Abstract
1. Responses of cat retinal ganglion cells have been examined with a view to specifying the characteristics that limit the detection of light stimuli.
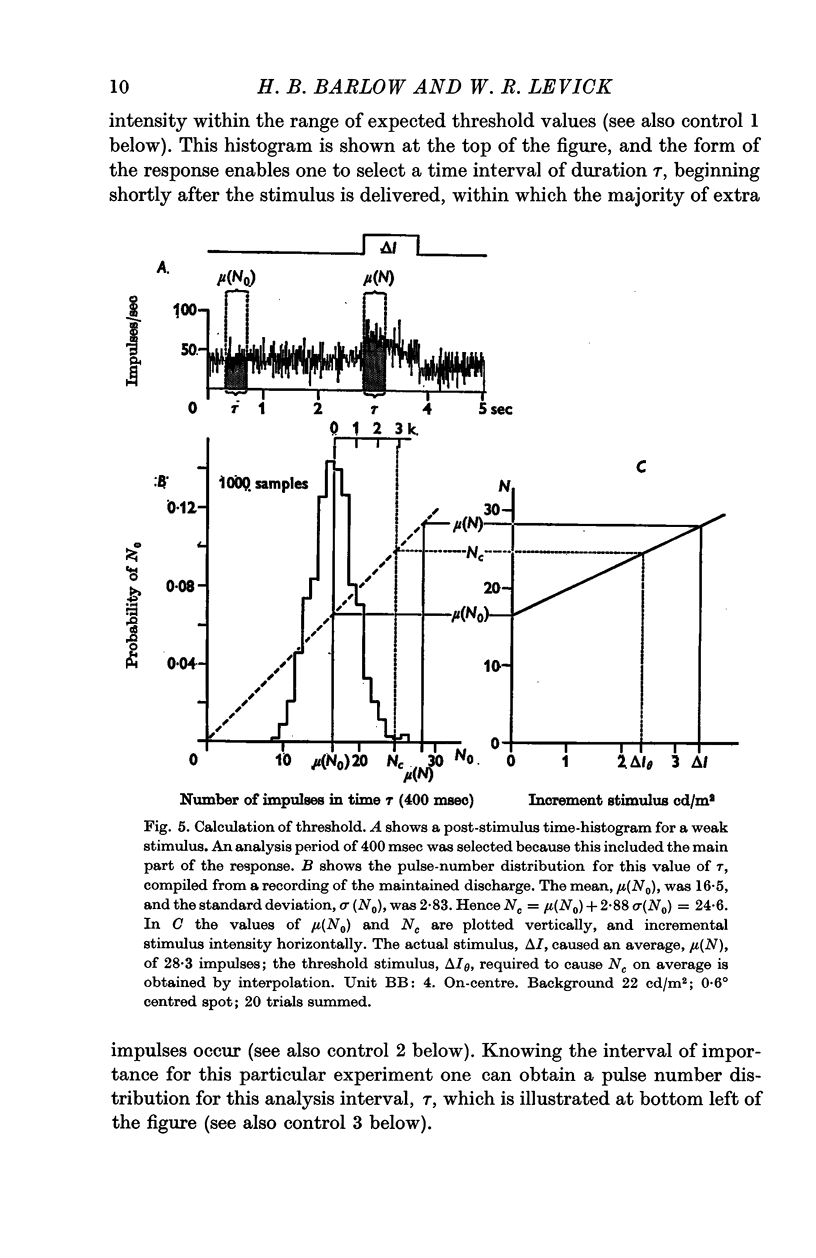
2. Threshold is defined as the weakest stimulus that can be reliably detected by examination of the output from a retinal ganglion cell; it depends upon (a) the quantum/spike ratio, which is the mean number of additional quantal absorptions required to produce an additional impulse, (b) the temporal course of the response, which determines the time interval within which the maintained discharge is modified, and (c) the statistical distribution of the number of impulses that occur in this time interval in the absence of the stimulus.
