Abstract
Background
The characteristic ECG pattern of ST-segment elevation in V1 and V2 in the Brugada syndrome is dynamic; it is often intermittently present in affected individuals and can be unmasked by sodium channel blockers, including antiarrhythmic drugs and tricyclic antidepressants. We report here 2 patients who developed the Brugada ECG pattern after administration of lithium, a commonly used drug not previously reported to block cardiac sodium channels.
Methods and Results
Lithium induced transient ST-segment elevation (type 1 Brugada pattern) in right precordial leads at therapeutic concentrations in 2 patients with bipolar disorder. Lithium withdrawal in the patients resulted in reversion to type 2 or 3 Brugada patterns or resolution of ST-T abnormalities. In Chinese hamster ovary cells transfected with SCN5A, which encodes the cardiac sodium channel, lithium chloride caused concentration-dependent block of peak INa at levels well below the therapeutic range (IC50 of 6.8±0.4 μmol/L).
Conclusions
The widely used drug lithium is a potent blocker of cardiac sodium channels and may unmask patients with the Brugada syndrome.
Keywords: Brugada syndrome, lithium, drugs, genetics, ion channels
The Brugada syndrome (BS) is a familial disease that displays an autosomal dominant mode of transmission, with incomplete penetrance of the characteristic ECG phenotype and an incidence ranging between 5 and 66 per 10 000.1,2 Sudden death in patients with BS is thought to be caused by polymorphic ventricular tachycardia or fibrillation. Mutations in SCN5A, the α-subunit of the cardiac sodium channel, account for ≈20% of cases.3
The ECG signature of the BS is dynamic and often concealed, but it can be unmasked by sodium channel blockers such as flecainide, ajmaline, and procainamide.4 Other agents that have been shown to unmask or accentuate ST-segment elevation in patients with BS include vagotonic agents, α-adrenergic agonists, β-adrenergic blockers, first-generation antihistamines (dimenhydrinate), cocaine toxicity, and tricyclic antidepressants.4–12 We report here 2 cases of lithium-associated BS. Although this widely used drug has been available for decades for the treatment of bipolar disorder13,14 and can compete with sodium for permeation through sodium channels,15–17 it has not been studied as a blocker of the cardiac sodium channel.
Methods
Patient Presentations
Patient 1
A 26-year-old man was referred after a routine ECG was noted to show ≥2-mm J-wave amplitude, coved ST-T configuration with negative T wave in leads V1 to V3 (Figure 1, top), consistent with type 1 BS ECG pattern. The patient had a long history of bipolar disorder that continued to worsen despite trials of bupropion, sertraline, and lamotrigine. Two months before presentation, the patient was started on escalating doses of lithium. An ECG before lithium initiation was normal (Figure 1, bottom). At the time of evaluation, he was on a maintenance dose of 900 mg once per day, and the lithium level was 1.0 mEq/L, within the therapeutic range of 0.8 to 1.2 mEq/L (800 to 1200 μmol/L). Although the patient denied a recent history of syncope, he did describe “fading out” episodes that started as a teenager. A witnessed account by his wife (a nurse) described episodes that typically occurred at rest; a glazed look would come over the patient’s eyes, he would become unresponsive and then slide to the ground. These episodes had never occurred with exercise or when standing. The patient denied a preceding history of palpitations, lightheadedness, dizziness, or presyncope. His last episode of syncope occurred 2 years before evaluation. The patient denied other cardiac symptoms. There was a family history of hypertension and diabetes mellitus and sudden death in a paternal cousin at age 6 months.
Figure 1.
