro binding of the purified MalE-GadX protein to the promoter regions of gadA (PgadA, left) and gadBC (PgadB, right) genes. The DNA fragments were labeled with [α-32P]dATP by fill-in of 5′ protruding ends. In each binding reaction, 10 fmol of the DNA probe was incubated in a 10-μl volume with increasing amounts (0.5 to 10 pmol) of the MalE-GadX protein, under the conditions described in Materials and Methods. MalE-GadX-bound DNA fragments (forms I, II, and III) were separated from the unbound probe on a 5% polyacrylamide gel run in 0.5× TAE buffer. (B) DNase I footprinting assays. The 265-bp DNA fragments carrying the promoter regions of gadA (left) and gadBC (right) were incubated with the indicated amounts (picomoles) of MalE-GadX. Samples were processed as described in Materials and Methods using gadAfrw (left panel) and gadABrev (right panel) as the primers. Lanes G and A represent TaqI polymerase sequencing reactions using the same primers. The MalE-GadX-protected sites are indicated with vertical lines and labeled with roman numbers from I to IV. Arrows indicate DNase I-hypersensitive sites. (C) Sequence alignment of gadA and gadBC promoter regions showing the DNase I-protected sites on the coding (full line) and noncoding (dotted line) DNA strands. Sites are indicated above the corresponding sequence. The −35 and −10 hexamers for both gadA and gadBC are shown in bold type.
A less complex pattern of MalE-GadX binding was observed with the PgadB probe (Fig. 7A, right panel), with the generation of only two forms (II and III). In this case, form II appeared to be prevalent, but the binding affinity of the promoter was apparently lower than that of PgadA, as indicated by the fact that a substantial amount of the PgadB remained unbound in the presence of 10 pmol of MalE-GadX. Under the same experimental conditions, the mobility of the PgadC promoter was not affected by the addition of up to 10 pmol of MalE-GadX, and a shift of PgadA and PgadB was not observed after replacement of the fusion protein with an equimolar amount of the MalE protein (data not shown).
The observation that MalE-GadX specifically interacts with the promoter sequences of the gadA and gadBC genes, but not with the intergenic region between gadB and gadC, prompted us to map the binding sites of GadX on gadA and gadB promoters by DNase I footprinting assays. Four MalE-GadX binding sites were identified on PgadA and two on PgadB (Fig. 7B). MalE-GadX extensively protected the gadA promoter region (left panel) at positions −4 to −20 (site I), −49 to −62 (site II), −70 to −107 (site III), and −112 to −135 (site IV), relative to the transcription start site. The protected sites have an average length of approximately 22 nucleotides and show, within the limit of accuracy of our analysis, different affinities for MalE-GadX. Almost complete DNase I protection occurred at sites III and IV upon the addition of 8 pmol of MalE-GadX, while a twofold increase in the amount of the protein (16 pmol) was required for binding at sites I and II.
As expected from the gel retardation assay, MalE-GadX also bound the gadB promoter region, but only at two sites and with a lower affinity compared with the gadA promoter region (Fig. 7B, right panel). MalE-GadX preferentially binds to the distal upstream promoter region of gadB, as inferred by the protection exerted by 14 pmol of the protein on an extended region spanning from positions −87 to −131 (site II). Upon addition of more than 30 pmol of MalE-GadX, another site extending from positions −17 to −58 (site I) became protected on PgadB.
DISCUSSION
In this work we present evidence for the direct involvement of the GadX protein in transcriptional activation of the gad gene system. GadX belongs to the AraC/XylS family of bacterial transcriptional regulators, known to be activators of functions as diverse as sugar catabolism, responses to stress, and virulence (15, 23). The positive regulatory role of GadX was inferred both by transactivation assays of gad structural genes under physiological and nonphysiological conditions and by reduced transcription of gadA and gadBC in a gadX mutant. Differences in gadA and gadBC expression levels under the above conditions were confirmed by the direct measurement of glutamic acid decarboxylase (GadA/B) and GABA export (GadC) activities. Expression analysis of the gad gene system in the gadX mutant indicates that GadX is required for maximal expression during the stationary phase and acid induction, though it is not essential for basal expression of the system.
