Abstract
1. The water content, extracellular (60CoEDTA) space, ionic composition and ultrastructure of several mammalian smooth muscles were studied after incubation in solutions of varying ionic compositions and osmolarities.
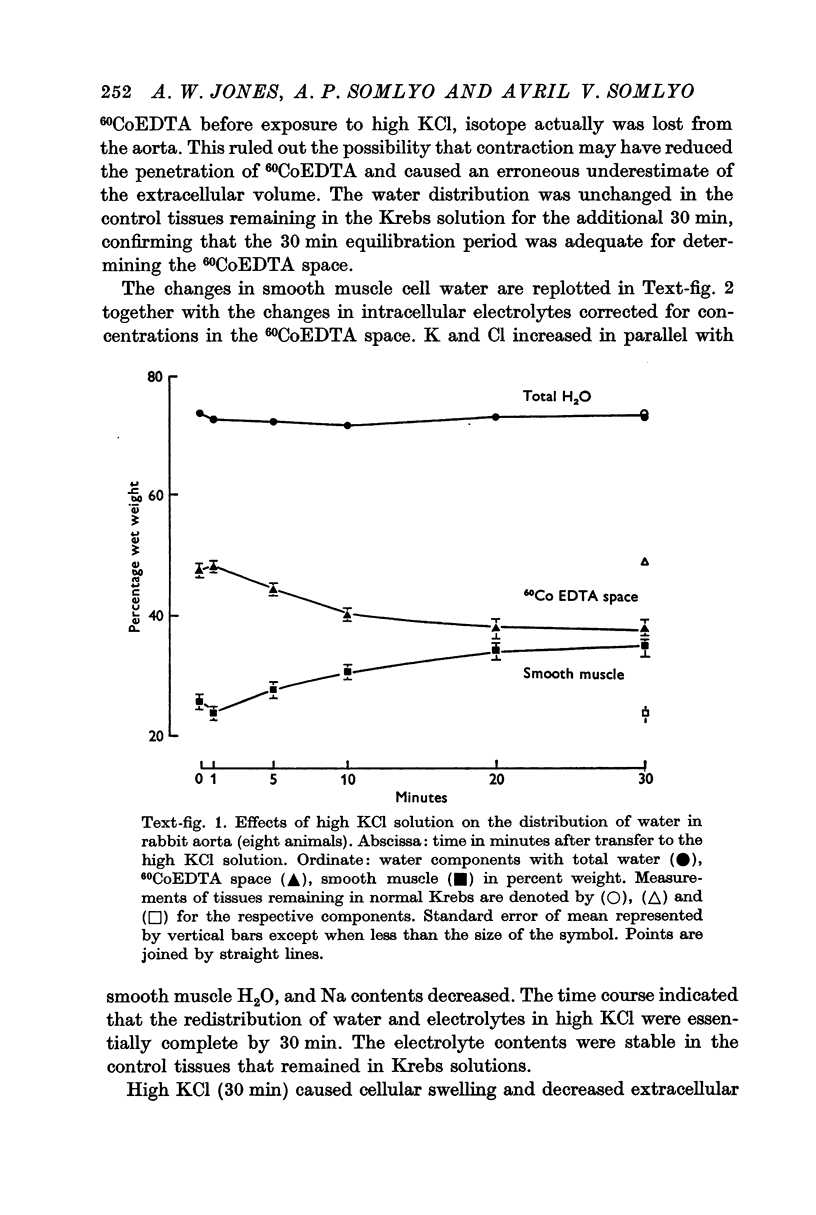
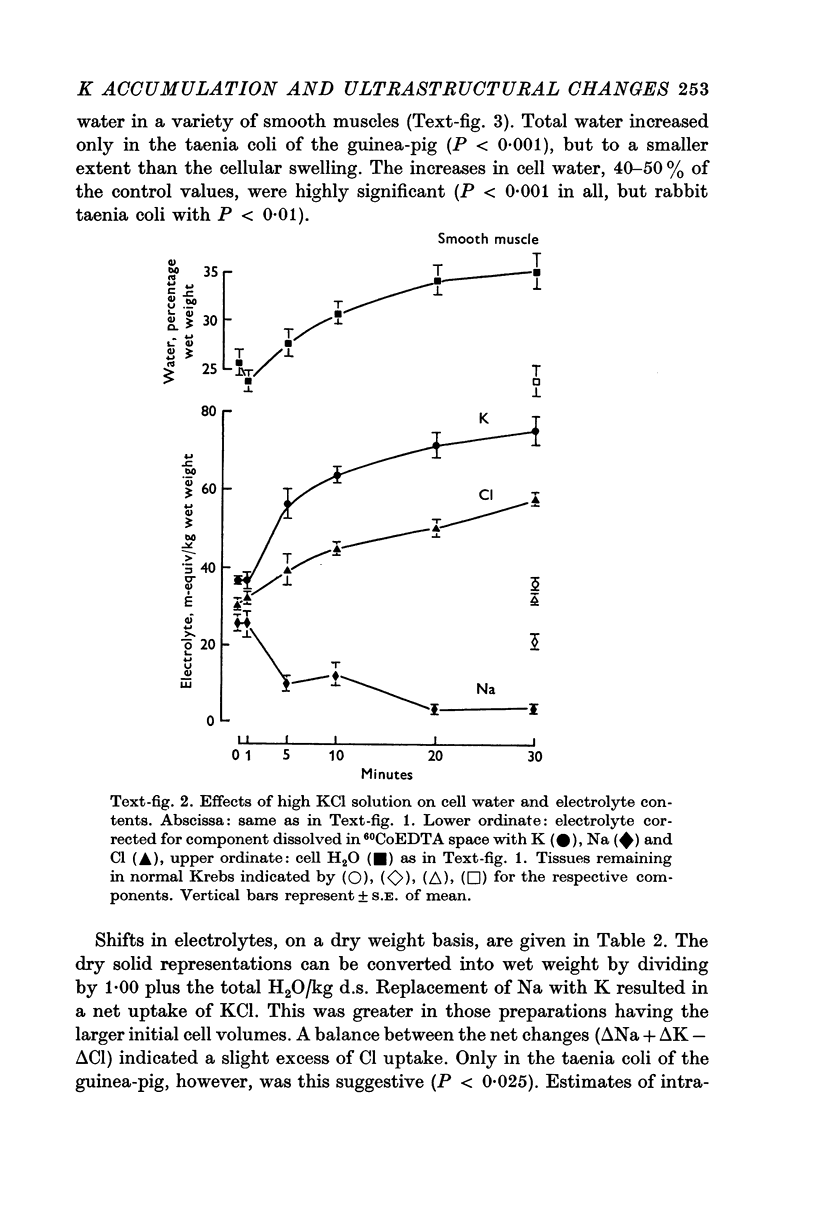
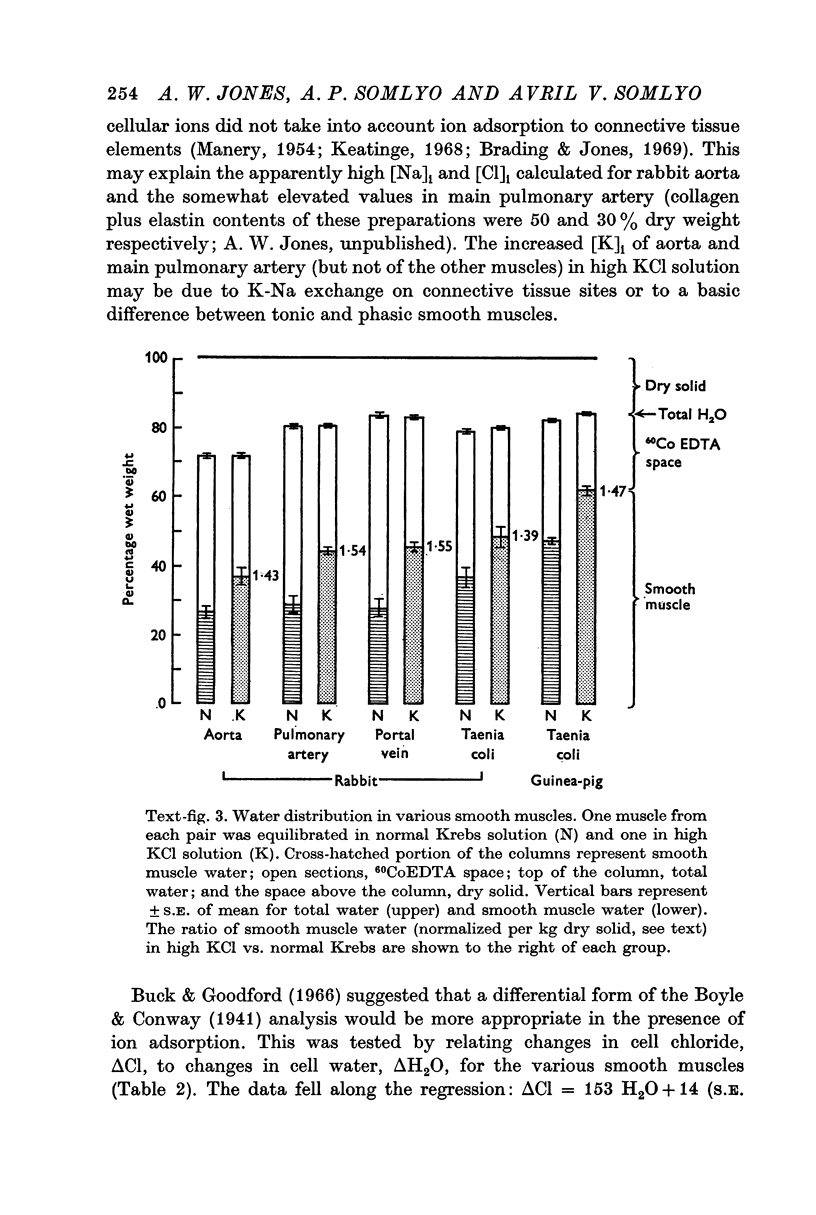
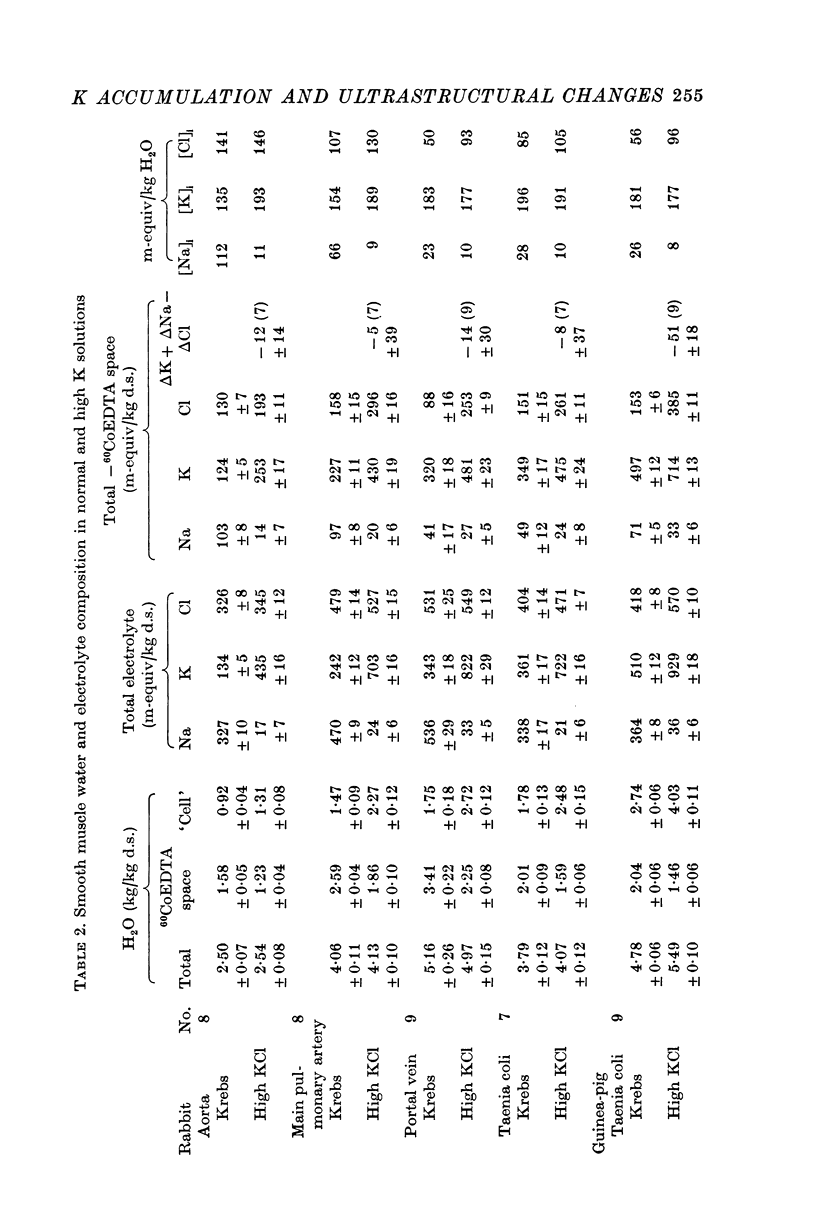
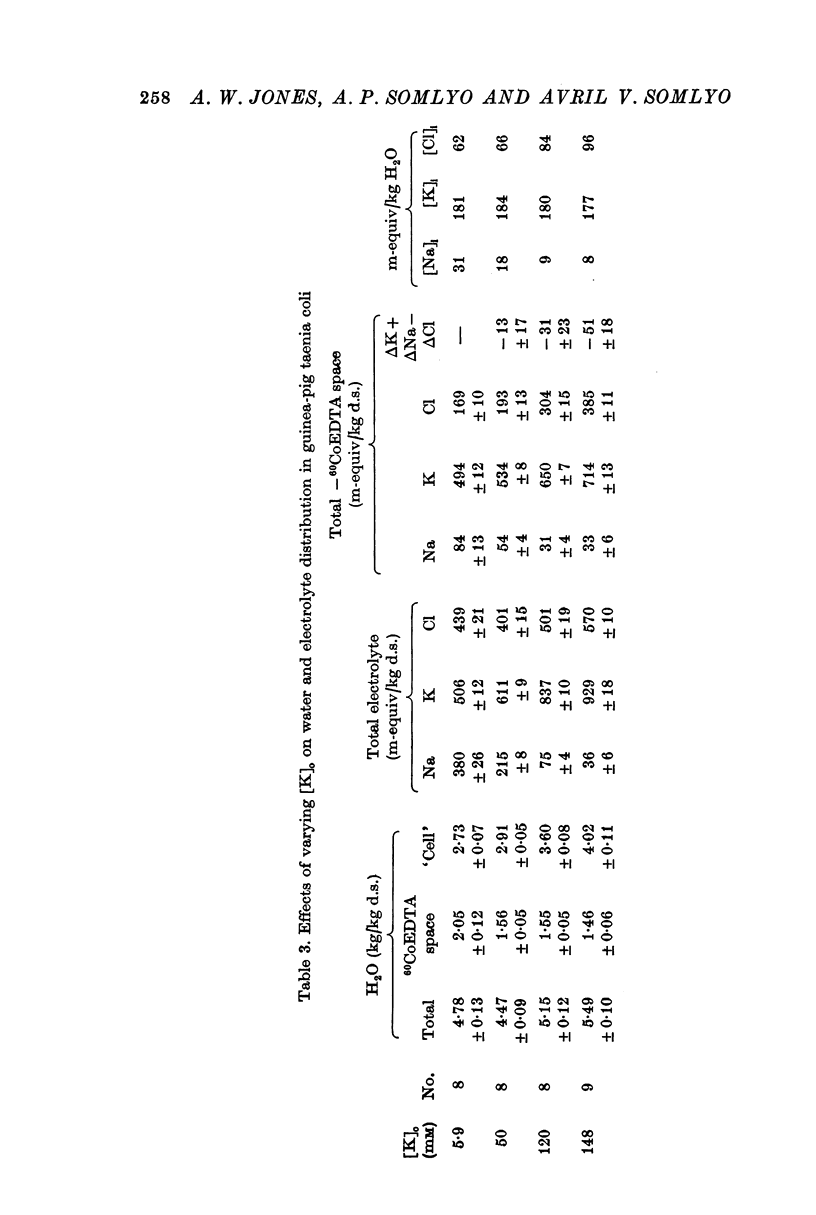
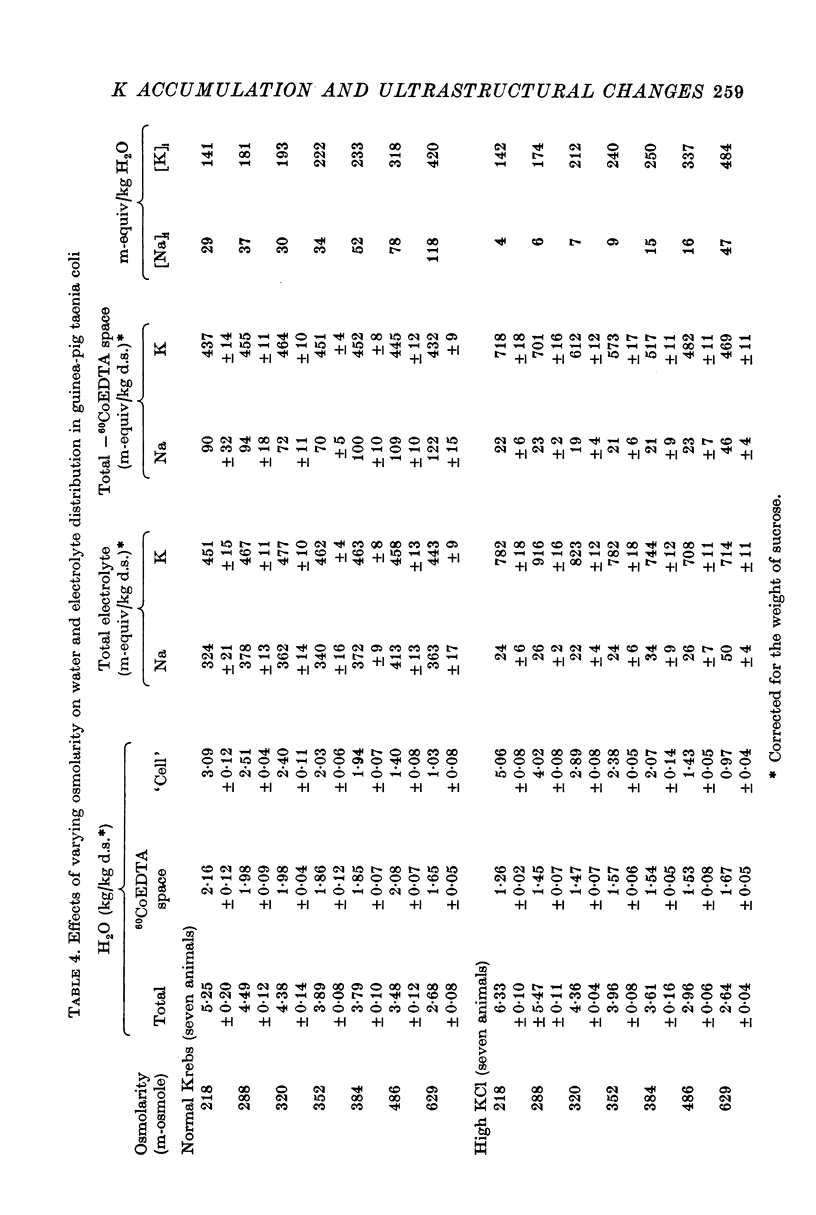
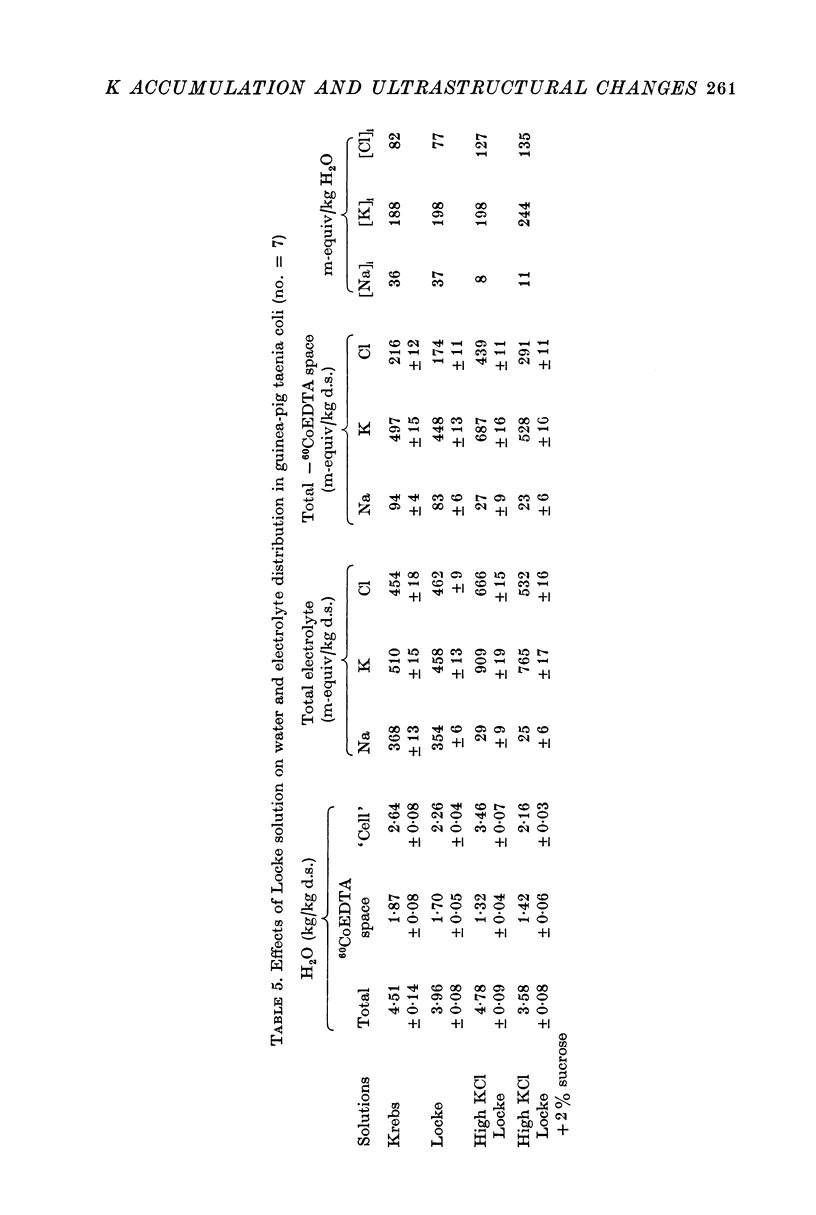
