Abstract
1. Intracellular and intercellular concentrations of sodium and potassium have been measured in pregastrular embryos of Xenopus laevis and Amblystoma mexicanum. Calcium and magnesium contents have also been determined.
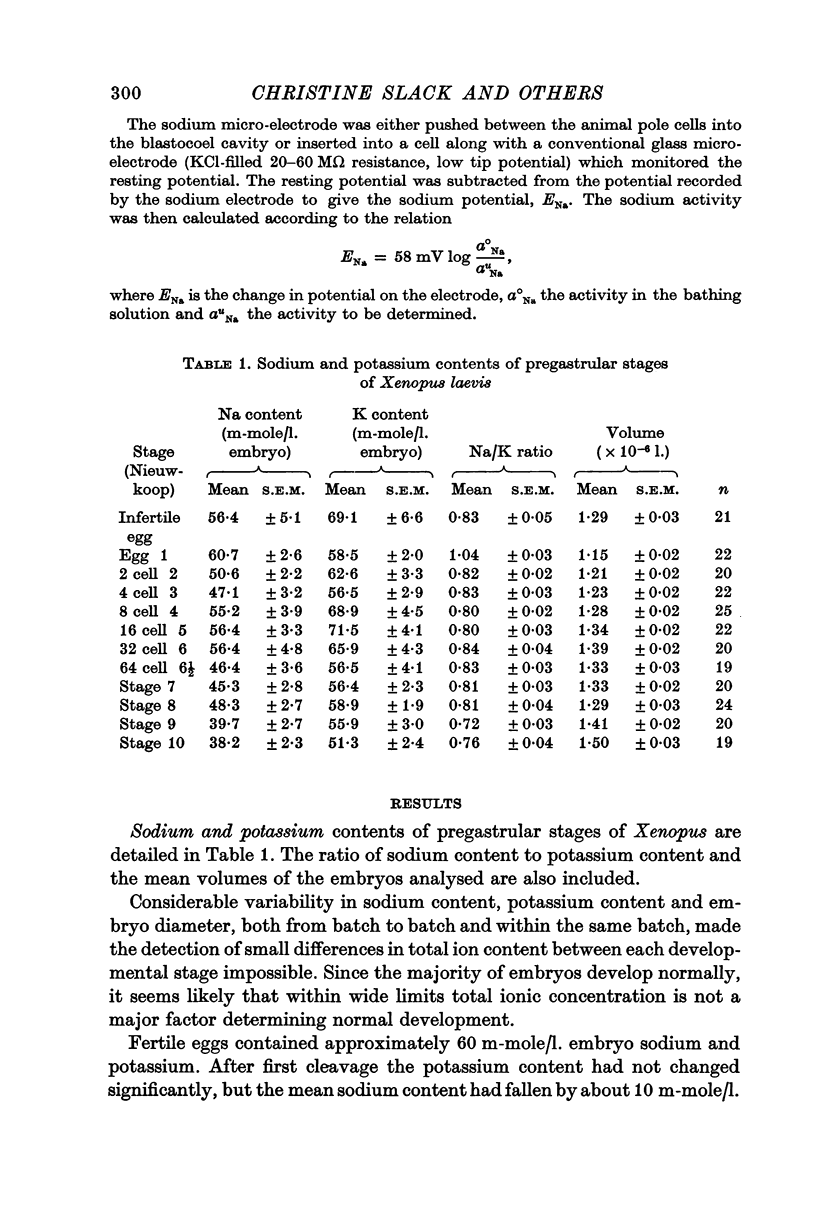
2. Between egg and gastrula stages of development the potassium concentration is near to 60 m-mole/l. embryo. Up to the blastula stage the sodium concentration is near to 50 m-mole/l. embryo; the amount of sodium in the embryo begins to fall just before gastrulation.