Given that expression of gad structural genes is silenced during the exponential phase in neutral rich (LB) medium, it was possible to monitor GadX activity only when the culture had entered the stationary phase. At this growth stage in neutral LB medium, gadA and gadBC are transcribed exclusively by RpoS (9, 7), and their reduced expression in the gadX mutant points to an interplay between GadX and RpoS. This is not surprising, inasmuch as other proteins of the AraC/XylS family have been shown to activate transcription directed by alternative sigma factors (21). Our data also suggest that RpoS is not the only sigma factor involved in GadX-mediated activation of the gad gene system. In fact, GadX-mediated recruitment of vegetative (RpoD-dependent) RNA polymerase at the gadA and gadBC promoters could explain the observation that expression of gadX under an exogenous promoter suffices for transactivation of the gad gene system during the exponential phase in neutral rich (LB) medium. Accordingly, gadX-dependent activation of gad structural genes was also observed in an rpoS background (A. Tramonti and D. De Biase, unpublished data). Moreover, an involvement of GadX in the RpoS-independent, acid-responsive pathway of induction of the gad genes (7) is inferred by the evidence that stationary-phase expression of gadA and gadBC genes in the gadX mutant is more drastically reduced at acidic than at neutral pH. However, the observation that transcription of gadA and gadBC is only partially abrogated in the gadX mutant raises the possibility that additional positive regulators are also involved in the transcriptional tuning of the gad system, as found for other genes controlled by transcriptional regulators of the AraC/XylS family (22, 25).
As an activator of the gad system, gadX was expected to respond to one or more of the stimuli known to trigger expression of the gad structural genes. As far as stationary phase and acid induction are concerned, gadX expression paralleled that of the gad structural genes, being stationary phase dependent and acid inducible. Conversely, expression of gadX during the stationary phase was not affected by exposure of cells to hyper- and hypo-osmotic environments. The expression of gadX was found to be primarily dependent on RpoS, and under our experimental conditions, gadX expression was not appreciable in wild-type E. coli during the exponential phase, even upon acidic induction. RpoS dependence is also supported by the observation that the gadX promoter is endowed with both sequence and topological features predicting recognition by RpoS (11).
We and others (17) have observed that H-NS acts as a repressor of gadX expression, and this could explain silencing of gadX during the exponential phase. Given the hierarchy of regulation of the whole gad system, a direct activity of H-NS on the gadX promoter can be envisaged, resulting in repression of the gad structural genes during the exponential phase. However, an antagonistic effect of GadX on H-NS repression, likely resulting from competition at the gadA and gadBC promoters, should also be taken into account. An additional level of repression of gad genes is provided by the cyclic AMP receptor protein (CRP), which was demonstrated to repress RpoS-dependent gad transcription during the exponential phase in rich (LB) medium (7). Since the region predicted to interact with CRP overlaps GadX binding sites II and III on PgadA (Fig. 7), displacement of the CRP repressor by the GadX activator could account, at least in part, for the upregulation of the system during the exponential phase.
Based on primer extension results and sequence analysis, the gadX-specific mRNA is predicted to be approximately 930 nucleotides in length. Accordingly, RNA blot experiments showed that an mRNA species with an apparent size of 1 kb was specifically recognized by the gadX probe. In addition to the 1-kb gadX transcript, a less abundant gadAX bicistronic transcript of approximately 2.7 kb was also detected. Thus, autoregulation of gadX expression can be expected to occur through the direct activity of GadX at the gadA promoter, leading to gadAX cotranscription. Such an autoregulatory loop would imply that expression of both GadA and GadX is coordinated and responsible for superinduction of the whole gad system; primary stimuli (stationary phase and acid) acting on PgadX would lead to increased expression of GadX and, in turn, to an amplification of the response through GadX-dependent activation of the gadAX and gadBC promoters.
Conclusive evidence for direct recognition of the gad promoters by GadX derives from electrophoretic mobility shift assays, which indicate that MalE-GadX specifically binds PgadA and PgadB and that binding occurs through the formation of several DNA-protein complexes. These forms were not detected in a preliminary analysis of MalE-GadX binding to the gadA and gadBC promoters (30). However, the complex profile of promoter recognition by MalE-GadX was confirmed herein by DNase I footprinting analysis. Binding was observed to occur with different affinity at four sites on PgadA and two on PgadB. The highest-affinity binding was detected at sites III and IV of PgadA, the former site encompassing at least 60% of the DNA element which was predicted to be pH responsive (9). Binding of MalE-GadX at sites III and/or IV of PgadA could account for the formation of the two predominant protein-DNA complexes (forms II and/or I) observed at the same protein concentration in the electrophoretic mobility shift assay (Fig. 7, left panels).