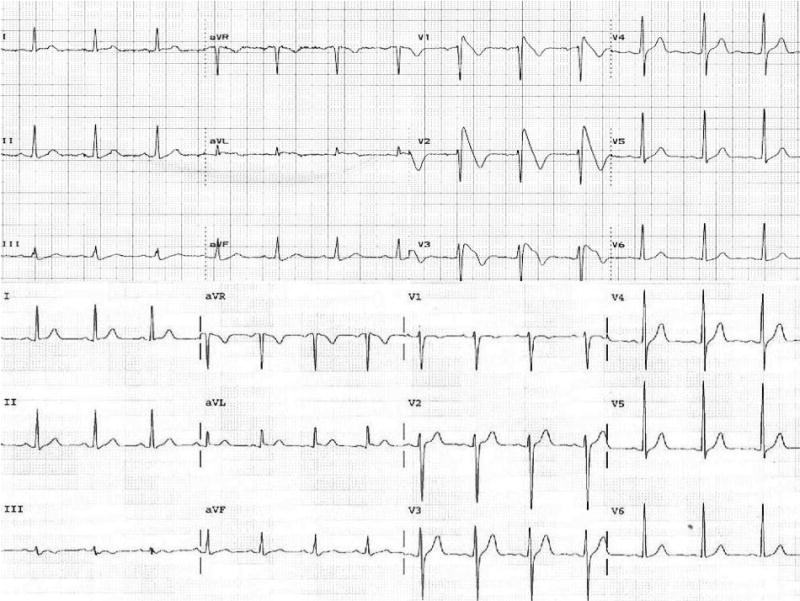
Top, Twelve-lead surface ECG recorded 1 month after lithium therapy was started in patient 1 showing coved-type ST-segment elevation (type 1) in leads V1 to V3 typical of that described for BS. Bottom, ECG taken before initiation of lithium in patient 1 showing no repolarization abnormalities.
Lithium was stopped and the patient was started on clonazepam and lamotrigine 2 days after the ECG shown in Figure 1 (top). Physical examination was unremarkable. An ECG performed 6 weeks later showed a type 2 (J-wave amplitude ≥2 mm, gradually descending ST-T segment with biphasic or “saddleback” appearance) Brugada pattern (Figure 1, top). A cardiac MRI showed normal left and right ventricular size and function and no evidence for arrhythmogenic right ventricular dysplasia. At electrophysiology study, basal measurements of conduction intervals were normal, and programmed ventricular stimulation with 3 premature extrastimuli induced sustained ventricular fibrillation that required defibrillation (Figure 2, bottom). An implantable cardioverter-defibrillator (ICD) was implanted. Molecular genetic analysis revealed no mutations in SCN5A.
Figure 2.
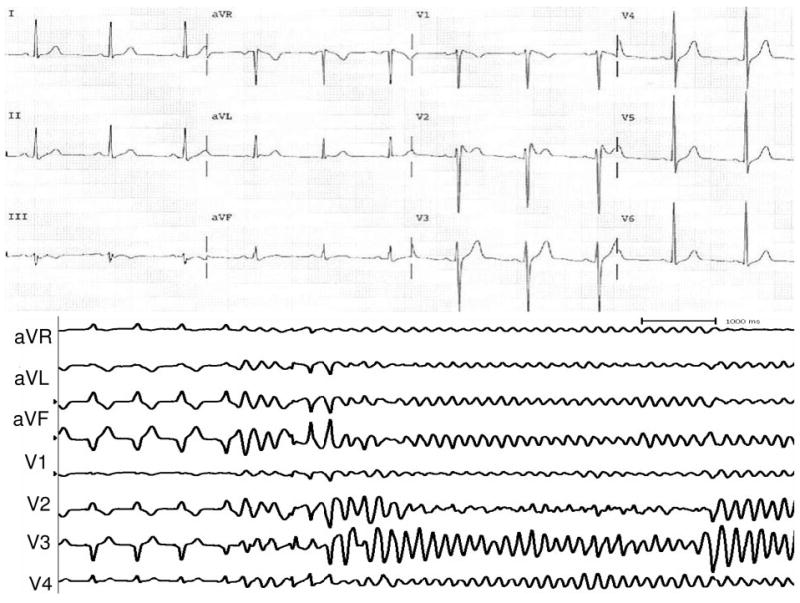
Top, Repeat ECG after withdrawal of lithium showed persistent ST elevation in precordial leads but a “saddleback” (type 2) pattern in patient 1. Bottom, Programmed ventricular stimulation with 3 premature extrastimuli induced sustained ventricular fibrillation requiring defibrillation in patient 1.
Although an ECG recording 1 month after ICD implantation continued to show repolarization changes with a type 3 (ST segment of <1 mm of saddleback type, coved type, or both) Brugada pattern, numerous ECGs over the 6-month follow-up period have revealed no ST-T abnormalities. No ventricular arrhythmias have been recorded by the ICD to date. The patient continues to take lamotrigine and clonazepam for the bipolar disorder.
