Abstract
We have examined the functional role of two internal cysteine residues of the F-plasmid TraV outer membrane lipoprotein. Each was mutated to a serine separately and together to yield three mutant traV genes: traVC10S, traVC18S, and traVC10S/C18S. All three cysteine mutations complemented a traV mutant for DNA donor activity and for sensitivity to donor-specific bacteriophage; however, when measured by a transduction assay, the donor-specific DNA bacteriophage sensitivities of the traVC18S and, especially, traVC10S/C18S mutant strains were significantly less than those of the traV+ and traVC10S strains. Thus, unlike the Agrobacterium tumefaciens T-plasmid-encoded VirB7 outer membrane lipoprotein, TraV does not require either internal cysteine to retain significant biological activity. By Western blot analysis, all three mutant TraV proteins were shown to accumulate in the outer membrane. However, by nonreducing gel electrophoresis, wild-type TraV and especially the TraVC18S mutant were shown to form mixed disulfides with numerous cell envelope proteins. This was not observed with the TraVC10S or TraVC10S/C18S proteins. Thus, it appears that TraV C10 is unusually reactive and that this reactivity is reduced by C18, perhaps by intramolecular oxidation. Finally, whereas the TraVC10S and TraVC18S proteins fractionated primarily with the outer membrane, as did the wild-type protein, the TraVC10S/C18S protein was found in osmotic shock fluid and inner membrane fractions as well as outer membrane fractions. Hence, at least one cysteine is required for the efficient localization of TraV to the outer membrane.
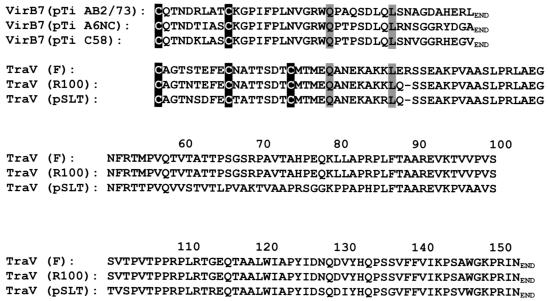
The F-plasmid outer membrane lipoprotein TraV anchors a multiprotein complex required for cells to elaborate F pili (5, 7), perhaps among other functions. In this respect, it resembles the VirB7 outer membrane lipoprotein encoded by Ti plasmids of Agrobacterium tumefaciens (2, 4). In other respects, however, the two proteins are quite different (Fig. 1). VirB7 polypeptides are only about 25% the size of TraV polypeptides, and their primary structures provide no evidence of homology.
FIG. 1.
Alignment of VirB7 encoded by the A. tumefaciens T plasmid and TraV encoded by F plasmid. The sequences begin with the N-terminal cysteine characteristic of outer membrane lipoproteins; leader peptide sequences have been omitted. All cysteines are indicated by dark shading, and two other identical residues are indicated by light shading. Accession numbers or references are as follows: for VirB7 from pTiAB2/73, AF329849; for VirB7 from pTiA6NC, J03216; for VirB7 from pTiC58, X53264; for TraV from F plasmid, Frost et al. (6); for TraV from R100, NP_052954; and for TraV from pSLT, NP_490570.
VirB7 and TraV do have four identical amino acids, two of which are cysteines (Fig. 1). As outer membrane lipoproteins, TraV and VirB7 are both characterized by N-terminal cysteines (after cleavage of their leader peptides), which are presumably modified by fatty acylation and serve to anchor the respective proteins in the outer membrane (15). Outer membrane VirB7 contains one internal cysteine, C10. This cysteine forms intermolecular disulfide bonds with a second VirB7 molecule or with C262 of VirB9. These bonds are critical to the stabilization of VirB7 in vivo (13). A VirB7C10S mutant failed to accumulate to levels detectable by Western blot analysis. However, when fused to PhoA, which dimerizes by way of noncovalent interactions, the VirB7C10S::PhoA polypeptide did accumulate, indicating that at least one major role for C10 of VirB7 is to stabilize the molecule through formation of disulfide-linked dimers (13).
TraV also contains internal cysteines (Fig. 1). One is at the same position relative to the N-terminal cysteine of the mature protein as the C10 of VirB7 is, and the other is 8 residues beyond, at position 18. We have examined the role of these internal cysteines in the function, accumulation, and localization of TraV.