By doubling the amount of MalE-GadX, two additional protected sites (I and II) were detected on PgadA. Interestingly, this is also the concentration threshold at which transition from complex II to complex III occurs. Thus, while complex I is stably retained up to saturating concentrations of MalE-GadX, complexes II and III appear to be mutually exclusive. A plausible explanation for these observations is that, at low protein-DNA ratios, high-affinity binding of MalE-GadX either to site III or IV generates complex I (1:1 protein-DNA ratio), while binding to both sites results in complex II (2:1 protein-DNA ratio). At higher protein-DNA ratios, the coexistence of both forms I and III (Fig. 7, left panels) indicates that MalE-GadX can occupy either one or three of four sites at the same time.
The protection pattern of MalE-GadX on PgadB is different from and less puzzling than that observed for PgadA. Protection of site II from DNase I digestion occurred within the concentration range used to observe the formation of the DNA-protein complex II in the electrophoretic mobility shift assay (Fig. 7, right panels). The lower electrophoretic mobility of complex II compared with that of complex I in PgadA suggests that it originates from binding of two MalE-GadX molecules, and this would be in line with the stoichiometry predicted for complex II of PgadA. The amount of MalE-GadX required to protect site I on PgadB, however, can also reasonably explain the appearance of complex III in the gel mobility shift assay. In fact, site I is fully protected and complex III is formed when 1 μM MalE-GadX is used, a concentration which is far too high to be significant.
Thus, while both the gadA and gadB promoters are characterized by the presence of multiple sites of interaction with GadX, they differ significantly in the overall organization (number, position, size, and sequence) of binding sites, which might reflect relevant differences in the regulation of these genes by GadX. Preferential targeting of GadX to gadA was inferred from gel mobility shift and DNase I footprinting assays and by the observation that the multicopy gadA can titrate out the intracellular pool of the chromosomally encoded GadX activator. Careful analysis of the data shown in Fig. 1B indicates that introduction of multicopy gadA (pBsA) in E. coli results in exuberant glutamate decarboxylase activity (12.4 U/mg) but low GABA export (1.2 mM). This can be interpreted as the GadX-dependent expression of chromosomal gadBC being limited due to withholding of the available GadX activator by the multicopy gadA promoter sequence. Conversely, when gadB is expressed as a multicopy gene (pBsB), the glutamate decarboxylase activity is noticeably lower (4.7 U/mg), consistent with the lower affinity of GadX for the gadBC promoter, but the amount of exported GABA is increased (2.2 mM). This would mean that the intracellular GadX level is sufficient to activate transcription of the chromosomal copies of the gad structural genes, including gadC, resulting in increased expression of the GadC antiporter. The results obtained with the multicopy gadAX contruct (pBsAX) further corroborate this conclusion; in fact, they demonstrate that the titrating effect of the multicopy gadA promoter is relieved by virtue of GadX overexpression, and this results in high GadC activity (as inferred from GABA export), similar to what was observed with the multicopy gadX (pBsX) and gadBC (pBsBC) constructs.
Identification of the effectors involved in GadX activation and further investigation of the interactions between regulatory components of the gad system (H-NS, RpoS, CRP, and GadX) may provide further insight into the molecular circuitry underlying the glutamic acid-based acid stress response of E. coli.
Acknowledgments
A. Tramonti and P. Visca contributed equally to this work.
This work was supported by grants from the Istituto Pasteur Fondazione Cenci Bolognetti to D.D.B. and from the Italian Ministry of University and Scientific Research to P.V., M.F., and D.D.B.
We thank F. Bossa and D. Barra for helpful discussions and encouraging advice.
Footnotes
This manuscript is dedicated to the memory of Franco Tatò, prematurely deceased 7 July 2001.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. John Wiley and Sons, New York, N.Y.