Patient 2
A 65-year-old man with manic depression had been taking lithium (1200 mg once per day) for almost 20 years when he developed paroxysmal atrial fibrillation associated with sick sinus syndrome. The patient also gave a history of recurrent syncope. An ECG performed after 1 syncopal episode showed a type 1 BS pattern associated with PR-interval prolongation (Figure 3, top). There was a family history of sudden cardiac death, with a brother and twin maternal uncles all dying suddenly at age 50 years. Physical examination was unremarkable. An echocardiogram revealed only mild aortic stenosis (maximal gradient 34 mm Hg). At electrophysiology study, the His to ventricle interval was prolonged (69 ms), and programmed electrical stimulation from the right ventricular outflow tract induced only nonsustained ventricular tachycardia. An ICD was implanted. Although the patient had been maintained on the same dose of lithium for many years, 1 year after ICD implantation, the patient presented with symptoms consistent with lithium toxicity. An ECG continued to show a type 1 BS ECG pattern. However, when the dose of lithium was lowered (400 mg once per day), the ECG normalized (Figure 3, bottom), which suggests that lithium was responsible for unmasking the BS in this patient. Molecular genetic analysis in this patient has revealed no mutations in SCN5A.
Figure 3.
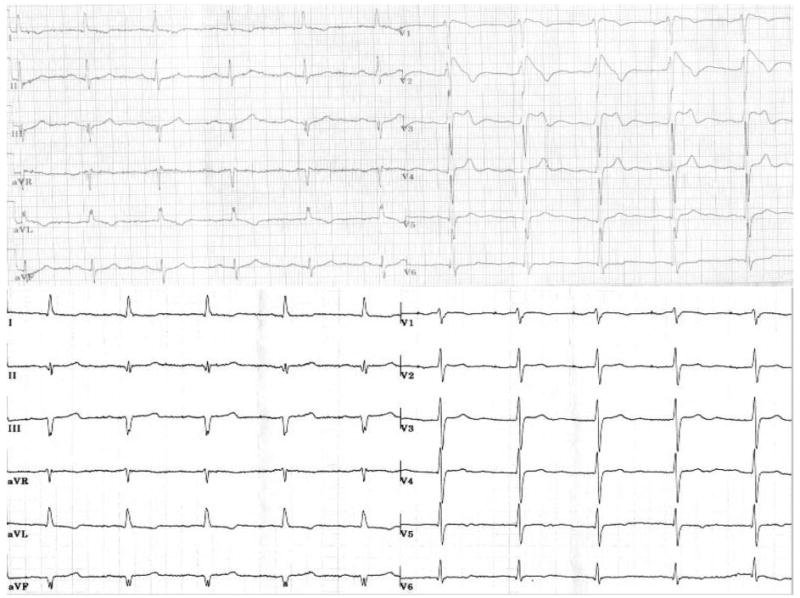
Top, Twelve-lead surface ECG recorded from patient 2 during lithium therapy (1200 mg), showing typical type 1 BS pattern with PR-interval prolongation. Bottom, ECG taken after the dose of lithium was lowered (400 mg), showing resolution of ST-T abnormalities.
Heterologous Expression of Human SCN5A Sodium Channels
The cardiac sodium channel was studied in Chinese hamster ovary cells by cotransfection of 2 μg of a plasmid encoding the α-subunit, pRcCMV-hSCN5A (SuperFect, Qiagen Inc), and 2 μg of a second bicistronic plasmid, pGFP-IRES-hβ1, encoding green fluorescent protein (GFP) as a marker and the human β1-subunit.18 Forty-eight hours after transfection, cells expressing GFP were selected for electrophysiology studies.
Electrophysiology and Solutions
SCN5A channel currents (INa) were recorded with whole-cell voltage clamp as described previously.19 Glass microelectrode resistance ranged from 1.0 to 1.5 MΩ. Data acquisition was performed with an Axopatch 200A patch-clamp amplifier and pCLAMP 7.0 software (Axon Instruments Inc). Currents were filtered at 5 kHz (−3 dB, 4-pole Bessel filter) and digitized with an analog-to-digital interface (Digidata 1200B, Axon Instruments). To minimize the capacitive transients, 70% to 80% of the cell capacitance and series resistance were compensated. Maximal INa was elicited with 50-ms repetitive pulses at a rate of 2 Hz (120 bpm) to −30 mV from a holding potential of −120 mV. The repetitive pulses were repeated at a rate of 1 Hz (60 bpm) to assess whether lithium-induced block of INa was use-dependent. The bath solution contained (in mmol/L) NaCl 130, KCl4, CaCl2 1.8, MgCl2 1, HEPES 10, and glucose 10, pH 7.35 (adjusted with NaOH). The electrode-filling solution contained (in mmol/L) KCL 140, MgCl2 1, MgATP 4, NaCl 5, EGTA 5, and HEPES 10, pH 7.4 (adjusted with CsOH).18 A stock solution of 10 mmol/L LiCl (Sigma) was made to examine the effect of LiCl on INa at different concentrations. Peak sodium current was ≈3 nA and could be controlled in these cells (average size 20 to 40 pF; large microelectrodes [≈1 MΩ]).