We first mutated each cysteine in TraV to a serine. Mutant traV genes were synthesized by oligonucleotide-directed mutagenesis with three primers. In this method, two outside primers encompassing the entire traV sequence and encoding appropriate restriction sites were added at the usual concentration to three amplification PCR mixtures. The forward primer [GTGAACTGC(G→A)GATGAGAAAGG] corresponded to tra coordinates 6665 to 6684 (6); the G→A transition introduced a PstI site. The reverse primer [GGCTGATATA(C→G)AACTTCAGGGC] corresponded to tra coordinates 7234 to 7213; the C→G transition and deletion of a C (subscript) introduced an EcoRI site. A third, mutagenic primer was also added but in limiting amounts, usually 0.1, 0.2, and 0.4 mol/mol of outside primer. The mutagenic primers were GAATTTGAGT(G→C)TAACGCCACC for C10S and CCGATACCT(G→C)TATGACGATGG for C18S. After 30 cycles of amplification, the reaction products were combined, digested with PstI and EcoRI, and cloned into pUC19. Random transformants were then sequenced; the frequency of mutants was 10 to 50%. The traV double mutant was synthesized from traVC18S in a second PCR. The respective pUC19 traV derivatives were transformed into Escherichia coli strain RD17/pOX38 traV::cat. The traV::cat allele was constructed essentially as described previously (8). For functional assays, cells, including recipient cells for mating experiments, were routinely grown with aeration to optical densities (at 600 nm) of 0.3 to 0.6 in nutrient broth (1% tryptone, 0.1% yeast extract, 0.08% NaCl, 0.2% glucose, 5 mM concentrations each of MgCl2 and CaCl2). For cell fractionation experiments, cells were grown in Luria-Bertani broth to optical densities of 0.6 to 0.8. All cultures were grown in the absence of IPTG (isopropyl-β-d-thiogalactopyranoside). Without induction, TraV protein levels in cells containing pUC19 traV plasmids were shown by Western blot analysis to be similar to those observed in RD17/pOX38 tra+ cells.
We carried out four functional assays which determined the following characteristics: DNA donor activity (1); transduction to kanamycin resistance by donor-specific, filamentous bacteriophage M13K07 (1, 14); sensitivity to RNA bacteriophage R17 by plaque assay; and sensitivity to M13K07 by plaque assay. In all assays, the wild-type traV gene fully complemented the pOX38 traV::cat allele; this required increases in donor activity and bacteriophage sensitivity of at least 6 to 7 orders of magnitude (Table 1). The traVC10S allele likewise complemented the pOX38 traV mutation to a level that was within the same order of magnitude as wild-type traV. This observation, along with the data shown below, establishes that C10 of TraV and C10 of VirB7 have very different effects on the function and stability of the respective lipoproteins.
TABLE 1.
Effect of traV cysteine mutations on biological functions
| Plasmid(s) | Phage titer (PFU/ml)a
|
No. of Kanr reductants/cell by M13K07c | No. of Camr Tetr transconjugants cell for DNA donor activityd | |
|---|---|---|---|---|
| R17 | M13K07 | |||
| pOX38 traV::cat | <105 | <105 | <1.2 × 10−7 | <1 × 10−6 |
| + pUC traV | 1 × 1011 | 1 × 1011 | 0.21 | 1.0 |
| + pUC traVC10S | 0.4 × 1011 | 1 × 1011 | 0.41 | 0.22 |
| + pUC traVC18S | NDb | ND | 0.014 | 0.14 |
| + pUC traVC10/18S | 0.3 × 1011 | 0.5 × 1011 | 6.8 × 10−4 | 0.09 |
Titers on RD17/pOX38 tra+ cells were 1 × 1011 PFU/ml for R17 and 1.3 × 1011 PFU/ml for M13K07.
ND, not determined (see text).
Measured as described in reference 1.
The recipient strain was AE2248 (F− thr-34::Tn10).
