Abstract
1. Micro-electrode recordings have been made from single neurones in the dorsal horn of male rats anaesthetized with urethane. Scrotal temperature was altered within the range 13-43° C by means of a thermode. The mean firing rate of neurones was correlated with step and ramp changes of temperature.
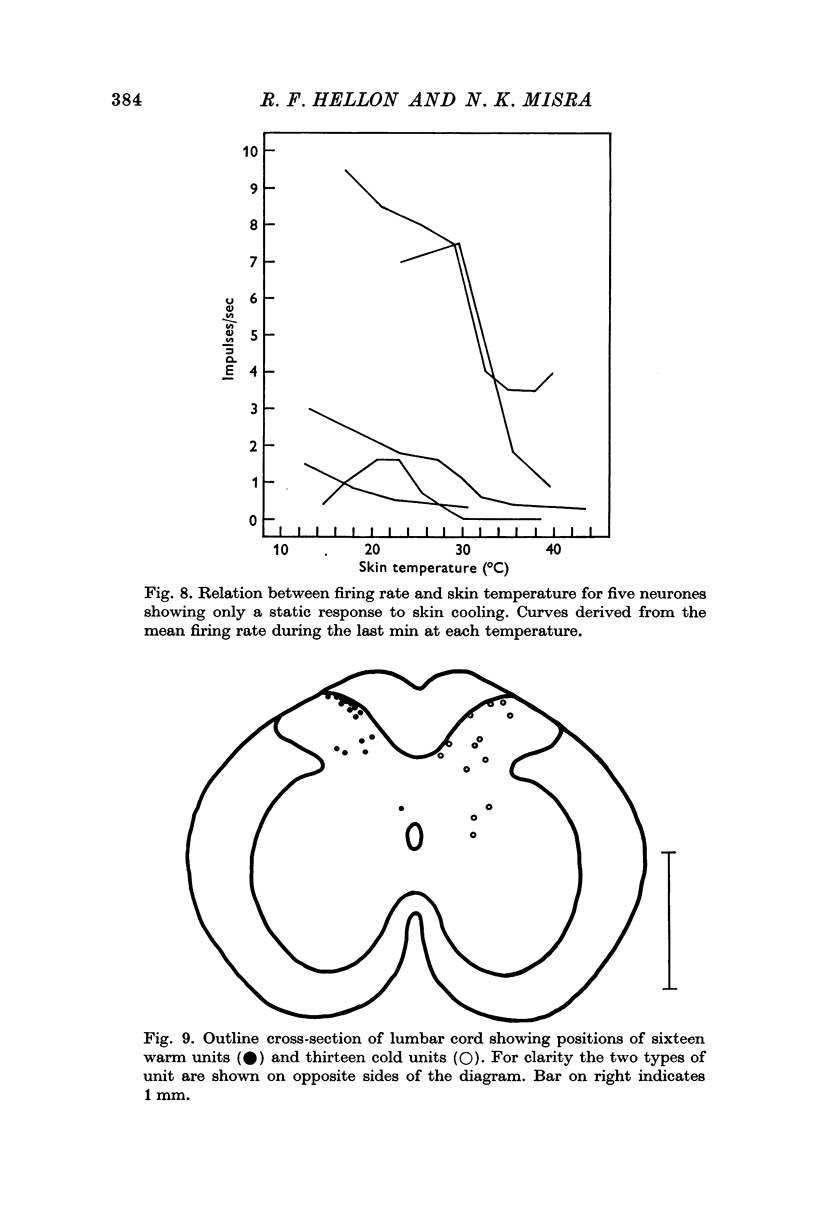
2. In the region where the scrotal nerve enters the cord, 47% of the neurones were responsive to scrotal temperature: half were excited by warming and half by cooling. Most of these thermally responding units were not affected when the scrotal skin was touched and only one-fifth responded to both modalities.
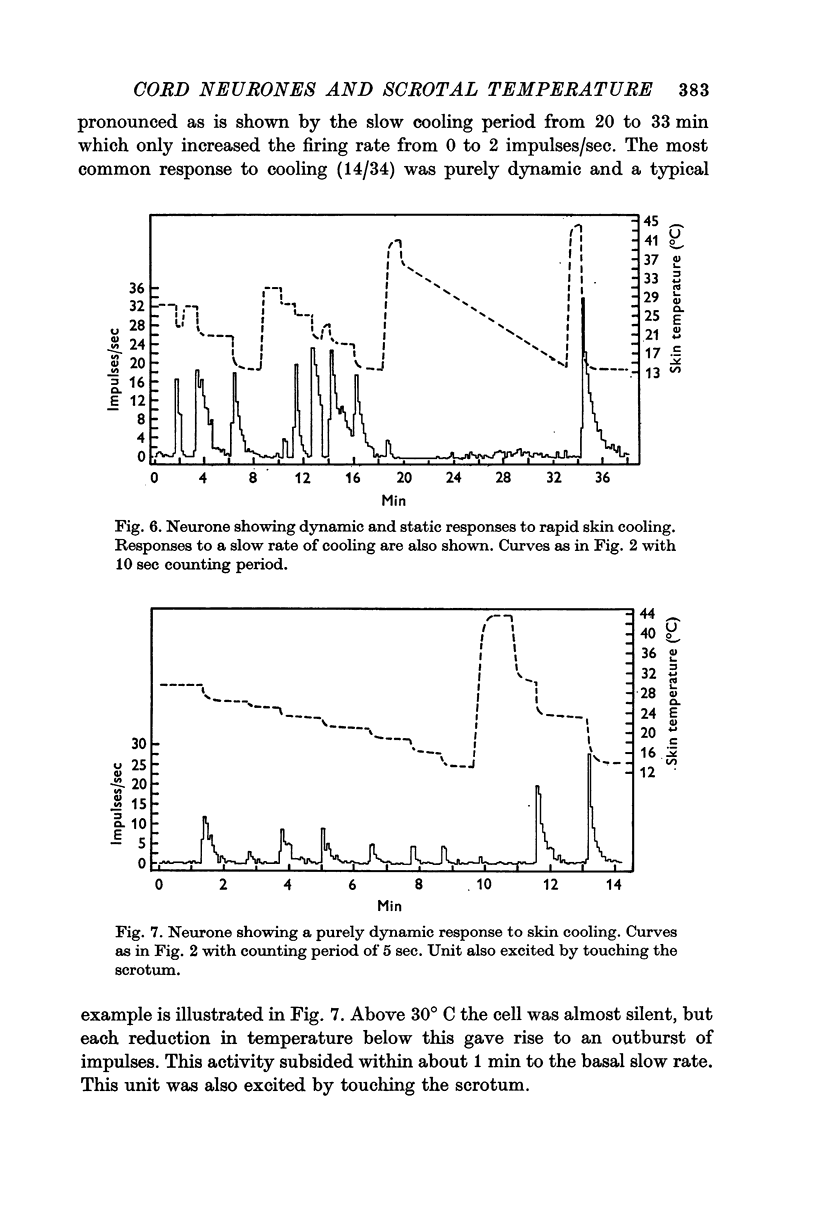
3. Both the `warm' and `cold' groups of neurones showed responses to step changes of temperature which were classified as dynamic plus static, dynamic only or static only. Comparison of these responses with those published for the scrotal thermoreceptors showed that the incoming thermal information was being processed in the dorsal horn.