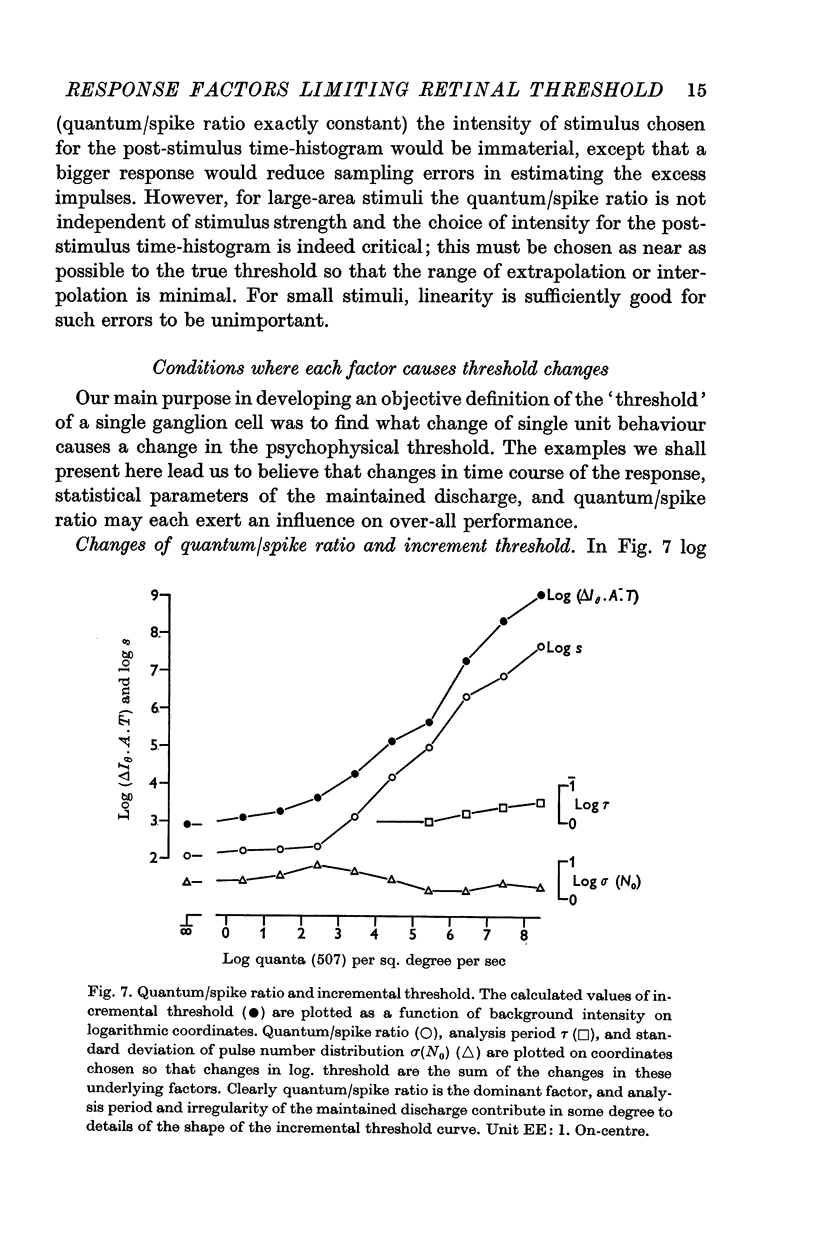
3. The quantum/spike ratio changes greatly when adapting luminance is changed, and this is the predominant factor accounting for changes in increment threshold.
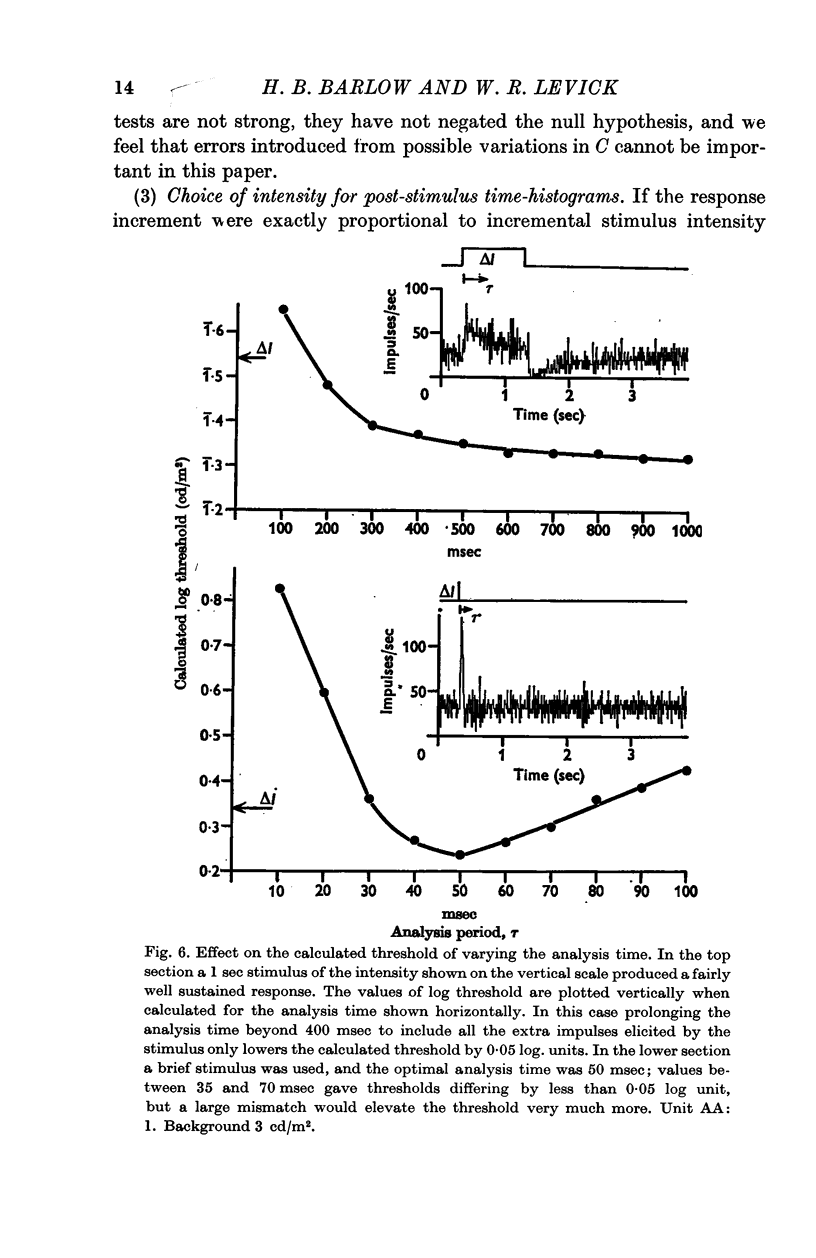