The other two traV mutants were defective in one or another of the assays, though each did complement the pOX38 traV::cat mutation to a significant degree. The traVC18S allele was substantially normal in donor activity but exhibited reduced activity as a host for the M13K07 transducing bacteriophage. We were unable to do plaque assays with the traVC18S strain because the cells did not grow well enough to make this test reliable. The traVC18S strain also grew poorly in liquid culture. We suggest a possible explanation for these results later in this paper.
The double mutant also complemented the traV::cat allele for DNA donor activity, though perhaps not quite fully. However, the double mutant was even less active as a host for the M13K07 transducing bacteriophage than the traVC18S strain. Surprisingly, this failure to complement fully was not as evident in a plaque assay on solid medium; by this test, the traVC10S/C18S plasmid restored the sensitivities of both M13K07 and R17 to near normal levels. In summary, it does not appear that the internal cysteines of TraV are absolutely required for any of the functions associated with the role of TraV in F-pilus assembly and function. The data do not exclude the possibility that these cysteines play an as-yet-unidentified role in sensitivity to donor-specific DNA bacteriophage, at least under some conditions.
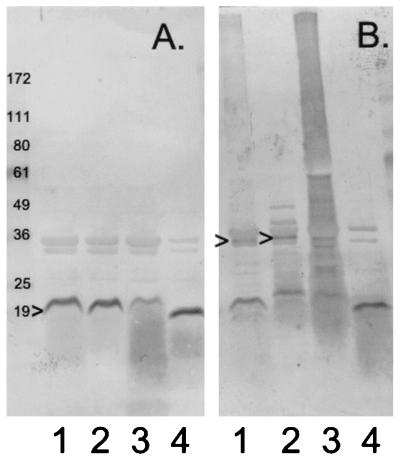
From the data in Table 1, we were able to infer that all of the TraV proteins should accumulate in the outer membrane of the cell. Western blot analysis (7) of purified outer membrane fractions confirmed this prediction (Fig. 2A). However, while TraV could be detected in outer membrane preparations from all four strains, TraV from the traVC18S strain contained the least amount. Moreover, the smudge that is visible below the TraVC18S band, but not, or to a lesser degree, beneath the other TraV bands, suggests that one reason for this quantitative difference is that TraVC18S is degraded. Hence, TraV C18 might contribute to TraV stability.
FIG. 2.
Accumulation of TraV proteins in the outer membrane. (A) Standard (reducing) gel; (B) nonreducing gel. Outer membrane fractions were obtained by banding in sucrose density gradients as described previously (9). Gels contained 4 to 20% polyacrylamide gradients. For nonreducing gels, mercaptoethanol was omitted from the sample buffer. Lanes 1, outer membrane from pOX38 traV::cat/pUC traV (wild-type) cells; lanes 2, outer membrane from pOX38 traV::cat/pUC traVC10S cells; lanes 3, outer membrane from pOX38 traV::cat/pUC traVC18S cells; lanes 4, outer membrane from pOX38 traV::cat/pUC traVC10S/C18S cells. The caret at the left of panel A indicates the TraV protein. The carets in panel B indicate possible TraV dimers. Numbers to the left of the figure are molecular masses in kilodaltons of marker proteins with the indicated mobilities.
We also examined these outer membrane fractions in nonreducing gels to determine whether either or both of the TraV cysteines reacted with other cell envelope proteins (Fig. 2B). In comparing the corresponding lanes of reducing gels, wild-type TraV and TraVC10S both appeared to form at least one disulfide-linked multimer corresponding to a molecular mass of about 36 kDa, twice the mass of TraV itself. Preferential formation of a TraV dimer would indicate that at least some TraV in the outer membrane is normally clustered. The TraVC10S protein also formed two distinct multimers, presumably heterodimers, with masses in the range of 36 to 49 kDa. Interestingly, there was no evidence for wild-type TraV trimers or higher-order multimers, even though, with two cysteines per TraV molecule, such multimers are possible.