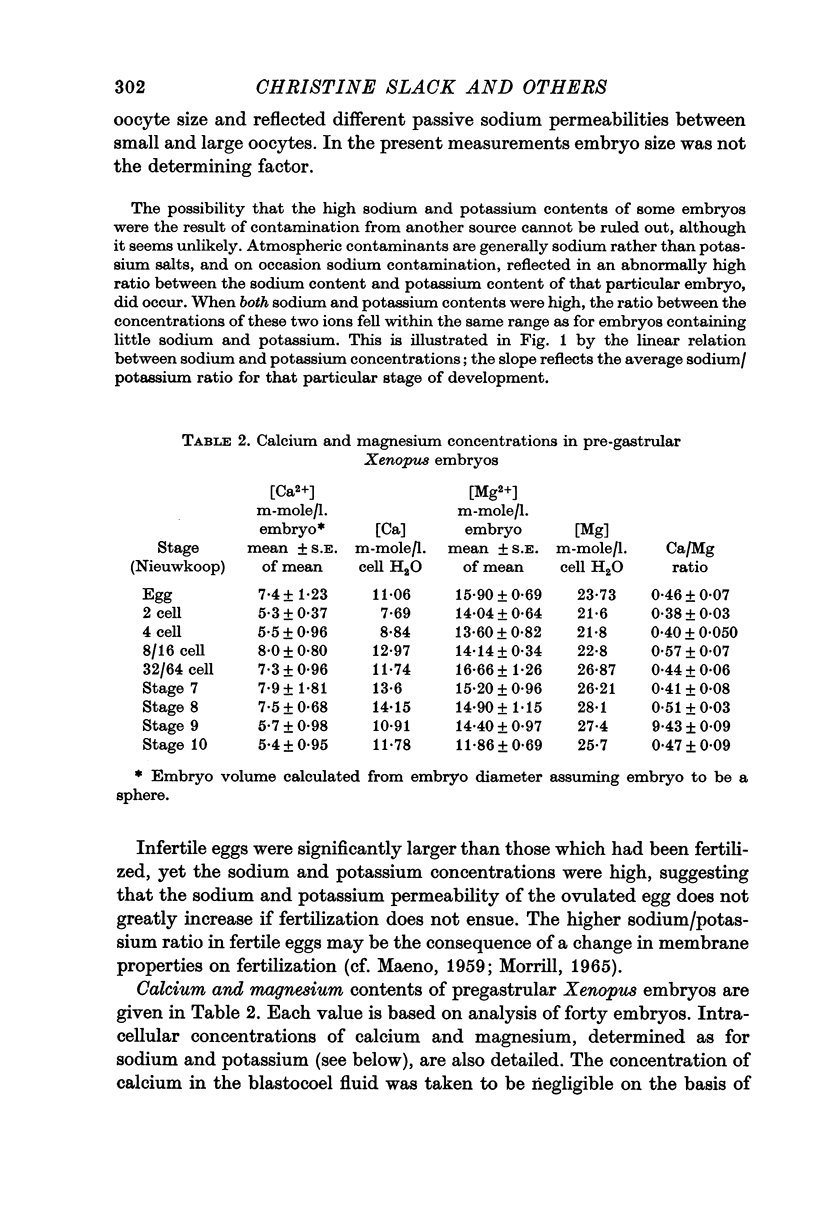
3. Embryo calcium and magnesium concentrations show no significant variations prior to gastrulation. Average calcium concentrations for the different stages range from 5 to 8 m-mole/l. embryo; magnesium concentrations lie between 12·4 and 17 m-mole/l. embryo.
4. The intercellular fluid contains 100 mM sodium and 1 mM potassium; the majority of the sodium is ionically active. As no net uptake of sodium or potassium occurs before gastrulation these cations must have been transferred from the cells to the cavity.