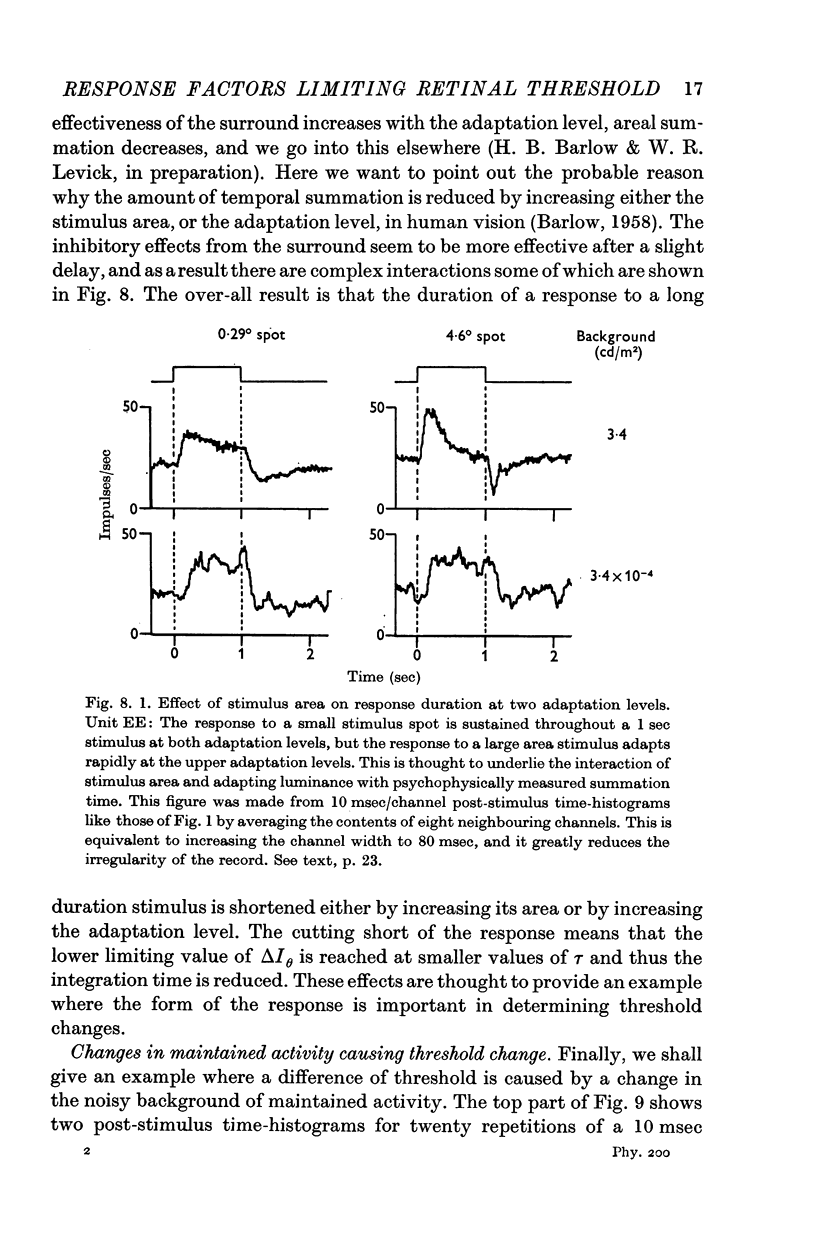
4. The time course of the response changes with adaptation level and area of the stimulus. This may account for the changes in temporal integration that occur in analogous psychophysical experiments.