Strikingly, outer membrane TraVC18S protein appeared to be promiscuous in its ability to form disulfide-linked complexes with other proteins. The lane of the nonreducing gel containing outer membrane protein from the traVC18S mutant (Fig. 2B, lane 3) exhibited a broad smear extending to the top of the gel. This smear was not evident in a reducing gel or in the nonreducing gel containing protein from the traVC10S mutant or the traVC10S/C18S double mutant. It was evident in the lane containing outer membrane protein from traV+ cells (Fig. 2B, lane 1) but to a much lesser degree; in some experiments, this material could be resolved into finely spaced bands near the top of the gel. To determine whether this high-molecular-mass material contained full-length TraV, we isolated it from pOX38 tra+ cell outer membrane by electrophoresis through a 2% agarose gel in sodium dodecyl sulfate gel running buffer. Fractions of this gel corresponding to masses of >172 kDa, which did contain material reactive with anti-TraV antibodies on a Western blot, were then electrophoresed through a 4 to 20% polyacrylamide gradient gel with or without mercaptoethanol. With mercaptoethanol, all of the fractions yielded full-length TraV as shown by Western blotting, with no evidence of degradation products; without mercaptoethanol, immunoreactive material remained at the top of the gel (data not shown). Hence, outer membrane TraV can form disulfide linkages, though most of the wild-type protein was recovered as the monomer after electrophoresis through nonreducing gels (Fig. 2, lanes 1).
Our data indicate that TraV C10 alone is extremely reactive (Fig. 2B, lane 3) but that the presence of C18 reduces C10 reactivity, perhaps by maintaining C10 in C10-C18 intramolecular disulfide linkage. Under this hypothesis, the traVC18S strain might grow poorly because of the accumulation of abnormal protein aggregates formed by disulfide bridges between C10 of outer membrane TraV and cysteine sulfhydryls of other periplasmic proteins.
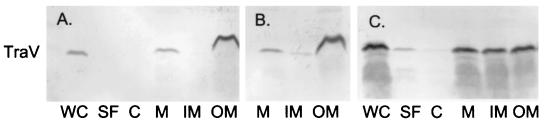
The internal cysteines of TraV are also required for efficient TraV localization to the outer membrane. We fractionated different strains into periplasmic proteins, cytoplasmic proteins, crude membrane proteins, inner membrane proteins, and outer membrane proteins as described previously (7). As expected (7), wild-type TraV fractionated exclusively with the outer membrane (Fig. 3A). Similar results were obtained with the TraVC10S and TraVC18S mutants (data not shown), except that in the former case, a small amount of TraV fractionated with the inner membrane via sucrose gradient sedimentation (Fig. 3B). In contrast, TraVC10S/C18S was found in all fractions, including shock fluid and soluble (cytoplasmic) material (Fig. 3C). Even so, most protein fractionated with crude membrane, although roughly equal quantities appeared in inner and outer membrane fractions separated by banding in sucrose gradients (9). Thus, for efficient localization of TraV to the outer membrane, either C10 or C18 is required in addition to the N-terminal cysteine characteristic of outer membrane lipoproteins (15), including TraV (3).
FIG. 3.
TraV content of different subcellular fractions. Cells were fractionated as described previously (7). WC, whole-cell extract; SF, osmotic shock fluid; C, cytoplasmic proteins; M, crude membranes; IM, purified inner membrane; OM, purified outer membrane. (A) pOX38 traV::cat/pUC traV (wild-type) cells; (B) pOX38 traV::cat/pUC traVC10S cells (membrane fractions only); (C) pOX38 traV::cat/pUC traVC10S/C18S cells.
To summarize, unlike C10 of VirB7, neither of the internal cysteines of F-plasmid-encoded TraV is absolutely required for formation of conjugative pili, as inferred from functional assays, or for protein accumulation or stability. These cysteines together do play a role in the efficient localization of TraV to the outer membrane. Perhaps most interestingly, one of these cysteines, C10, appears to be relatively reactive in the absence of C18, forming mixed disulfides with many different cell envelope proteins. Presumably, C18 destabilizes these mixed disulfides by its ability to form an intramolecular disulfide with C10, as proposed for the E. coli thiol oxidase DsbA (16). Our data (Fig. 3B, lanes 1 and 3) may be related to these observations regarding DsbA. While the reactivity of TraV C10 and the apparent effect of C18 on C10 reactivity suggests that TraV may have thiol oxidase or disulfide isomerase activity (16), the sequence between the TraV cysteines is four times longer than the dipeptide separating the cysteines of DsbA and other members of the thioredoxin superfamily of thiol:disulfide oxidoreductases (10). Finally, it may seem strange that mixed disulfides accumulate in the presence of disulfide bond isomerases, such as DsbC, in the E. coli periplasm (12). However, these enzymes work preferentially on intramolecular disulfides, whereas abnormal intermolecular disulfides may trigger extracytoplasmic stress responses instead (11).