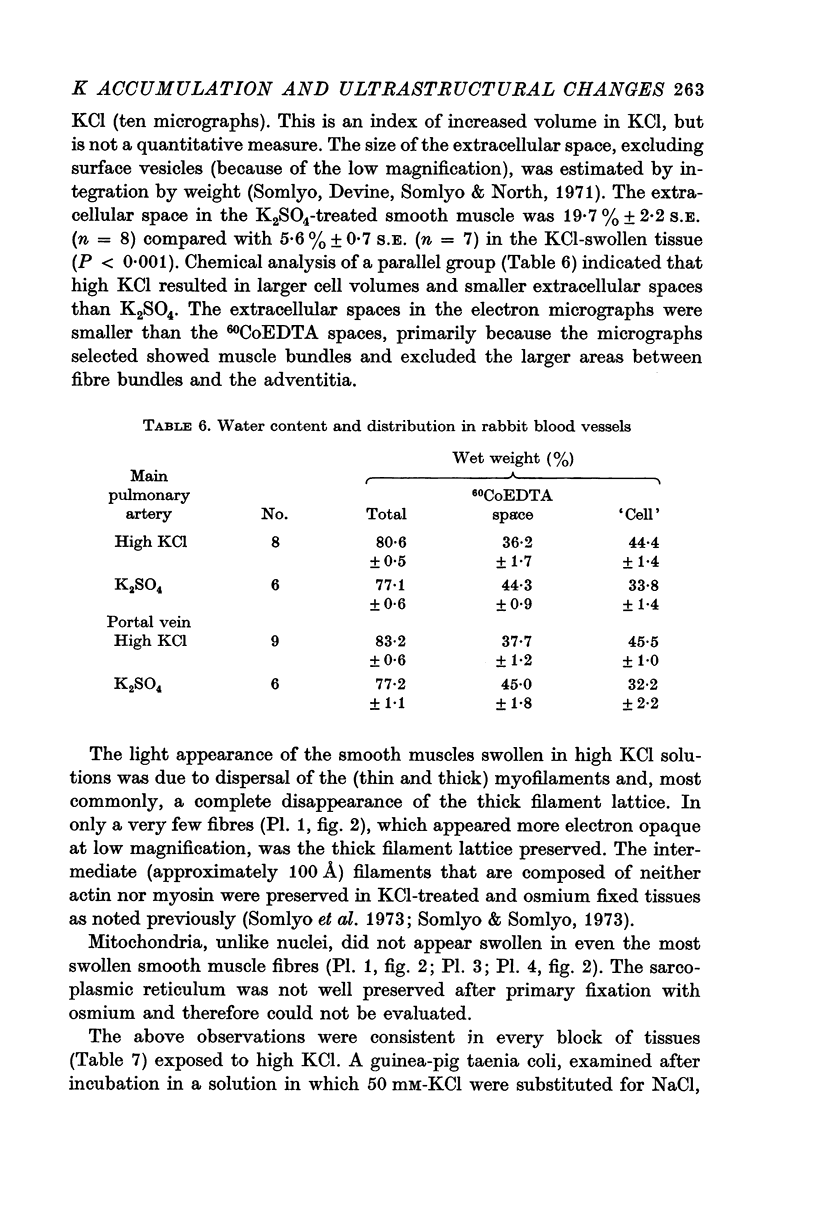
2. Substitution of KCl for NaCl resulted in an increase in cell water, K and Cl, accompanied by little change in total wet weight. This was due to a reduction in the extracellular space.