4. Histological examination of the cord showed that recording sites were in laminae I to V of the dorsal horn.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP P. O., BURKE W., DAVIS R. The identification of single units in central visual pathways. J Physiol. 1962 Aug;162:409–431. doi: 10.1113/jphysiol.1962.sp006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K., Brengelman G., Sundsten J. W. Evidence for spinal cord unit activity responsive to peripheral warming in the primate. Brain Res. 1972 Aug 25;43(2):657–661. doi: 10.1016/0006-8993(72)90425-8. [DOI] [PubMed] [Google Scholar]
- Duclaux R., Kenshalo D. R. The temperature sensitivity of the type I slowly adapting mechanoreceptors in cats and monkeys. J Physiol. 1972 Aug;224(3):647–664. doi: 10.1113/jphysiol.1972.sp009917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor M., Zimmermann M. Characteristics of spinal neurones responding to cutaneous myelinated and unmyelinated fibres. J Physiol. 1972 Mar;221(3):555–576. doi: 10.1113/jphysiol.1972.sp009767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENSEL H., ZOTTERMAN Y. The response of mechanoreceptors to thermal stimulation. J Physiol. 1951 Sep;115(1):16–24. doi: 10.1113/jphysiol.1951.sp004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales J. R., Hutchinson J. C. Metabolic, respiratory and vasomotor responses to heating the scrotum of the ram. J Physiol. 1971 Jan;212(2):353–375. doi: 10.1113/jphysiol.1971.sp009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F. The marking of electrode tip positions in nervous tissue. J Physiol. 1971;214 (Suppl):12P–12P. [PubMed] [Google Scholar]
- Iggo A. Cutaneous thermoreceptors in primates and sub-primates. J Physiol. 1969 Feb;200(2):403–430. doi: 10.1113/jphysiol.1969.sp008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram D. L., Legge K. F. The influence of deep body and skin temperatures on thermoregulatory responses to heating of the scrotum in pigs. J Physiol. 1972 Jul;224(2):477–487. doi: 10.1113/jphysiol.1972.sp009906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINZELL J. L., BLIGH J. Polypnoea evoked by heating the udder of the goat. Nature. 1961 Apr 8;190:173–173. doi: 10.1038/190173a0. [DOI] [PubMed] [Google Scholar]
- Lewin J. E. A counter for recording the rate of firing of neurones. J Physiol. 1972 Apr;222(2):132P–133P. [PubMed] [Google Scholar]
- Poulos D. A., Lende R. A. Response of trigeminal ganglion neurons to thermal stimulation of oral-facial regions. I. Steady-state response. J Neurophysiol. 1970 Jul;33(4):508–517. doi: 10.1152/jn.1970.33.4.508. [DOI] [PubMed] [Google Scholar]
- Poulos D. A., Lende R. A. Response of trigeminal ganglion neurons to thermal stimulation of oral-facial regions. II. Temperature change response. J Neurophysiol. 1970 Jul;33(4):518–526. doi: 10.1152/jn.1970.33.4.518. [DOI] [PubMed] [Google Scholar]
- Rowe M. J., Sessle B. J. Responses of trigeminal ganglion and brain stem neurones in the cat to mechanical and thermal stimulation of the face. Brain Res. 1972 Jul 20;42(2):367–384. doi: 10.1016/0006-8993(72)90537-9. [DOI] [PubMed] [Google Scholar]
- SZENTAGOTHAI J. NEURONAL AND SYNAPTIC ARRANGEMENT IN THE SUBSTANTIA GELATINOSA ROLANDI. J Comp Neurol. 1964 Apr;122:219–239. doi: 10.1002/cne.901220207. [DOI] [PubMed] [Google Scholar]
- Steiner T. J., Turner L. M. Cytoarchitecture of the rat spinal cord. J Physiol. 1972 Apr;222(2):123P–125P. [PubMed] [Google Scholar]
- WAITES G. M. The effect of heating the scrotum of the ram on respiration and body temperature. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:314–323. doi: 10.1113/expphysiol.1962.sp001615. [DOI] [PubMed] [Google Scholar]