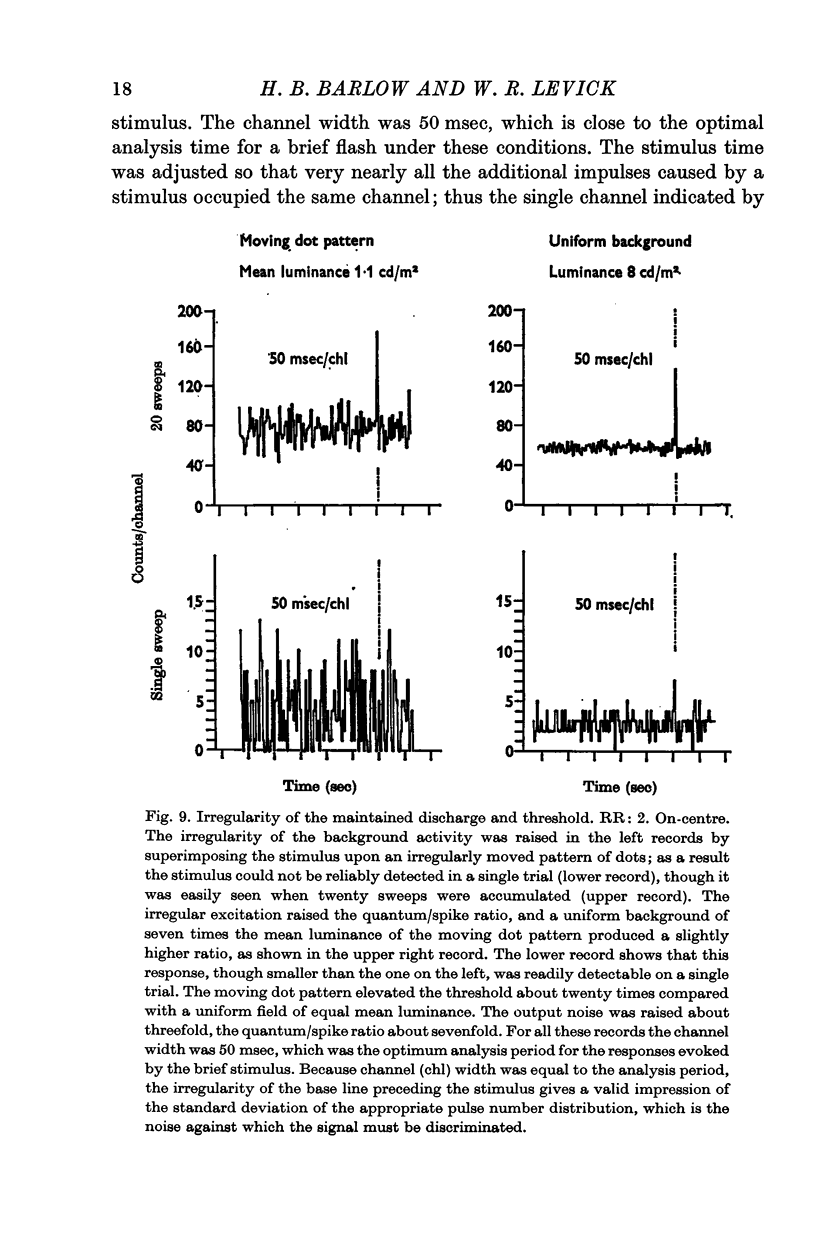
5. Changes in the irregularity of the maintained discharge also affect the threshold of single ganglion cells. This is only a minor factor in the conditions of most of our experiments, but it may be important when unstabilized images and non-equilibrium adaptation conditions are encountered.