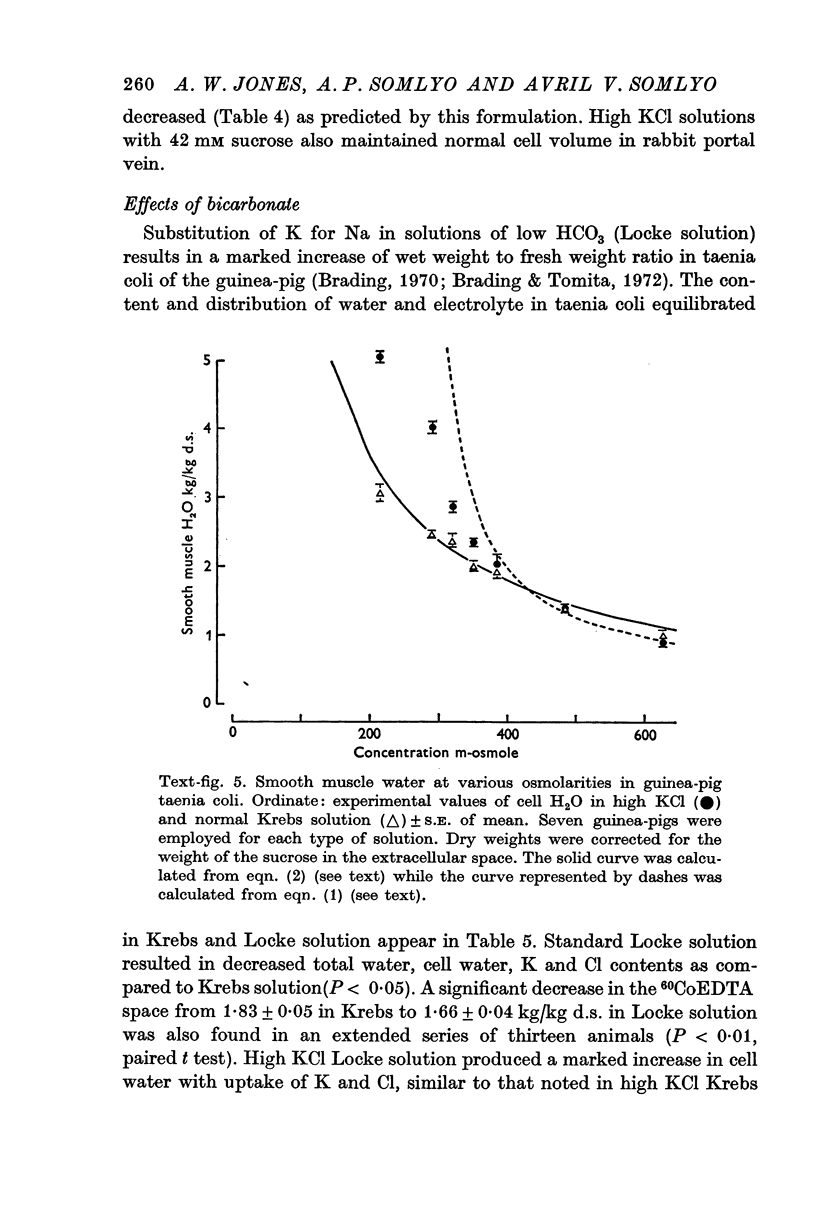
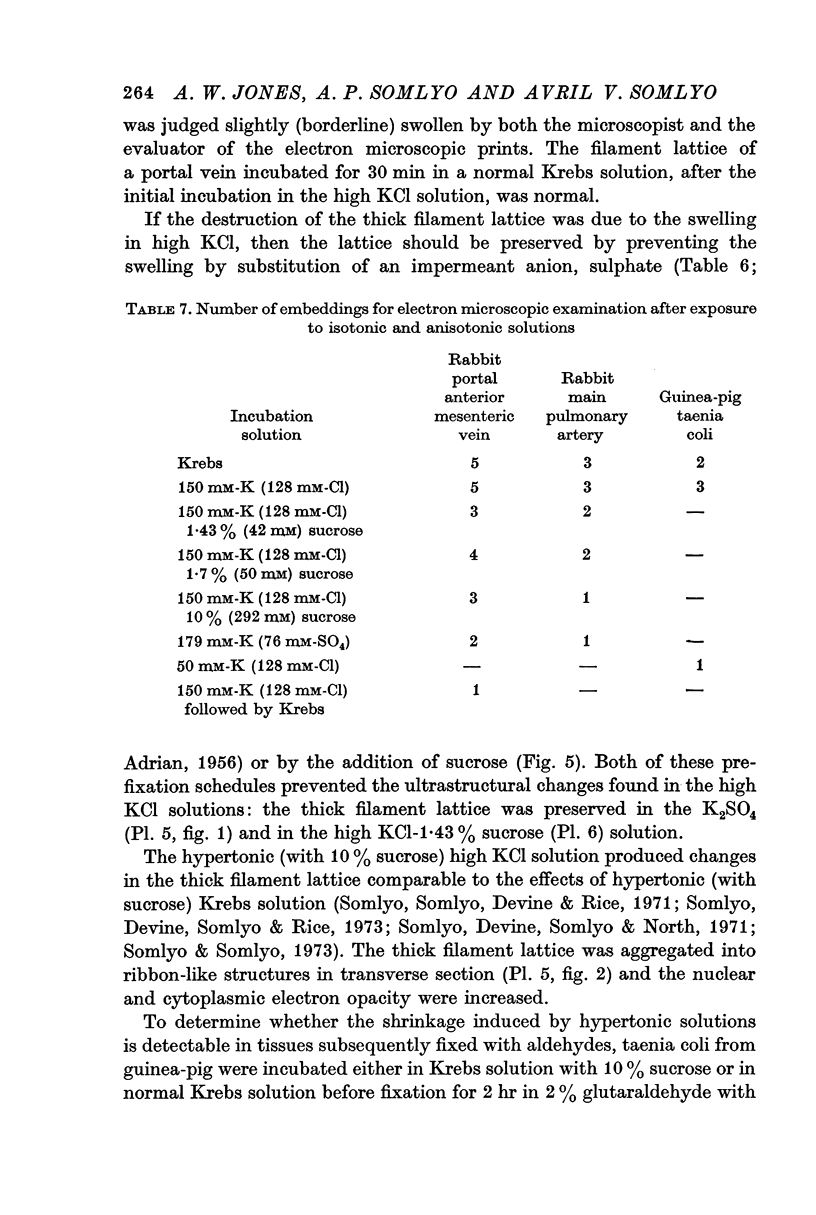
3. Changes in extracellular osmolarity produced a wider range of cell volumes in high KCl solutions than in Krebs. The addition of 29-58 mM sucrose to high KCl prevented the swelling.