- 3.Barth, M., C. Marschall, A. Muffler, D. Fischer, and R. Hengge-Aronis. 1995. Role of the histone-like protein H-NS in growth phase-dependent and osmotic regulation of σS and many σS-dependent genes in Escherichia coli. J. Bacteriol. 177:3455-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blattner, F. R., G. Plunkett, I. I. I., C. A. Bloch, N. T. Perna, V. Burland, M. Riley, et al. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 5.Casadaban, M. J., J. Chou, and S. N. Cohen. 1980. In vitro gene fusions that join an enzymatically active β-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J. Bacteriol. 143:971-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castanie-Cornet, M-P., T. A. Penfound, D. Smith, J. E. Elliott, and J. W. Foster. 1999. Control of acid resistance in Escherichia coli. J. Bacteriol. 181:3525-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castanie-Cornet, M-P., and J. W. Foster. 2001. Escherichia coli acid resistance: cAMP receptor protein and a 20 bp cis-acting sequence control pH and stationary phase expression of the gadA and gadBC glutamate decarboxylase genes. Microbiology 147:709-715. [DOI] [PubMed] [Google Scholar]
- 8.Cotter, P. D., C. G. M. Gahan, and C. Hill. 2001. A glutamate decarboxylase system protects Listeria monocytogenes in gastric fluid. Mol. Microbiol. 40:465-475. [DOI] [PubMed] [Google Scholar]
- 9.De Biase, D., A. Tramonti, F. Bossa, and P. Visca. 1999. The response to stationary-phase stress conditions in Escherichia coli: role and regulation of the glutamic acid decarboxylase system. Mol. Microbiol. 32:1198-1211. [DOI] [PubMed] [Google Scholar]
- 10.De Biase, D., A. Tramonti, R. A. John, and F. Bossa. 1996. Isolation, overexpression and biochemical characterization of the two isoforms of glutamic acid decarboxylase from Escherichia coli. Protein Expr. Purif. 8:430-438. [DOI] [PubMed] [Google Scholar]
- 11.Espinosa-Urgel, M., C. Chamizo, and A. Tormo. 1996. A consensus structure for sigmaS-dependent promoters. Mol. Microbiol 21:657-659. [DOI] [PubMed] [Google Scholar]
- 12.Falconi, M., B. Colonna, G. Prosseda, G. Micheli, and C. O. Gualerzi. 1998. Thermoregulation of Shigella and Escherichia coli EIEC pathogenicity. A temperature-dependent structural transition of DNA modulates accessibility of virF promoter to transcriptional repressor H-NS. EMBO J. 17:7033-7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fawcett, W. P., and R. E. Wolf. 1994. Purification of MalE-SoxS fusion protein and identification of control sites of Escherichia coli superoxide-inducible genes. Mol. Microbiol. 14:669-679. [DOI] [PubMed] [Google Scholar]
- 14.Foster, J. W. 2000. Microbial responses to acid stress, p. 99-115. In G. Storg and R. Hengge-Aronis (ed.) Bacterial stress responses. American Society for Microbiology, Washington, D.C.