Data Analysis
Peak INa was measured on pulsing from −120 to −30 mV. The concentration blocking half the current (IC50) was determined by fitting mean data to the logistic equation y=1/(1 + {[C]/IC50}h), where [C] is the LiCl concentration and h is the Hill coefficient. Results are presented as mean±SE.
Results
Figure 4 (inset) shows representative INa traces recorded before and after acute exposure to a range of concentrations of LiCl. The IC50 for INa block by LiCl was 6.8±0.4 μmol/L, with a Hill coefficient of 0.86. Although maximal INa was reduced in a dose-dependent manner, gating kinetics were not affected by lithium. Additional experiments showed no effect on steady state inactivation. When pulsing at a rate of 1 Hz (60 bpm), 10 μmol/L lithium blocked peak INa by 50% to 60%, similar to that at 2 Hz. Thus, lithium-induced INa block does not appear to be use dependent in this range.
Figure 4.
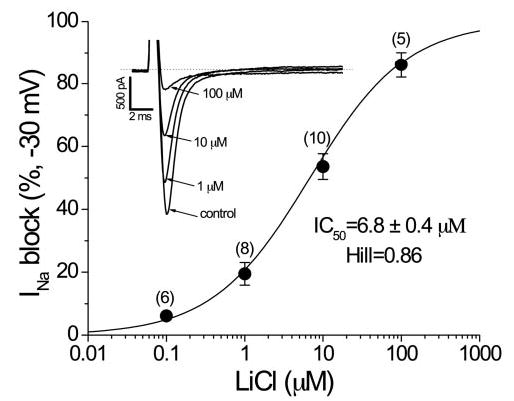
Inset shows representative INa traces recorded before and after acute exposure to different concentrations of LiCl. In these experiments, maximal INa was elicited with 50-ms repetitive pulses to −30 mV from a holding potential of −120 mV. An IC50 for INa block by LiCl was generated: 6.8±0.4 μmol/L with a Hill coefficient of 0.86.
Discussion
Previous case reports have documented that tricyclic antidepressants can unmask BS by sodium channel blockade.7,10 However, despite its widespread use in psychiatric therapy, there are no reports about the cardiac sodium channel–blocking properties of lithium and whether lithium may unmask the BS. We report here 2 cases of lithium-associated BS and demonstrate that lithium is a blocker of the cardiac sodium channel at concentrations below those achieved in therapy.
The BS should be strongly considered in patients with type 1 ST-segment elevation (coved type) in more than 1 right precordial lead (V1 to V3) in the presence or absence of a sodium channel blocker, in association with 1 or more clinical symptoms that include syncope, a family history of sudden cardiac death (<45 years) or electrophysiological inducibility and no other explanation for the ECG abnormality.2 In patient 1, the diagnostic type 1 Brugada ECG pattern only became apparent after lithium treatment was begun; an ECG before initiation was normal. In patient 2, a lower dose of lithium resulted in resolution of repolarization changes. Both of these cases strongly support a causal relationship between lithium and unmasking of type 1 BS ECG pattern. It is possible, however, that repolarization abnormalities seen in patient 1 after withdrawal of lithium could have been related to lamotrigine therapy. There is one report that suggests that lamotrigine affects the sodium channel.20 However, an ECG performed while the patient was taking lamotrigine, before lithium initiation, failed to demonstrate repolarization abnormalities. In addition, numerous ECGs after ICD implantation also have not shown any ST-T abnormalities. Thus, persistence of type 2 and type 3 BS ECG patterns after discontinuation of lithium in patient 1 is unlikely to be related to substitution of lamotrigine. There are no reports of clonazepam exacerbating or unmasking BS at therapeutic or toxic doses.