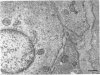
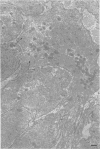
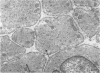
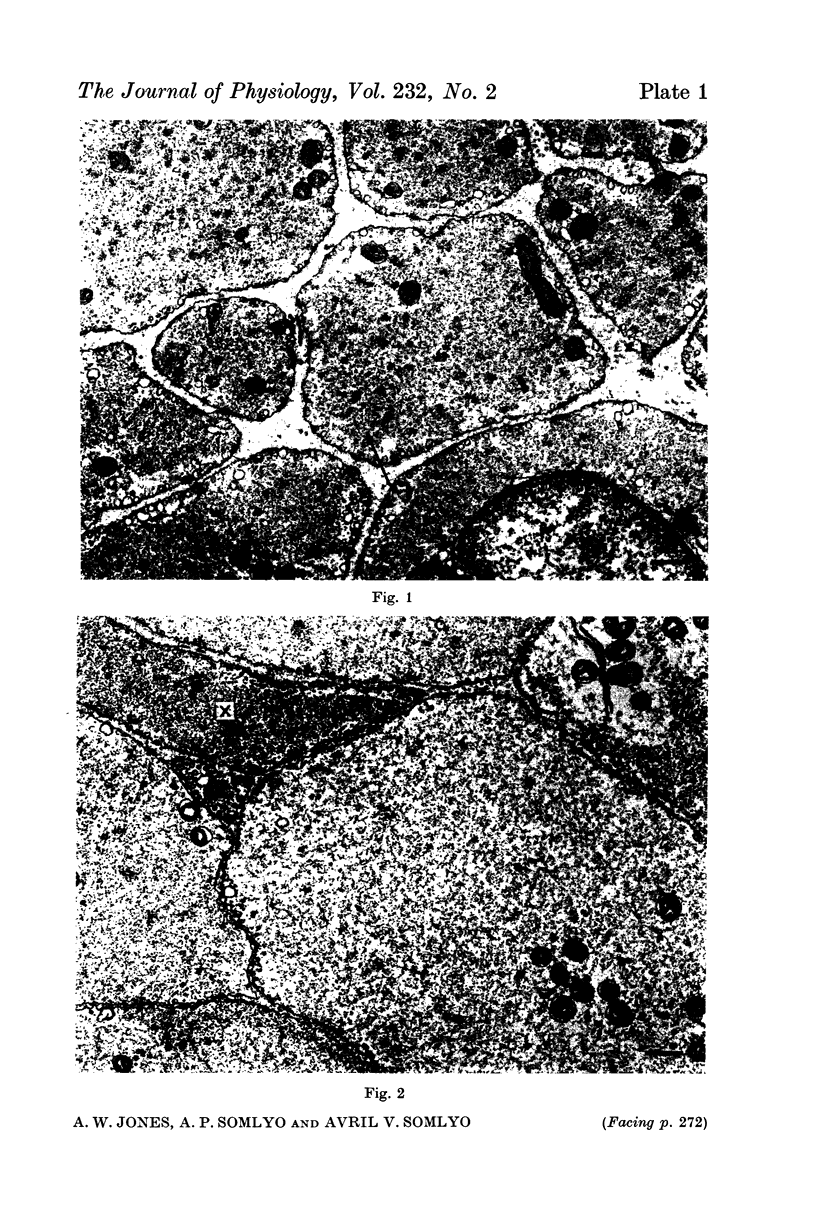
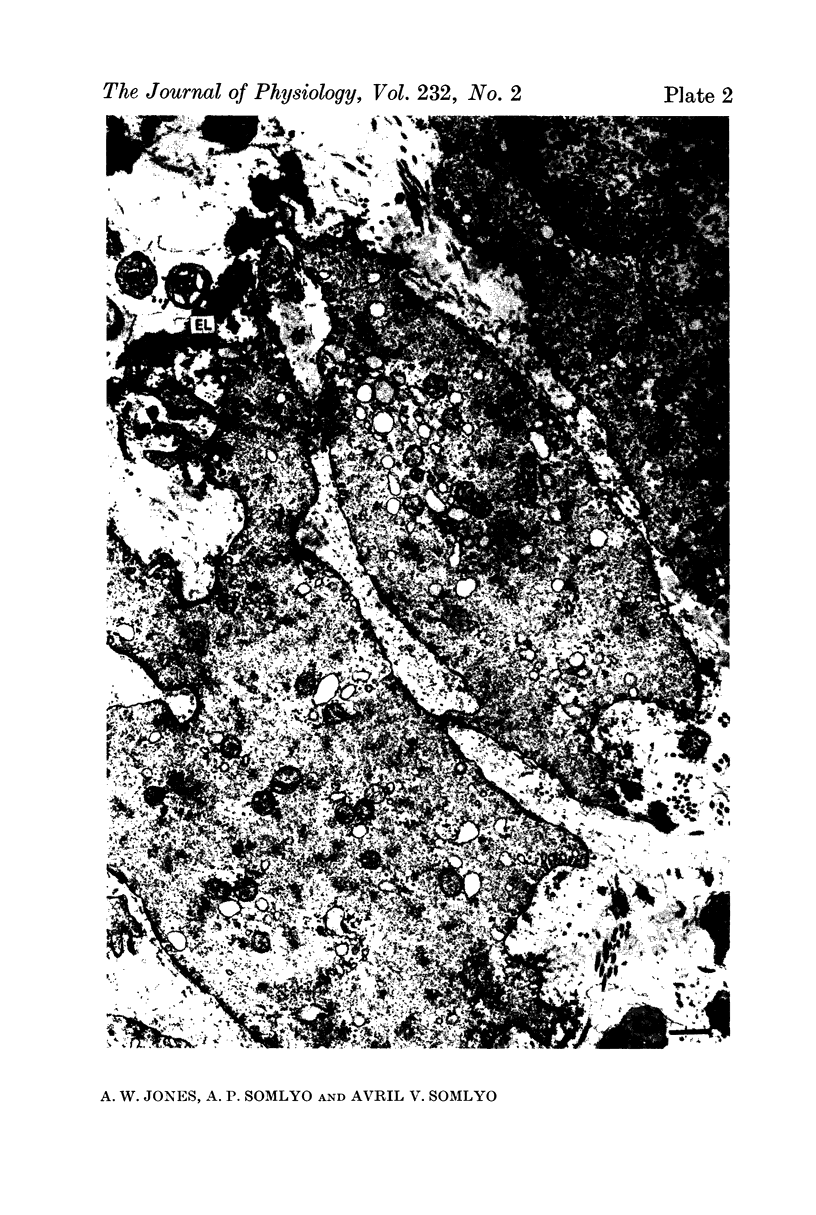
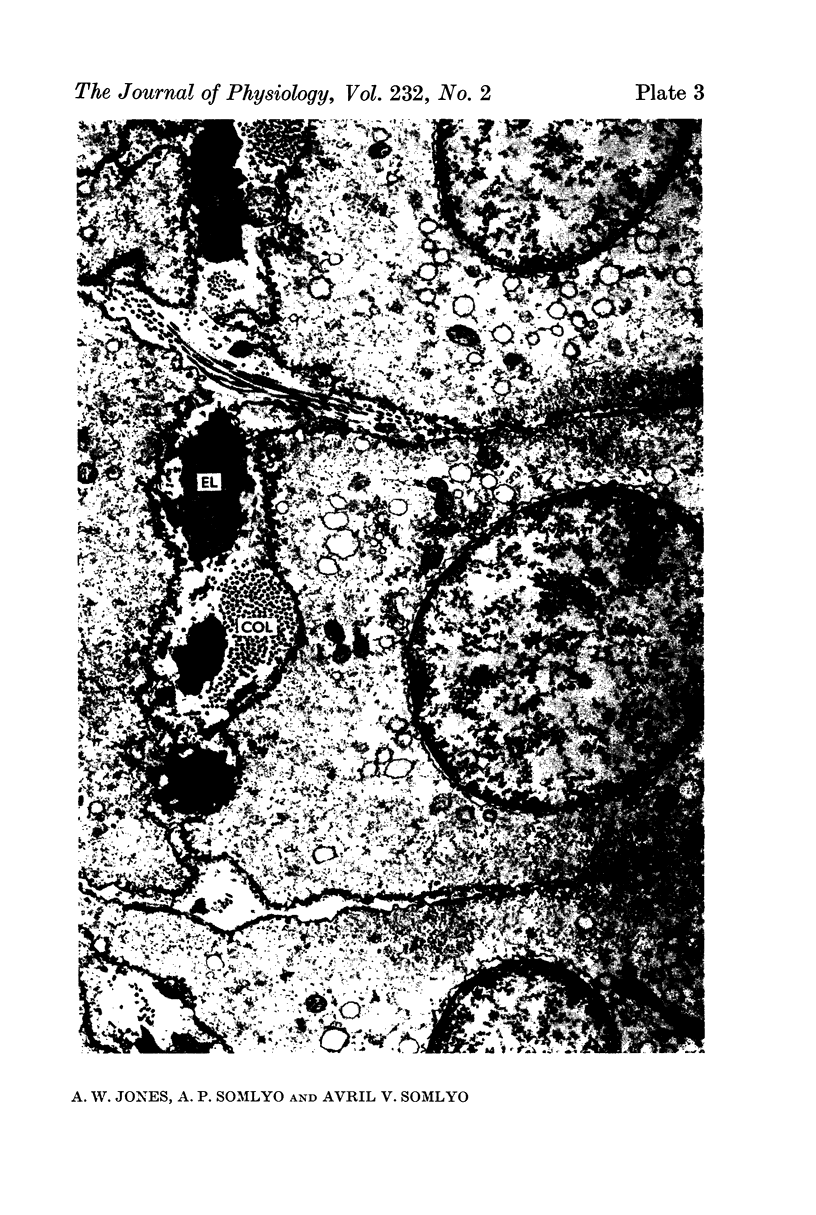
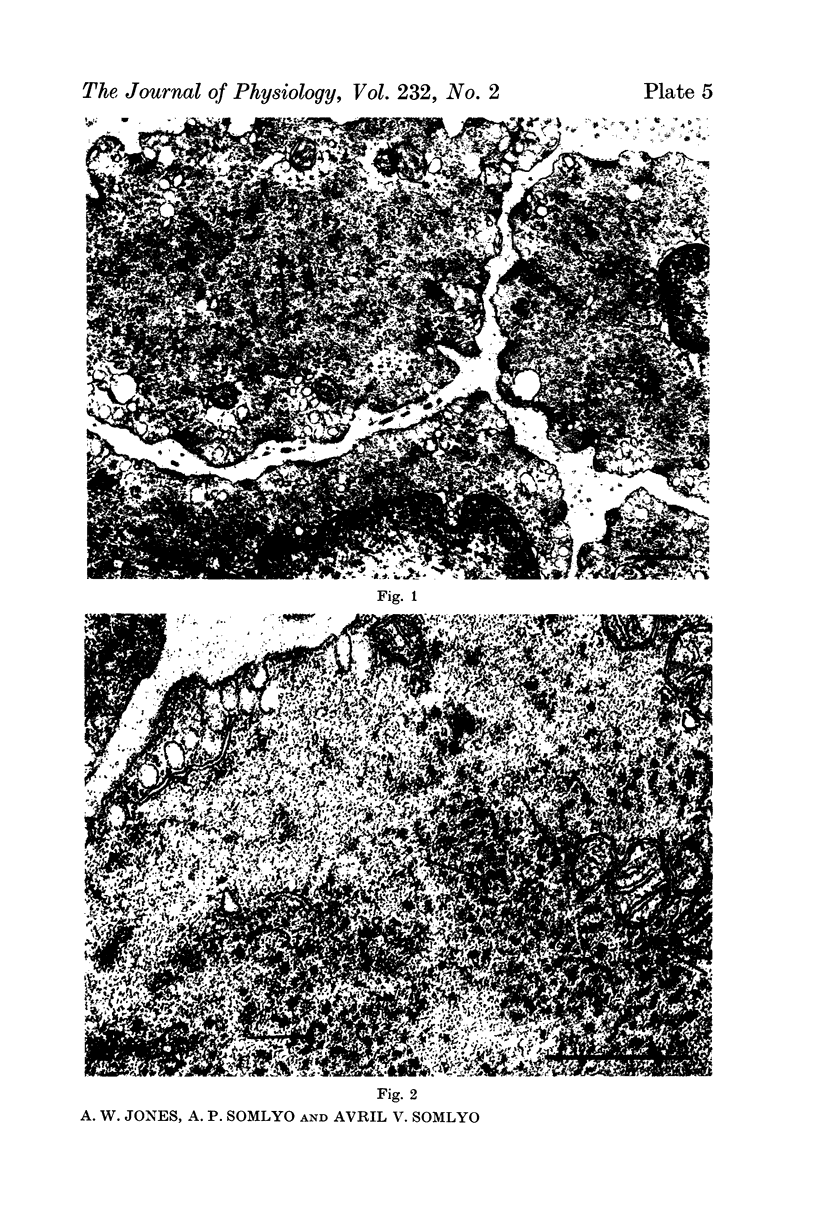
4. Electron microscopy of smooth muscle swollen in high KCl solution revealed light (less electron opaque than normal) fibres of increased diameter, reduction in extracellular space, and nuclear swelling. The normal thick filament lattice was destroyed in swollen, osmium-fixed smooth muscles.