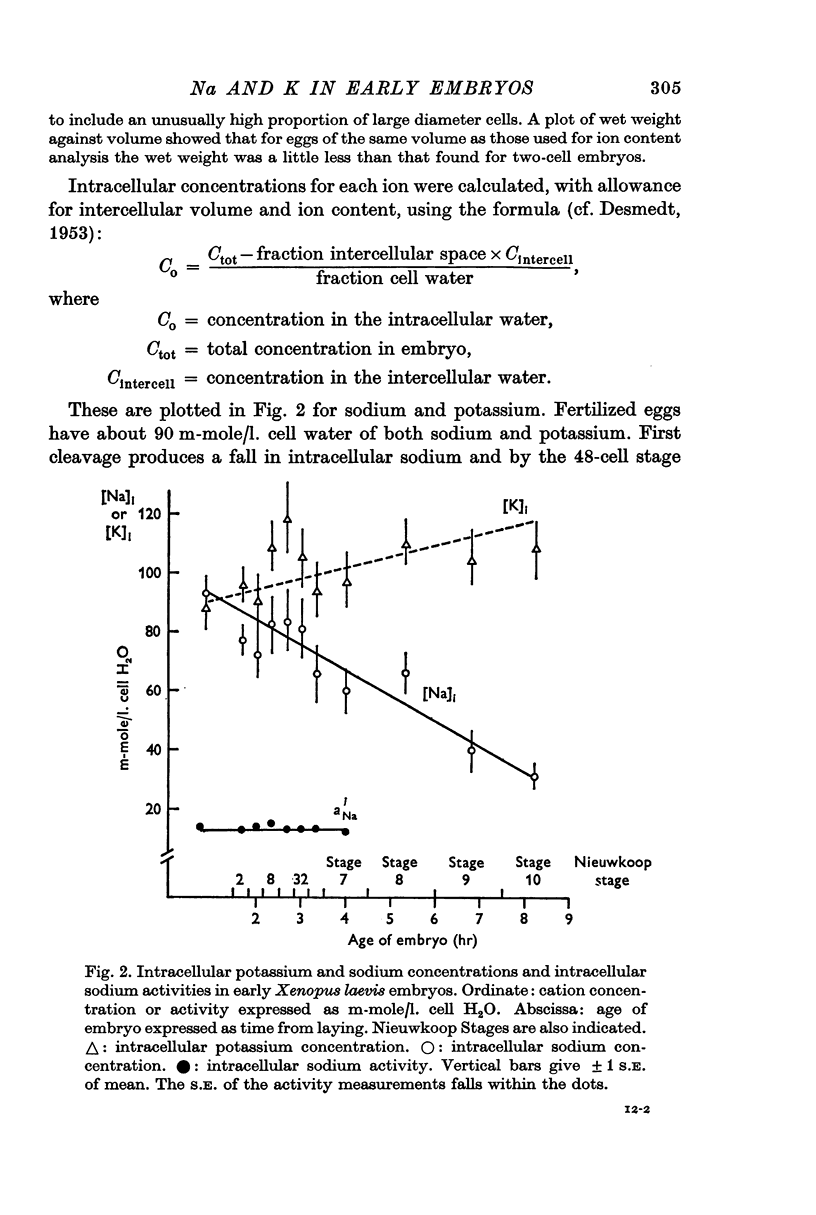
5. At all developmental stages studied, the intracellular potassium concentration is close to 100 m-mole/l. cell water. Intracellular sodium falls steadily from 80 m-mole/l. cell water in eggs to 30 m-mole/l. cell water at the beginning of gastrulation.