Acknowledgments
We are indebted to Marjorie Russel for providing information on the electrophoretic separation of proteins through agarose gels.
This work was supported by NSF grant MCB 9900553 and funds from the Oklahoma Medical Research Foundation. P.M.S. acknowledges support from the Marjorie Nichlos Chair in Medical Research.
REFERENCES
- 1.Anthony, K. G., W. A. Klimke, J. Manchak, and L. S. Frost. 1999. Comparison of proteins involved in pilus synthesis and mating pair stabilization from the related plasmids F and R100-1: insights into the mechanism of conjugation. J. Bacteriol. 181:5149-5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christie, P. J. 1997. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in eubacteria. J. Bacteriol. 179:3085-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doran, T. J., S. M. Loh, N. Firth, and R. A. Skurray. 1994. Molecular analysis of the F plasmid traVR region: traV encodes a lipoprotein. J. Bacteriol. 176:4182-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez, D., G. M. Spudich, X.-R. Zhou, and P. J. Christie. 1996. The Agrobacterium tumefaciens VirB7 lipoprotein is required for stabilization of VirB proteins during assembly of the T-complex transport apparatus. J. Bacteriol. 178:3168-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Firth, N., K. Ippen-Ihler, and R. A. Skurray. 1996. Structure and function of the F factor and mechanism of conjugation, p. 2377-2401. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 6.Frost, L. S., K. Ippen-Ihler, and R. A. Skurray. 1994. Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol. Rev. 58:162-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris, R. L., V. Hombs, and P. M. Silverman. 2001. Evidence that F-plasmid proteins TraV, TraK and TraB assemble into an envelope-spanning structure in Escherichia coli. Mol. Microbiol. 42:757-766. [DOI] [PubMed] [Google Scholar]
- 8.Moore, D., K. Maneewannakul, S. Maneewannakul, J. H. Wu, K. Ippen-Ihler, and D. E. Bradley. 1990. Characterization of the F-plasmid conjugative transfer gene traU. J. Bacteriol. 172:4263-4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osborne, M., J. Gander, E. Parisi, and J. Carson. 1972. Mechanism of assembly of the outer membrane of Salmonella typhimurium. J. Biol. Chem. 247:3962-3972. [PubMed] [Google Scholar]
- 10.Raina, S., and D. Missiakis. 1997. Making and breaking disulfide bonds. Annu. Rev. Microbiol. 51:179-202. [DOI] [PubMed] [Google Scholar]
- 11.Raivio, T. L., and T. J. Silhavy. 2001. Periplasmic stress and ECF sigma factors. Annu. Rev. Microbiol. 55:591-624. [DOI] [PubMed] [Google Scholar]
- 12.Ritz, D., and J. Beckwith. 2001. Roles of thiol-redox pathways in bacteria. Annu. Rev. Microbiol. 55:21-48. [DOI] [PubMed] [Google Scholar]
- 13.Spudich, G. M., D. Fernandez, X.-R. Zhou, and P. J. Christie. 1996. Intermolecular disulfide bonds stabilize VirB7 homodimers and VirB7/VirB9 heterodimers during biogenesis of the Agrobacterium tumefaciens T-complex transport apparatus. Proc. Natl. Acad. Sci. USA 93:7512-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vieira, J., and J. Messing. 1987. Production of single-stranded plasmid DNA. Methods Enzymol. 153:3-11. [DOI] [PubMed] [Google Scholar]
- 15.Wu, H. C. 1996. Biosynthesis of lipoproteins, p. 1005-1014 In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 16.Zapun, A., L. Cooper, and T. E. Creighton. 1994. Replacement of the active-site cysteine residues of DsbA, a protein required for disulfide bond formation in vivo. Biochemistry 33:1907-1914. [DOI] [PubMed] [Google Scholar]