5. The ultrastructural changes ascribed to swelling were absent in smooth muscles, (a) depolarized in high K2SO4 solutions, (b) in high KCl solutions with 29-58 mM sucrose, and (c) returned to normal Krebs solution for recovery from swelling.
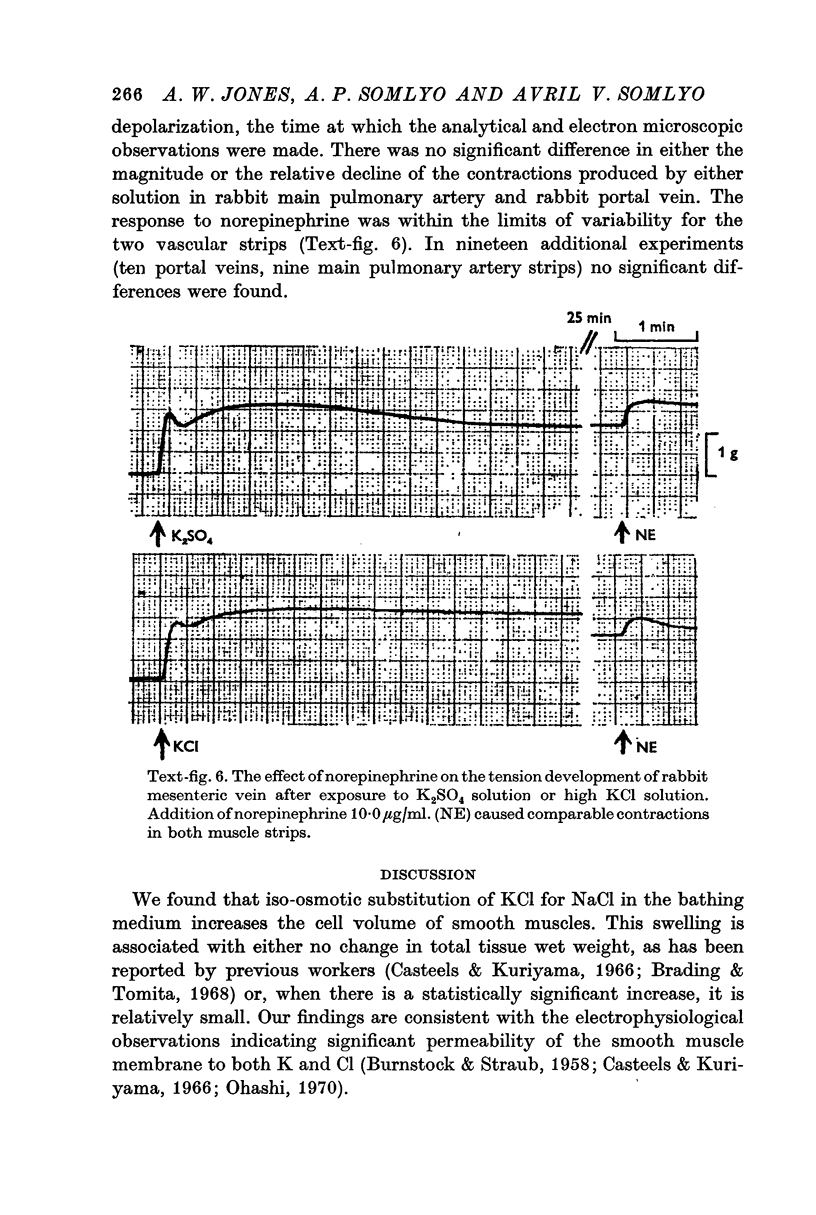
6. Smooth muscles incubated in high KCl (swollen) and high K2SO4 (unswollen) exhibited similar contractile responses, suggesting the filament lattice was intact until fixation, and that the contractile mechanism can operate over a relatively wide range of actin to myosin separations.