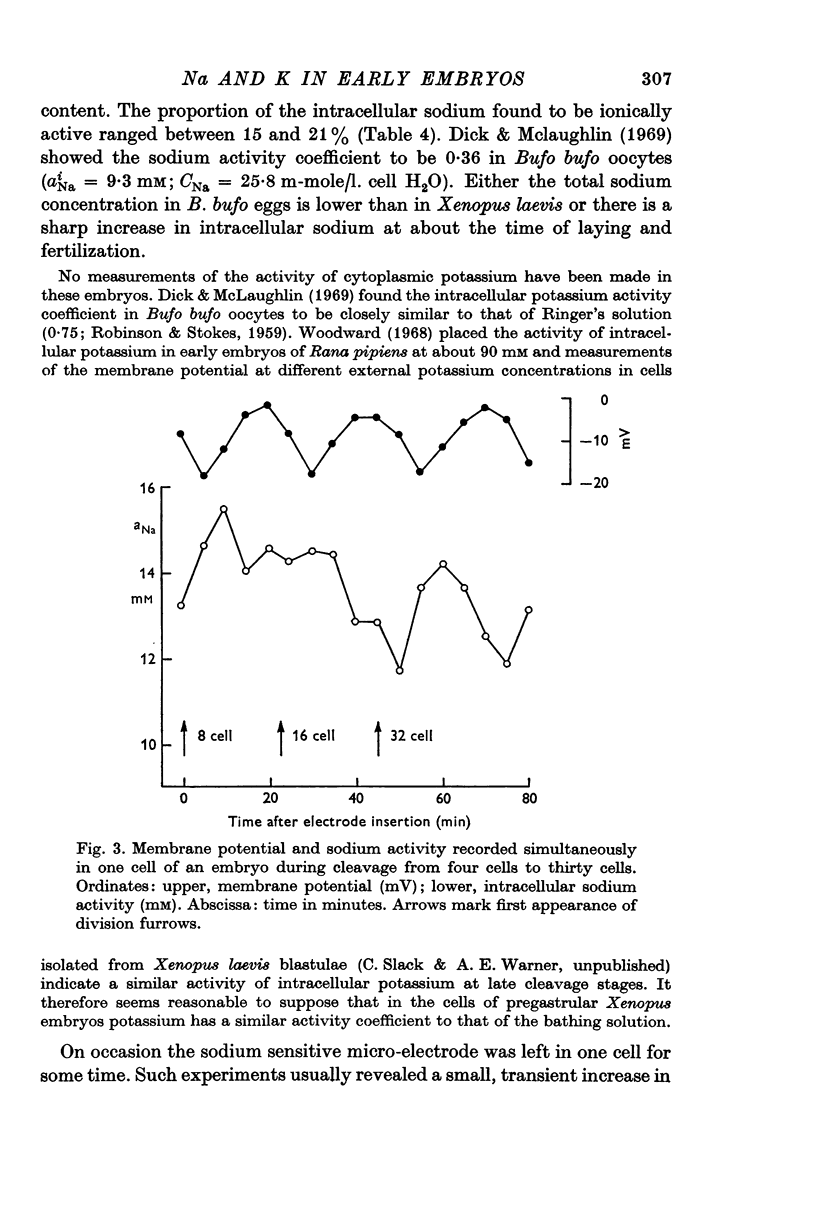
6. The intracellular sodium activity, measured with sodium sensitive intracellular micro-electrodes, is relatively constant between egg and blastula stages at about 14 mM. During each cell division cycle the intracellular sodium activity rises transiently by 2-3 mM.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol. 1956 Sep 27;133(3):631–658. doi: 10.1113/jphysiol.1956.sp005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTH L. G., BARTH L. J. Differentiation of cells of the Rana pipiens gastrula in unconditioned medium. J Embryol Exp Morphol. 1959 Jun;7:210–222. [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Warner A. E. Intracellular calcium and cell cleavage in early embryos of Xenopus laevis. J Cell Biol. 1972 May;53(2):579–581. doi: 10.1083/jcb.53.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher S. K., Warren R. L. Observations on the electrochemistry of the cochlear endolymph of the rat: a quantitative study of its electrical potential and ionic composition as determined by means of flame spectrophotometry. Proc R Soc Lond B Biol Sci. 1968 Nov 5;171(1023):227–247. doi: 10.1098/rspb.1968.0066. [DOI] [PubMed] [Google Scholar]
- Century T. J., Fenichel I. R., Horowitz S. B. The concentrations of water, sodium and potassium in the nucleus and cytoplasm of amphibian oocytes. J Cell Sci. 1970 Jul;7(1):5–13. doi: 10.1242/jcs.7.1.5. [DOI] [PubMed] [Google Scholar]