The Brugada ECG pattern has been attributed to differences in action potential duration and configuration between the endocardium and epicardium, mainly in the right ventricle.3 Recently, it has also been shown that accentuation of the right ventricular epicardial notch may be sufficient to explain development of the Brugada ECG.21 In theory, a reduction in INa or L-type ICa or an increase in Ito (dominant at epicardium) would produce a striking abbreviation of the epicardial action potential, which would lead to an epicardial-endocardial heterogeneity of repolarization that would cause ST-segment elevation. To date, not only reductions in sodium current caused by drugs or mutations in SCN5A but also electrolyte abnormalities and trauma to the right ventricle have been reported to induce the syndrome.22
Patients with proven BS can have normal ECG recordings at other times.1 In these cases, the abnormal ECG often can be reproduced by antiarrhythmic drugs, such as flecainide, procainamide, or ajmaline, which block the sodium channel.4,5 Autonomic influence also is important. β-Adrenergic blockade increases ST-segment elevation, whereas β-adrenergic stimulation is expected to do the opposite.5 Enhanced ST-segment elevation has also been observed after α-adrenergic and muscarinic stimulation.5
Lithium is a commonly used drug in the treatment of depressive and bipolar affective disorders.13,14 Its cardiac side effects, ranging from benign to severe, have been described at both therapeutic and toxic serum levels in adult patients.23 Asymptomatic ECG changes are commonly seen and include nonspecific T-wave changes. Other conduction defects and rhythm disturbances have also been reported, including sinus node dysfunction, atrial flutter, atrioventricular block, right bundle-branch block, left anterior hemiblock, ventricular tachycardia, ventricular fibrillation, and QT-interval prolongation.23,24 However, to the best of our knowledge, we describe the first cases of BS that were clearly unmasked by lithium therapy. Furthermore, in at least 1 of the patients in the present study, the augmentation or production of type 1 Brugada ECG pattern occurred when the lithium level was within the therapeutic range. It would follow logically that unmasking of the coved ST-segment elevation in patients with BS may place this group of patients at increased risk for sudden cardiac death. Indeed, the increased mortality rate in patients with bipolar disorders25 may be related in part to the proarrhythmic effects of psychotropic drugs in patients with underlying cardiac disease.26 Unmasking of BS by lithium may therefore be a potential, although rare, contributor to the increased risk of sudden cardiac death in patients with bipolar disorder.
Although lithium has been used as a probe of cardiac sodium channel activity and is described as a “partial congener of sodium,”15–17 there is a lack of specific information on cardiac sodium channel–blocking properties of lithium. We show here that lithium not only blocks peak INa in a dose-dependent manner but does so at concentrations well below therapeutic levels. The fact that repolarization and conduction defects are not seen more often in patients taking lithium may relate to limitations in the applicability of cell-line experiments to the whole organism. It is well known that ion-current reduction varies not only according to species, cardiac cell type, and region but is also highly dependent on in vitro and in vivo conditions. To date, a variety of cell lines are being broadly used to heterogeneously study ion channel electrophysiology and pharmacology. These in vitro studies cannot hope to reproduce the exact effects of drug block in the intact human heart. Furthermore, in vitro conditions are optimized for a particular current to obtain reproducible and reliable data. Although the Chinese hamster ovary cell line may not be the ideal expression system, it has been used extensively to study cardiac ion channels and did provide evidence for lithium-induced INa blockade.
In conclusion, lithium, a commonly prescribed drug for bipolar disorder, can unmask latent BS by blockade of the cardiac sodium channel.
Acknowledgments
This work was supported by National Institutes of Health grants HL075255 to Dr Darbar and UO1 HL65962 to Dr Roden, and National Health Service 2003B195 grant to Dr Wilde. We are also grateful to Dr A.J. Funke-Küpper, Kennemer Gasthuis, Haarlem, for referring patient 2 to Dr Wilde.
Footnotes
Guest Editor for this article was Douglas P. Zipes, MD.