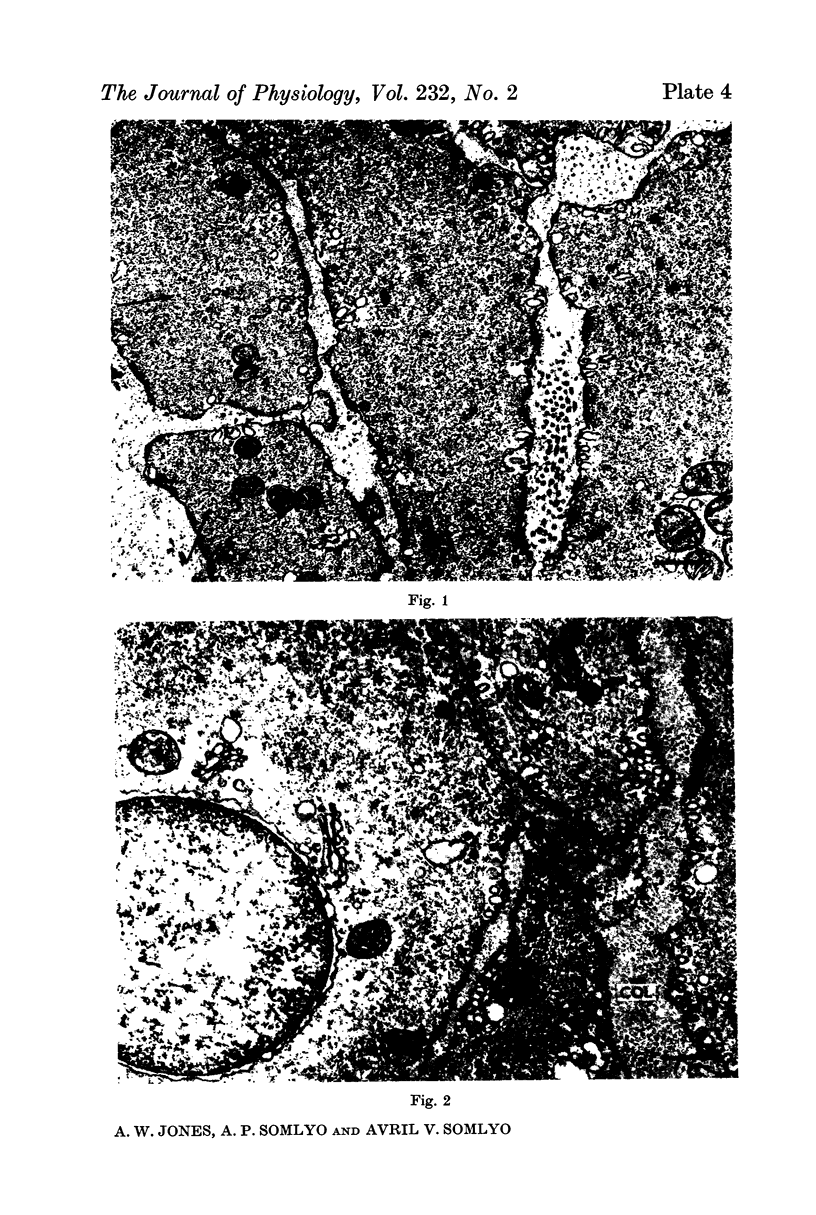
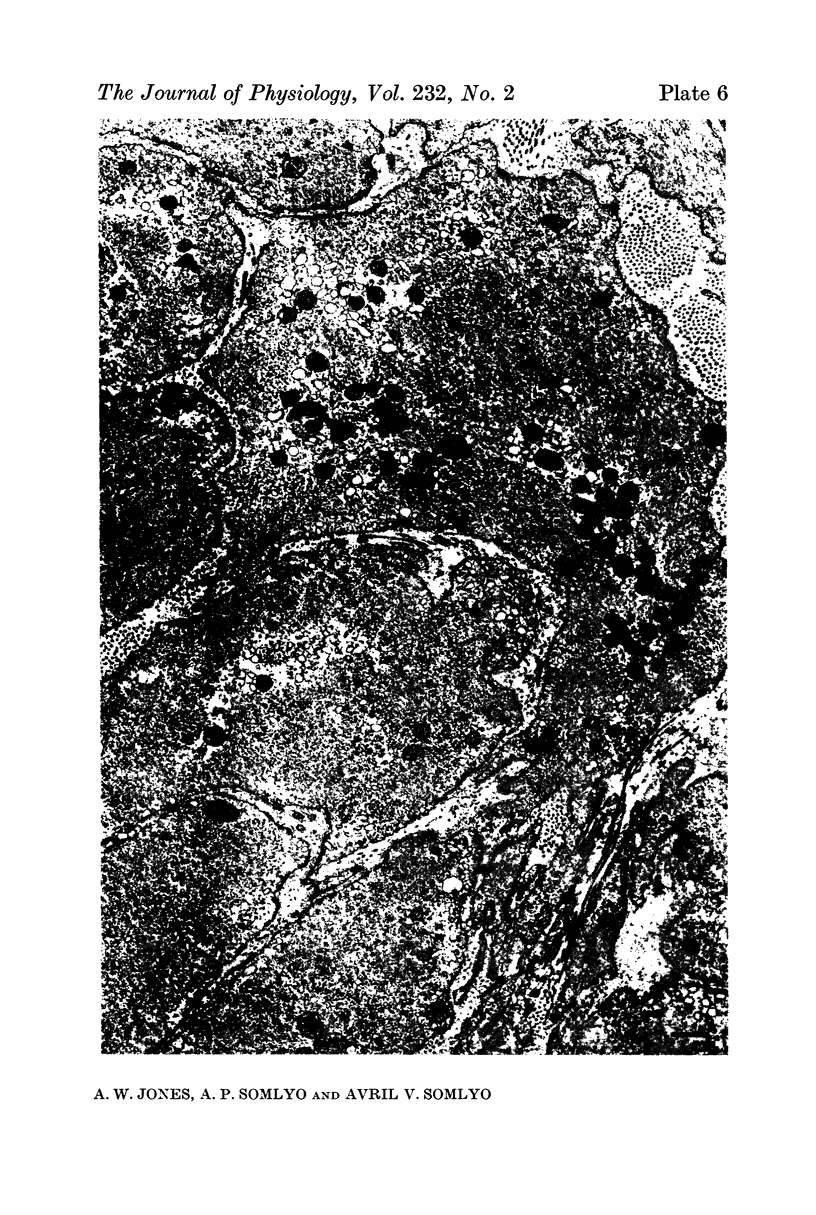
7. Shrinkage of smooth muscles in high KCl solutions made hypertonic with the addition of 10% sucrose was accompanied by an aggregation of the thick filaments.
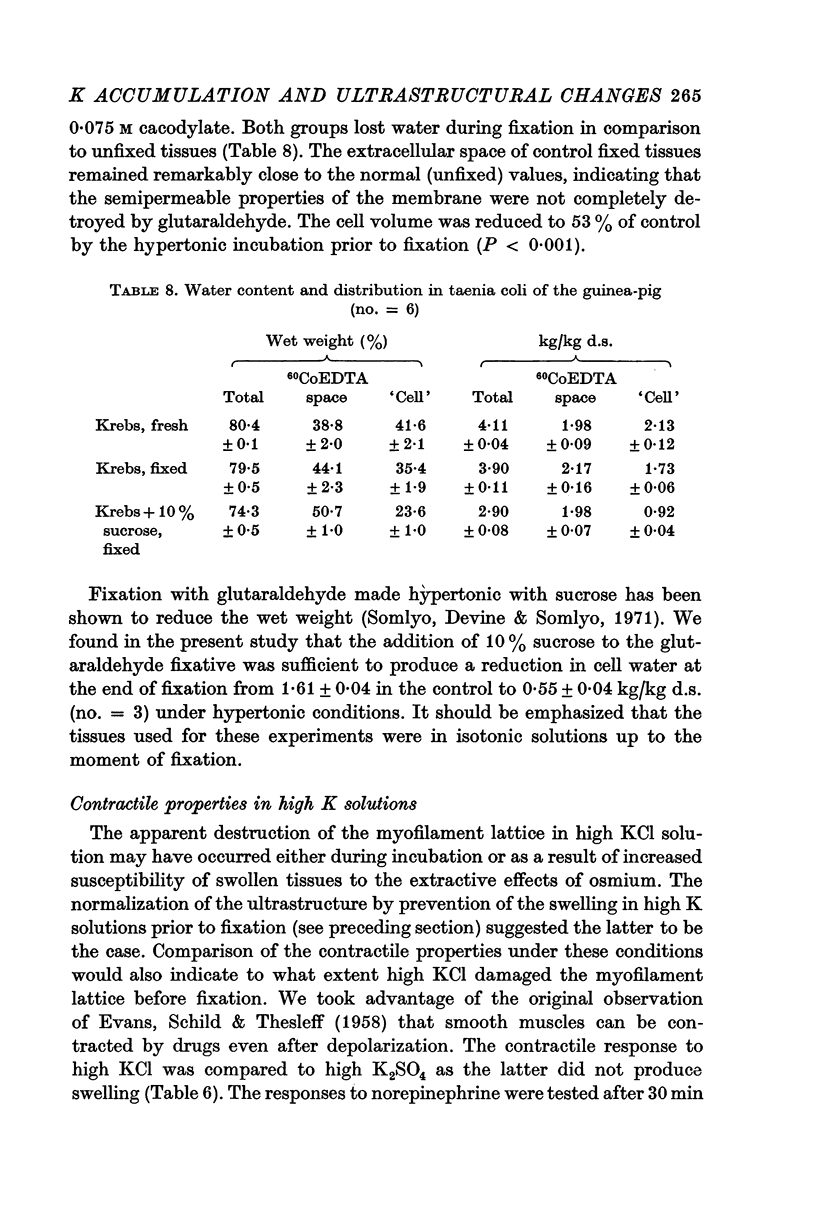
8. The cell water of fixed taenia coli was reduced (a) by incubation in hypertonic solution followed by fixation in normal glutaraldehyde, or (b) by fixation of normal tissue in hypertonic glutaraldehyde. Osmotic responses during aldehyde fixation may be a source of artifact in the visualization of the normal filament lattice.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol. 1956 Sep 27;133(3):631–658. doi: 10.1113/jphysiol.1956.sp005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvill A., Johansson B., Jonsson O. Effects of hyperosmolarity on the volume of vascular smooth muscle cells and the relation between cell volume and muscle activity. Acta Physiol Scand. 1969 Mar;75(3):484–495. doi: 10.1111/j.1748-1716.1969.tb04402.x. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., STRAUB R. W. A method for studying the effects of ions and drugs on the resting and action potentials in smooth muscle with external electrodes. J Physiol. 1958 Jan 23;140(1):156–167. doi: 10.1113/jphysiol.1958.sp005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F. Analysis of the effluxes of sodium, potassium and chloride ions from smooth muscle in normal and hypertonic solutions. J Physiol. 1971 May;214(3):393–416. doi: 10.1113/jphysiol.1971.sp009440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Jones A. W. Distribution and kinetics of CoEDTA in smooth muscle, and its use as an extracellular marker. J Physiol. 1969 Feb;200(2):387–401. doi: 10.1113/jphysiol.1969.sp008700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Setekleiv J. The effect of hypo- and hypertonic solutions on volume and ion distribution of smooth muscle of guinea-pig taenia coli. J Physiol. 1968 Mar;195(1):107–118. doi: 10.1113/jphysiol.1968.sp008449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Tomita T. Effect of anions on the volume of smooth muscle. Nature. 1968 Apr 20;218(5138):276–277. doi: 10.1038/218276a0. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Tomita T. Ionically induced volume changes of the smooth muscle of the guinea-pig taenia coli. Experientia. 1972 May 15;28(5):521–523. doi: 10.1007/BF01931854. [DOI] [PubMed] [Google Scholar]
- Buck B., Goodford P. J. The distribution of ions in the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Apr;183(3):551–569. doi: 10.1113/jphysiol.1966.sp007883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Increase of membrane conductance by adrenaline in the smooth muscle of guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):89–102. doi: 10.1098/rspb.1969.0013. [DOI] [PubMed] [Google Scholar]
- CASTEELS R., KURIYAMA H. MEMBRANE POTENTIAL AND IONIC CONTENT IN PREGNANT AND NON-PREGNANT RAT MYOMETRIUM. J Physiol. 1965 Mar;177:263–287. doi: 10.1113/jphysiol.1965.sp007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. Calculation of the membrane potential in smooth muscle cells of the guinea-pig's taenia coli by the Goldman equation. J Physiol. 1969 Nov;205(1):193–208. doi: 10.1113/jphysiol.1969.sp008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. Ion content and ion fluxes in the smooth muscle cells of the longitudinal layer of the guinea-pig's vas deferens. Pflugers Arch. 1969;313(2):95–105. doi: 10.1007/BF00586238. [DOI] [PubMed] [Google Scholar]
- Casteels R., Kuriyama H. Membrane potential and ion content in the smooth muscle of the guinea-pig's taenia coli at different external potassium concentrations. J Physiol. 1966 May;184(1):120–130. doi: 10.1113/jphysiol.1966.sp007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. The distribution of chloride ions in the smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1971 Apr;214(2):225–243. doi: 10.1113/jphysiol.1971.sp009429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke P. H., Fay F. S. Thick myofilaments in contracted and relaxed mammalian smooth muscle cells. Exp Cell Res. 1972;71(2):265–272. doi: 10.1016/0014-4827(72)90292-3. [DOI] [PubMed] [Google Scholar]
- DANIEL E. E., ROBINSON K. The secretion of sodium and uptake of potassium by isolated uterine segments made sodium-rich. J Physiol. 1960 Dec;154:421–444. doi: 10.1113/jphysiol.1960.sp006589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS D. H., SCHILD H. O., THESLEFF S. Effects of drugs on depolarized plain muscle. J Physiol. 1958 Oct 31;143(3):474–485. doi: 10.1113/jphysiol.1958.sp006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A., Secunda S. K., Mendels J. A method for the determination of sodium, potassium, magnesium and lithium concentrations in erythrocytes. Clin Chim Acta. 1972 Feb;36(2):499–509. doi: 10.1016/0009-8981(72)90026-5. [DOI] [PubMed] [Google Scholar]