- DESMEDT J. E. Electrical activity and intracellular sodium concentration in frog muscle. J Physiol. 1953 Jul;121(1):191–205. doi: 10.1113/jphysiol.1953.sp004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid I. B. Deoxyribonucleic acid in amphibian eggs. J Mol Biol. 1965 Jul;12(3):581–599. doi: 10.1016/s0022-2836(65)80313-8. [DOI] [PubMed] [Google Scholar]
- Dick D. A., McLaughlin S. G. The activities and concentrations of sodium and potassium in toad oocytes. J Physiol. 1969 Nov;205(1):61–78. doi: 10.1113/jphysiol.1969.sp008951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINKE J. A. The measurement of sodium and potassium activities in the squid axon by means of cation-selective glass micro-electrodes. J Physiol. 1961 Apr;156:314–335. doi: 10.1113/jphysiol.1961.sp006678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Hori N. Electrical characteristics of Triturus egg cells during cleavage. J Gen Physiol. 1966 May;49(5):1019–1027. doi: 10.1085/jgp.49.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES K. W., ELSDALE T. R. The culture of small aggregates of amphibian embryonic cells in vitro. J Embryol Exp Morphol. 1963 Mar;11:135–154. [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The intracellular calcium contents of some invertebrate nerves. J Physiol. 1956 Nov 28;134(2):399–407. doi: 10.1113/jphysiol.1956.sp005652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalt M. R. The relationship between cleavage and blastocoel formation in Xenopus laevis. I. Light microscopic observations. J Embryol Exp Morphol. 1971 Aug;26(1):37–49. [PubMed] [Google Scholar]
- Kalt M. R. The relationship between cleavage and blastocoel formation in Xenopus laevis. II. Electron microscopic observations. J Embryol Exp Morphol. 1971 Aug;26(1):51–66. [PubMed] [Google Scholar]