Full text
PDF



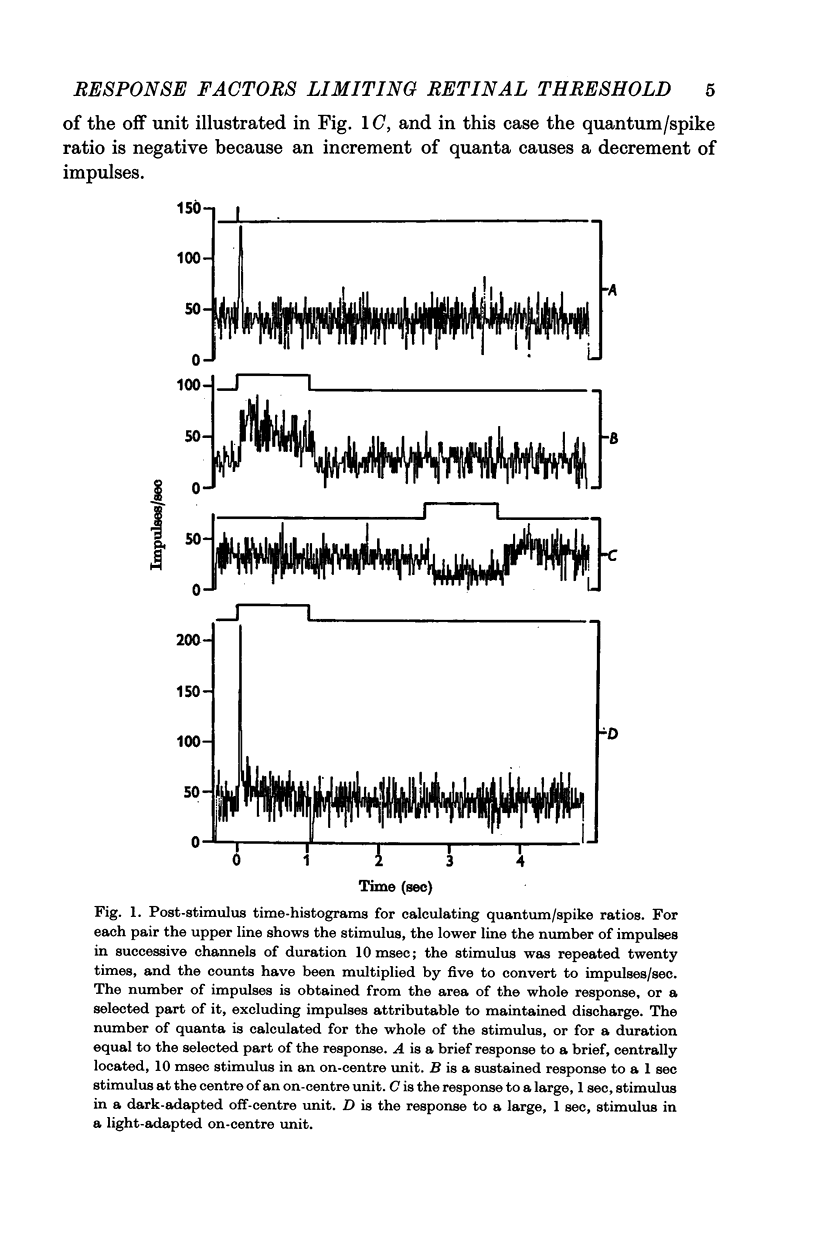

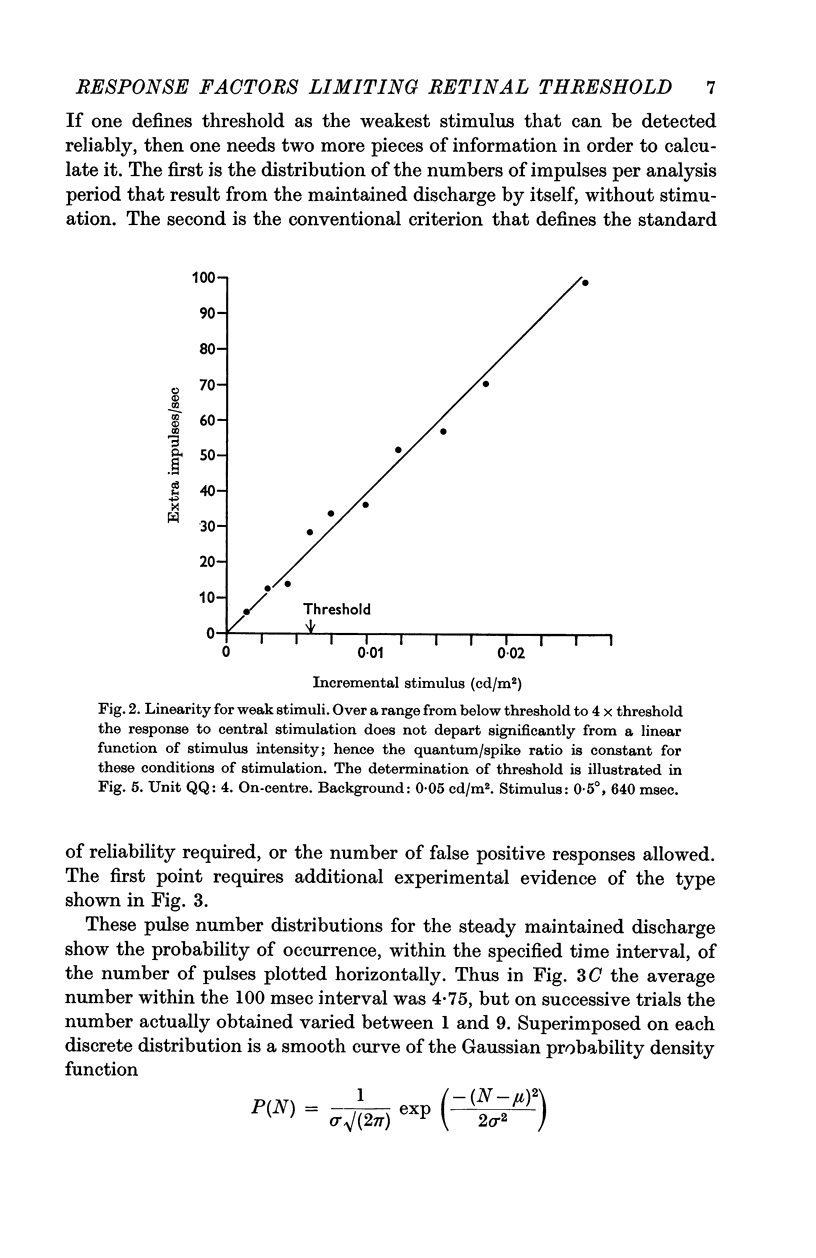
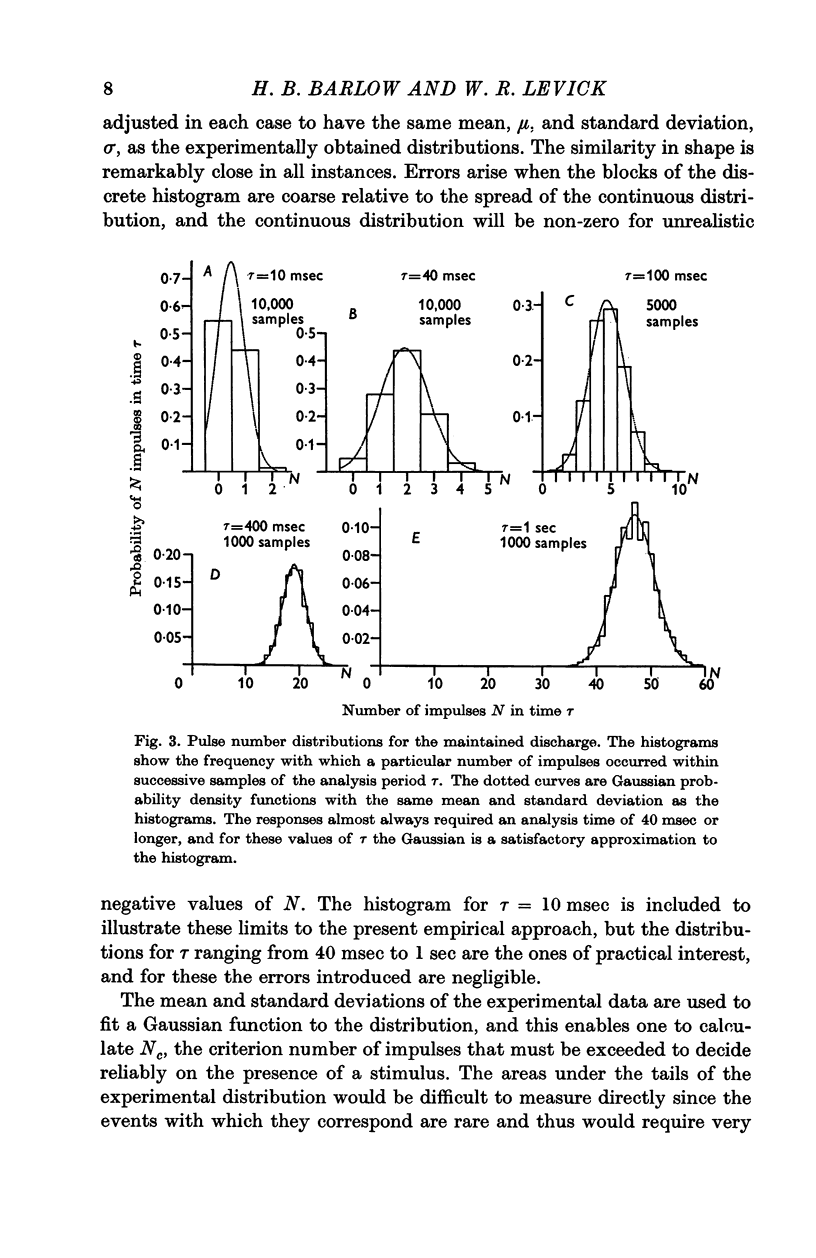
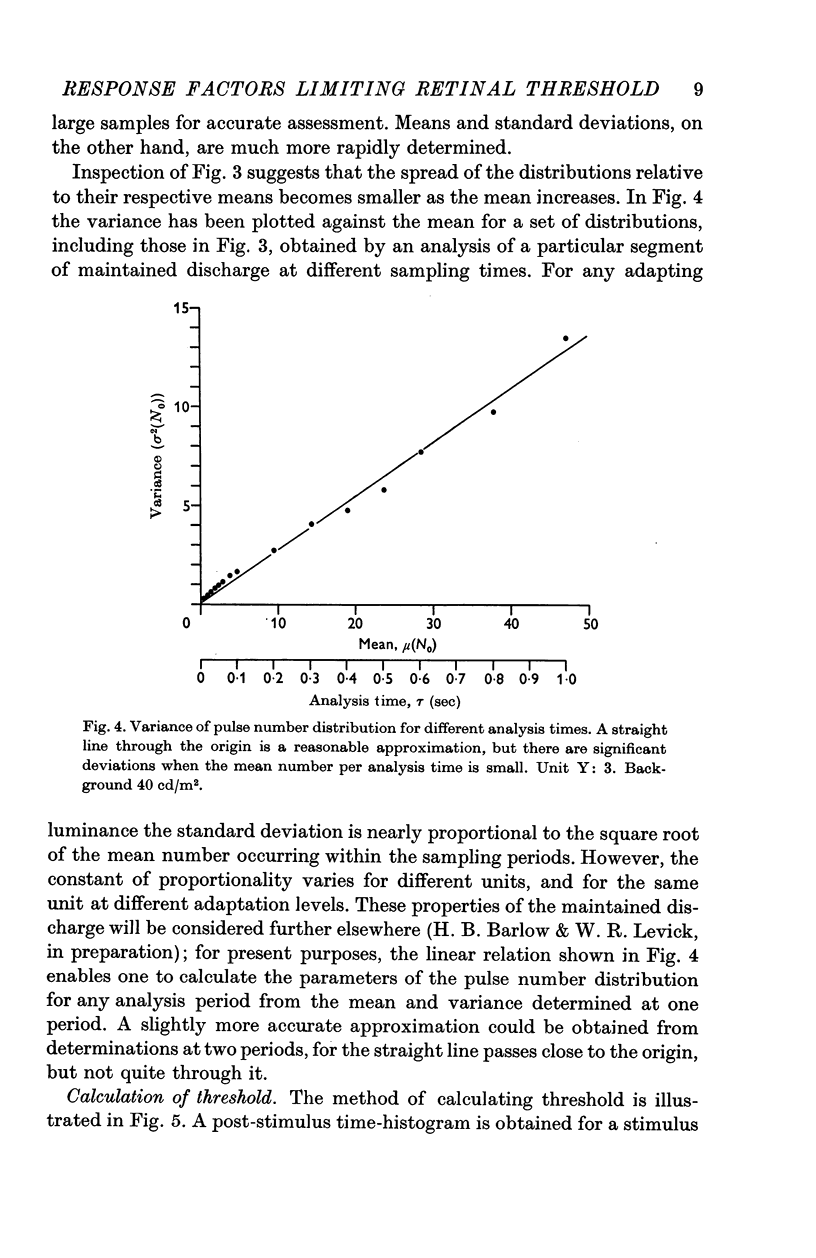










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARDUINI A., PINNEO L. R. Properties of the retina in response to steady illumination. Arch Ital Biol. 1962 Oct;100:425–448. [PubMed] [Google Scholar]
- BARLOW H. B. Increment thresholds at low intensities considered as signal/noise discriminations. J Physiol. 1957 May 23;136(3):469–488. doi: 10.1113/jphysiol.1957.sp005774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Retinal noise and absolute threshold. J Opt Soc Am. 1956 Aug;46(8):634–639. doi: 10.1364/josa.46.000634. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B. Temporal and spatial summation in human vision at different background intensities. J Physiol. 