- Grundfest H. Ionic permeability of the guinea pig taenia coli muscle. Nature. 1968 Aug 17;219(5155):732–733. doi: 10.1038/219732a0. [DOI] [PubMed] [Google Scholar]
- Gulati J. Cooperative interaction of external calcium, sodium, and ouabain with the cellular potassium in smooth muscle. Ann N Y Acad Sci. 1973 Mar 30;204:337–357. doi: 10.1111/j.1749-6632.1973.tb30789.x. [DOI] [PubMed] [Google Scholar]
- HUXLEY H. E. X-ray analysis and the problem of muscle. Proc R Soc Lond B Biol Sci. 1953 Mar 11;141(902):59–62. doi: 10.1098/rspb.1953.0017. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Jones A. W. Control of cooperative K accumulation in smooth muscle by divalent ions. Ann N Y Acad Sci. 1973 Mar 30;204:379–392. doi: 10.1111/j.1749-6632.1973.tb30792.x. [DOI] [PubMed] [Google Scholar]
- Jones A. W., Karreman G. Potassium accumulation and permeation in the canine carotid artery. Biophys J. 1969 Jul;9(7):910–924. doi: 10.1016/S0006-3495(69)86426-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge W. R. Sodium flux and electrical activity of arterial smooth muscle. J Physiol. 1968 Jan;194(1):183–200. doi: 10.1113/jphysiol.1968.sp008401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy J., Poulsen F. R., Vibert P. J. Myosin filaments in vertebrate smooth muscle. Nature. 1970 Mar 14;225(5237):1053–1054. doi: 10.1038/2251053a0. [DOI] [PubMed] [Google Scholar]
- Lowy J., Small J. V. The organization of myosin and actin in vertebrate smooth muscle. Nature. 1970 Jul 4;227(5253):46–51. doi: 10.1038/227046a0. [DOI] [PubMed] [Google Scholar]
- MANERY J. F. Water and electrolyte metabolism. Physiol Rev. 1954 Apr;34(2):334–417. doi: 10.1152/physrev.1954.34.2.334. [DOI] [PubMed] [Google Scholar]
- Pepe F. A. Some aspects of the structural organization of the myofibril as revealed by antibody--staining methods. J Cell Biol. 1966 Mar;28(3):505–525. doi: 10.1083/jcb.28.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R. V., McManus G. M., Devine O. F., Somlyo A. P. Regular organization of thick filaments in mammalian smooth muscle. Nat New Biol. 1971 Jun 23;231(25):242–243. doi: 10.1038/newbio231242a0. [DOI] [PubMed] [Google Scholar]
- Rome E. X-ray diffraction studies of the filament lattice of striated muscle in various bathing media. J Mol Biol. 1968 Oct 28;37(2):331–344. doi: 10.1016/0022-2836(68)90272-6. [DOI] [PubMed] [Google Scholar]
- Shoenberg C. F. The influence of temperature on the thick filaments of vertebrate smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):197–202. doi: 10.1098/rstb.1973.0023. [DOI] [PubMed] [Google Scholar]
- Small J. V., Squire J. M. Structural basis of contraction in vertebrate smooth muscle. J Mol Biol. 1972 Jun 14;67(1):117–149. doi: 10.1016/0022-2836(72)90390-7. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Devine C. E., Somlyo A. V., North S. R. Sarcoplasmic reticulum and the temperature-dependent contraction of smooth muscle in calcium-free solutions. J Cell Biol. 1971 Dec;51(3):722–741. doi: 10.1083/jcb.51.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. P., Devine C. E., Somlyo A. V., Rice R. V. Filament organization in vertebrate smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):223–229. doi: 10.1098/rstb.1973.0027. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Devine C. E., Somlyo A. V. Thick filaments in unstretched mammalian smooth muscle. Nature. 1971 Oct 13;233(5320):218–219. [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Devine C. E., Rice R. V. Aggregation of thick filaments into ribbons in mammalian smooth muscle. Nat New Biol. 1971 Jun 23;231(25):243–246. doi: 10.1038/newbio231243a0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exp Ther. 1968 Jan;159(1):129–145. [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Strontium accumulation by sarcoplasmic reticulum and mitochondria in vascular smooth muscle. Science. 1971 Nov 26;174(4012):955–958. doi: 10.1126/science.174.4012.955. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Vinall P., Somlyo A. P. Excitation-contraction coupling and electrical events in two types of vascular smooth muscle. Microvasc Res. 1969 Oct;1(4):354–373. doi: 10.1016/0026-2862(69)90014-4. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Taylor A. N., Wasserman R. H. Vitamin D-induced calcium-binding protein: comparative aspects in kidney and intestine. Am J Physiol. 1972 Jul;223(1):110–114. doi: 10.1152/ajplegacy.1972.223.1.110. [DOI] [PubMed] [Google Scholar]