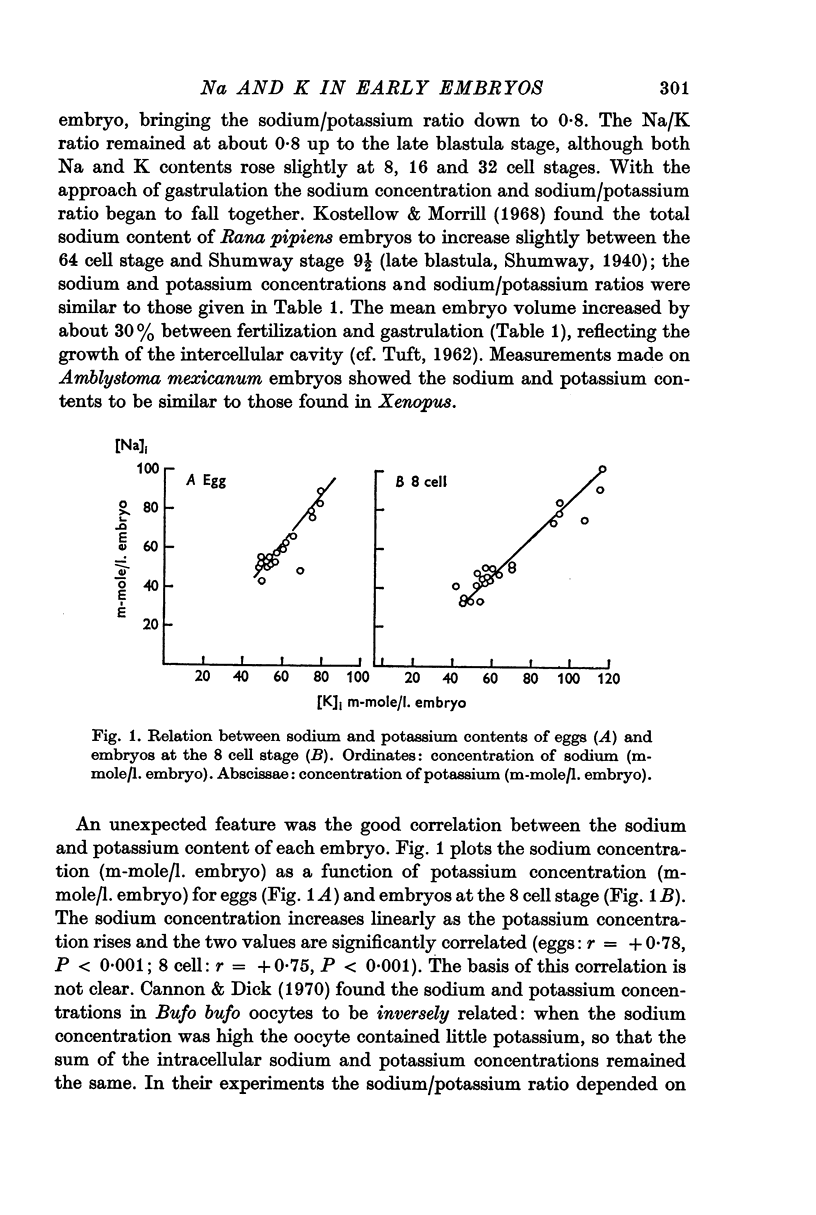
- Kostellow A. B., Morrill G. A. Intracellular sodium ion concentration changes in the early amphibian embryo and the influence on nuclear metabolism. Exp Cell Res. 1968 Jun;50(3):639–644. doi: 10.1016/0014-4827(68)90425-4. [DOI] [PubMed] [Google Scholar]
- Kroeger H., Lezzi M. Regulation of gene action in insect development. Annu Rev Entomol. 1966;11:1–22. doi: 10.1146/annurev.en.11.010166.000245. [DOI] [PubMed] [Google Scholar]
- Kuchler R. J. The role of sodium and potassium in regulating amino acid accumulation and protein synthesis in LM-strain mouse fibroblasts. Biochim Biophys Acta. 1967 Apr 25;136(3):473–483. doi: 10.1016/0304-4165(67)90006-2. [DOI] [PubMed] [Google Scholar]
- MAENO T. Electrical characteristics and activation potential of Bufo eggs. J Gen Physiol. 1959 Sep;43:139–157. doi: 10.1085/jgp.43.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill G. A. Water and electrolyte changes in amphibian eggs at ovulation. Exp Cell Res. 1965 Dec;40(3):664–667. doi: 10.1016/0014-4827(65)90245-4. [DOI] [PubMed] [Google Scholar]
- Muir C., Whitley J. E. Variation in the nuclear sodium concentration of newt oocytes during maturation. J Cell Sci. 1972 Mar;10(2):335–338. doi: 10.1242/jcs.10.2.335. [DOI] [PubMed] [Google Scholar]
- NAORA H., NAORA H., IZAWA M., ALLFREY V. G., MIRSKY A. E. Some observations on differences in composition between the nucleus and cytoplasm of the frog oocyte. Proc Natl Acad Sci U S A. 1962 May 15;48:853–859. doi: 10.1073/pnas.48.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