- 15.Gallegos, M., R. Schleif, A. Bairoch, K. Hofmann, and J. L. Ramos. 1997. AraC/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 61:393-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hengge-Aronis, R. 1996. Back to log phase: sigma S as a global regulator in the osmotic control of gene expression in Escherichia coli. Mol. Microbiol. 21:887-893. [DOI] [PubMed] [Google Scholar]
- 17.Hommais, F., E. Krin, C. Laurent-Winter, O. Soutourina, A. Malpertuy, J.-P. Le Caer, A. Danchin, and P. Bertin. 2001. Large-scale monitoring of pleiotropic regulation of gene expression by the procaryotic nucleoid-associated protein, H-NS. Mol. Microbiol. 40:20-36. [DOI] [PubMed] [Google Scholar]
- 18.Lange, R., and R. Hengge-Aronis. 1991. Identification of a central regulator of stationary phase gene expression in Escherichia coli. Mol. Microbiol. 5:49-59. [DOI] [PubMed] [Google Scholar]
- 19.Lin, J., I. S. Lee, J. Frey, J. L. Slonczewski, and J. W. Foster. 1995. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J. Bacteriol. 177:4097-4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchuck, D., M. Drumm, A. Saulino, and F. S. Collins. 1990. Construction of T-vectors, a rapid and general system for direct cloning unmodified PCR products. Nucleic Acids Res. 19:1154.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marqués, S., M. Manzanera, M. M. Gonzalez-Pérez, M. T. Gallegos, and J. L. Ramos. 1999. The XylS-dependent promoter is transcribed in vivo by RNA polymerase with sigma 32 or sigma 38 depending on the growth phase. Mol. Microbiol. 31:1105-1113. [DOI] [PubMed] [Google Scholar]
- 22.Martin, R. G., W. K. Gillette, S. Rhee, and J. L. Rosner. 1999. Structural requirements for marbox function in transcriptional activation of mar/sox/rob regulon promoters in Escherichia coli: sequence, orientation and spatial relationship to the core promoter. Mol. Microbiol. 34:431-441. [DOI] [PubMed] [Google Scholar]
- 23.Martin, R. G., and J. L. Rosner. 2001. The AraC transcriptional activators. Curr. Opin. Microbiol. 4:132-137. [DOI] [PubMed] [Google Scholar]
- 24.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 25.Munson, G. P., L. G. Holcomb, and J. R. Scott. 2001. Novel group of virulence activators within the AraC family that are not restricted to upstream binding sites. Infect. Immun. 69:186-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munson, G. P., and J. R. Scott. 1999. Binding site recognition by Rns, a virulence regulator in the AraC family. J. Bacteriol. 181:2110-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Najjar, V. A., and J. Fisher. 1952. Glutamic acid decarboxylase from E. coli. Fed. Proc. 11:264. [Google Scholar]
- 28.Penfold, R. J., and J. M. Pemberton. 1992. An improved suicide vector for construction of chromosomal insertion mutations in bacteria. Gene 118:145-146. [DOI] [PubMed] [Google Scholar]
- 29.Perna, N. T., G. III Plunkett, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. Dimalanta, K. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 30.Shin, S., M.-P. Castanie-Cornet, J. W. Foster, J. A. Crawford, K. Brinkley, and J. B. Kaper. 2001. An activator of glutamate decarboxylase genes regulates the expression of enteropathogenic Escherichia coli virulence genes through control of the plasmid-encoded regulator, Per. Mol. Microbiol. 41:1133-1150. [DOI] [PubMed] [Google Scholar]
- 31.Simon, R., U. Priefer, and A. Phuler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-790. [Google Scholar]
- 32.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 33.Tobe, T., M. Yoshikawa, T. Mizuno, and C. Sasakawa. 1993. Transcriptional control of the invasion regulatory gene virB of Shigella flexneri: activation by virF and repression by H-NS. J. Bacteriol. 175:6142-6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tramonti, A., P. Visca, F. Bossa, and D. De Biase. 2000. Environmental stimuli and regulatory factors affecting the expression of the glutamic acid decarboxylase system in Escherichia coli, p. 41-46. In A. Iriarte, H. M. Kagan, and M. Martinez-Carrion (ed.), Biochemistry and molecular biology of vitamin B6 and PQQ dependent proteins. Birkhaüser Verlag, Basel, Switzerland.
- 35.Waterman, S. R., and P. L. C. Small. 1996. Identification of σs-dependent genes associated with the stationary-phase acid-resistance phenotype of Shigella flexneri. Mol. Microbiol. 21:925-940. [DOI] [PubMed] [Google Scholar]
- 36.Yamashino, T., C. Ueguchi, and T. Mizuno. 1995. Quantitative control of the stationary phase sigma factor, σS, in Escherichia coli: involvement of the nucleoid protein H-NS. EMBO J. 14:594-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yasuzawa, K., N. Hayashi, N. Goshima, K. Kohno, F. Imamoto, and Y. Kano. 1992. Histone-like proteins are required for cell growth constraint of super coils in DNA. Gene 122:9-15. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida, T., T. Yamashino, C. Ueguchi, and T. Mizuno. 1993. Expression of the Escherichia coli dimorphic glutamic acid decarboxylases is regulated by the nucleoid protein H-NS. Biosci. Biotechnol. Biochem. 57:1568-1569. [DOI] [PubMed] [Google Scholar]