1958 Apr 30;141(2):337–350. doi: 10.1113/jphysiol.1958.sp005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORNSCHEIN H. Spontan- und Belichtungsaktivität in Einzelfasern des N. opticus der Katze. I. Der Einfluss kurzdauernder retinaler Ischämie. Z Biol. 1958 May;110(3):210–222. [PubMed] [Google Scholar]
- Barlow H. B. Optic nerve impulses and Weber's law. Cold Spring Harb Symp Quant Biol. 1965;30:539–546. doi: 10.1101/sqb.1965.030.01.052. [DOI] [PubMed] [Google Scholar]
- Craik K. J. The effect of adaptation on differential brightness discrimination. J Physiol. 1938 May 14;92(4):406–421. doi: 10.1113/jphysiol.1938.sp003612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E. The site of visual adaptation. Science. 1967 Jan 20;155(3760):273–279. doi: 10.1126/science.155.3760.273. [DOI] [PubMed] [Google Scholar]
- FITZHUGH A. A statistical analyzer for optic nerve messages. J Gen Physiol. 1958 Mar 20;41(4):675–692. doi: 10.1085/jgp.41.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZHUGH R. The statistical detection of threshold signals in the retina. J Gen Physiol. 1957 Jul 20;40(6):925–948. doi: 10.1085/jgp.40.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERBRANDS R., STEVENS J. C. A HIGH-INTENSITY FLASH-SOURCE. Am J Psychol. 1964 Dec;77:643–646. [PubMed] [Google Scholar]
- Heiss W. D., Milne D. C. Single fibers of cat optic nerve: "thresholds" to light. Science. 1967 Mar 24;155(3769):1571–1572. doi: 10.1126/science.155.3769.1571. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W., FITZHUGH R., BARLOW H. B. Maintained activity in the cat's retina in light and darkness. J Gen Physiol. 1957 May 20;40(5):683–702. doi: 10.1085/jgp.40.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVICK W. R., WILLIAMS W. O. MAINTAINED ACTIVITY OF LATERAL GENICULATE NEURONES IN DARKNESS. J Physiol. 1964 Apr;170:582–597. doi: 10.1113/jphysiol.1964.sp007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. Increment threshold and dark adaptation. J Opt Soc Am. 1963 Jan;53:104–109. doi: 10.1364/josa.53.000104. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. VISUAL ADAPTATION. Proc R Soc Lond B Biol Sci. 1965 Mar 16;162:20–46. doi: 10.1098/rspb.1965.0024. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W. Maintained activity of cat retinal ganglion cells. J Neurophysiol. 1967 Sep;30(5):1043–1071. doi: 10.1152/jn.1967.30.5.1043. [DOI] [PubMed] [Google Scholar]
- TANNER W. P., Jr, SWETS J. A. A decision-making theory of visual detection. Psychol Rev. 1954 Nov;61(6):401–409. doi: 10.1037/h0058700. [DOI] [PubMed] [Google Scholar]
- VAKKUR G. J., BISHOP P. O., KOZAK W. VISUAL OPTICS IN THE CAT, INCLUDING POSTERIOR NODAL DISTANCE AND RETINAL LANDMARKS. Vision Res. 1963 Nov;61:289–314. doi: 10.1016/0042-6989(63)90004-x. [DOI] [PubMed] [Google Scholar]