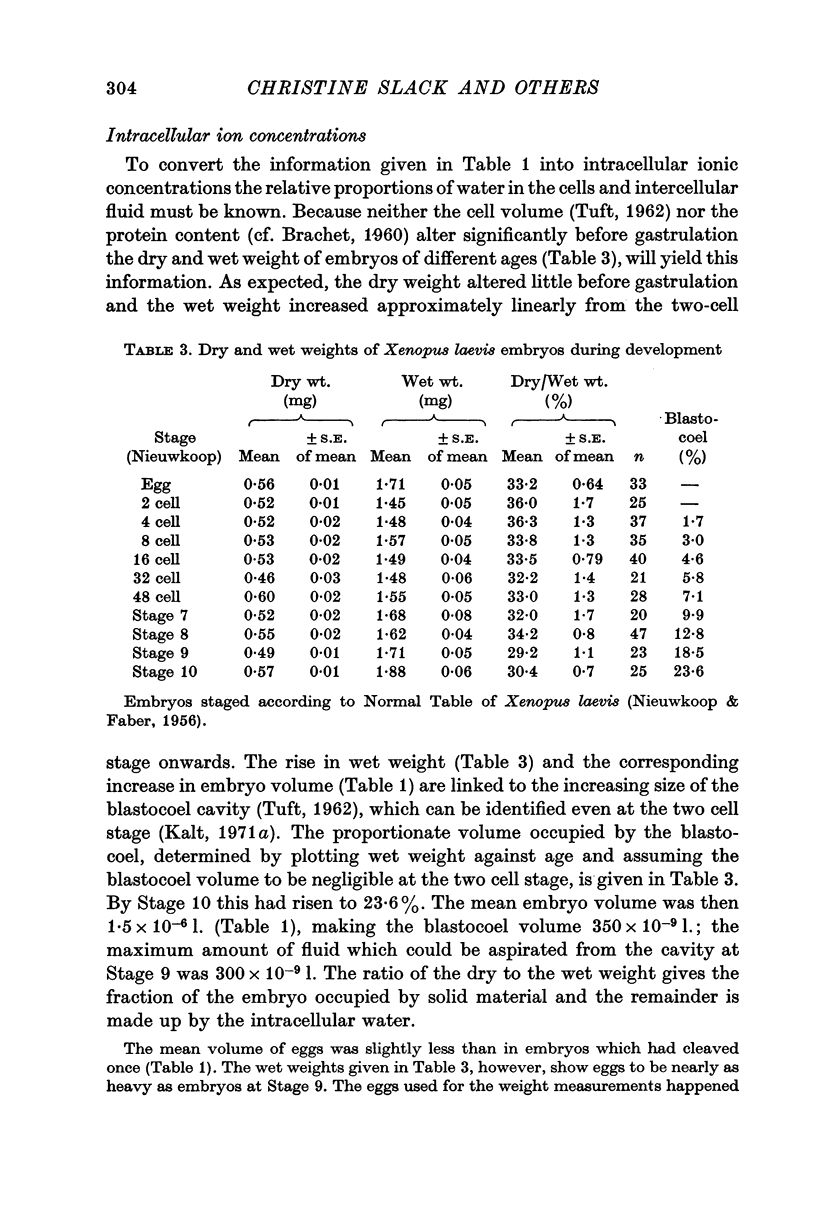
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Palmer J. F., Slack C. Some bio-electric parameters of early Xenopus embryos. J Embryol Exp Morphol. 1970 Nov;24(3):535–553. [PubMed] [Google Scholar]
- Riemann W., Muir C., Macgregor H. C. Sodium and potassium in oocytes of Triturus cristatus. J Cell Sci. 1969 Mar;4(2):299–304. doi: 10.1242/jcs.4.2.299. [DOI] [PubMed] [Google Scholar]
- Slack C., Warner A. E. Intracellular and intercellular potentials in the early amphibian embryo. J Physiol. 1973 Jul;232(2):313–330. doi: 10.1113/jphysiol.1973.sp010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stableford L. T. A study of calcium in the early development of the amphibian embryo. Dev Biol. 1967 Oct;16(4):303–314. doi: 10.1016/0012-1606(67)90044-9. [DOI] [PubMed] [Google Scholar]
- TUFT P. H. The uptake and distribution of water in the embryo of Xenopus laevis (Daudin). J Exp Biol. 1962 Mar;39:1–19. doi: 10.1242/jeb.39.1.1. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. New design for sodium-sensitive glass micro-electrode. J Physiol. 1970 Sep;210(2):82P–83P. [PubMed] [Google Scholar]
- Warren R. L. The determination of copper and magnesium in blood serum by high-resolution flame spectrophotometry. Analyst. 1965 Sep;90(74):549–553. doi: 10.1039/an9659000549. [DOI] [PubMed] [Google Scholar]
- Woodward D. J. Electrical signs of new membrane production during cleavage of Rana pipiens eggs. J Gen Physiol. 1968 Sep;52(3):509–531. doi: 10.1085/jgp.52.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]