References
- 1.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome: a multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 2.Wilde AA, Antzelevitch C, Borggrefe M, Brugada J, Brugada R, Brugada P, Corrado D, Hauer RN, Kass RS, Nademanee K, Priori SG, Towbin JA. Proposed diagnostic criteria for the Brugada syndrome. Eur Heart J. 2002;23:1648–1654. doi: 10.1053/euhj.2002.3382. [DOI] [PubMed] [Google Scholar]
- 3.Antzelevitch C, Brugada P, Brugada J, Brugada R, Towbin JA, Nademanee K. Brugada syndrome: 1992–2002: a historical perspective. J Am Coll Cardiol. 2003;41:1665–1671. doi: 10.1016/s0735-1097(03)00310-3. [DOI] [PubMed] [Google Scholar]
- 4.Brugada R, Brugada J, Antzelevitch C, Kirsch GE, Potenza D, Towbin JA, Brugada P. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation. 2000;101:510–515. doi: 10.1161/01.cir.101.5.510. [DOI] [PubMed] [Google Scholar]
- 5.Miyazaki T, Mitamura H, Miyoshi S, Soejima K, Aizawa Y, Ogawa S. Autonomic and antiarrhythmic drug modulation of ST segment elevation in patients with Brugada syndrome. J Am Coll Cardiol. 1996;27:1061–1070. doi: 10.1016/0735-1097(95)00613-3. [DOI] [PubMed] [Google Scholar]
- 6.Brugada P, Brugada J, Brugada R. Arrhythmia induction by antiarrhythmic drugs. Pacing Clin Electrophysiol. 2000;23:291–292. doi: 10.1111/j.1540-8159.2000.tb06751.x. [DOI] [PubMed] [Google Scholar]
- 7.Babaliaros VC, Hurst JW. Tricyclic antidepressants and the Brugada syndrome: an example of Brugada waves appearing after the administration of desipramine. Clin Cardiol. 2002;25:395–398. doi: 10.1002/clc.4950250809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldgran-Toledano D, Sideris G, Kevorkian JP. Overdose of cyclic antidepressants and the Brugada syndrome. N Engl J Med. 2002;346:1591–1592. doi: 10.1056/NEJM200205163462020. [DOI] [PubMed] [Google Scholar]
- 9.Rouleau F, Asfar P, Boulet S, Dube L, Dupuis JM, Alquier P, Victor J. Transient ST segment elevation in right precordial leads induced by psychotropic drugs: relationship to the Brugada syndrome. J Cardiovasc Electrophysiol. 2001;12:61–65. doi: 10.1046/j.1540-8167.2001.00061.x. [DOI] [PubMed] [Google Scholar]
- 10.Tada H, Sticherling C, Oral H, Morady F. Brugada syndrome mimicked by tricyclic antidepressant overdose. J Cardiovasc Electrophysiol. 2001;12:275. doi: 10.1046/j.1540-8167.2001.00275.x. Case report. [DOI] [PubMed] [Google Scholar]
- 11.Pastor A, Nunez A, Cantale C, Cosio FG. Asymptomatic Brugada syndrome case unmasked during dimenhydrinate infusion. J Cardiovasc Electrophysiol. 2001;12:1192–1194. doi: 10.1046/j.1540-8167.2001.01192.x. [DOI] [PubMed] [Google Scholar]
- 12.Ortega-Carnicer J, Bertos-Polo J, Gutierrez-Tirado C. Aborted sudden death, transient Brugada pattern, and wide QRS dysrhythmias after massive cocaine ingestion. J Electrocardiol. 2001;34:345–349. doi: 10.1054/jelc.2001.26318. [DOI] [PubMed] [Google Scholar]
- 13.Blanco C, Laje G, Olfson M, Marcus SC, Pincus HA. Trends in the treatment of bipolar disorder by outpatient psychiatrists. Am J Psychiatry. 2002;159:1005–1010. doi: 10.1176/appi.ajp.159.6.1005. [DOI] [PubMed] [Google Scholar]
- 14.Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351:476–486. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- 15.Sakmann BF, Spindler AJ, Bryant SM, Linz KW, Noble D. Distribution of a persistent sodium current across the ventricular wall in guinea pigs. Circ Res. 2000;87:910–914. doi: 10.1161/01.res.87.10.910. [DOI] [PubMed] [Google Scholar]
- 16.Hunter DR, Haworth RA, Berkoff HA. Cellular lithium uptake as a probe of sodium channels in the rat heart: modulation of lithium uptake by tetrodotoxin, verapamil, anthopleurin-A, isoproterenol and external stimulation. J Mol Cell Cardiol. 1984;16:1083–1090. doi: 10.1016/s0022-2828(84)80035-8. [DOI] [PubMed] [Google Scholar]
- 17.Kupriyanov VV, Xiang B, Yang L, Deslauriers R. Lithium ion as a probe of Na+ channel activity in isolated rat hearts: a multinuclear NMR study. NMR Biomed. 1997;10:271–276. doi: 10.1002/(sici)1099-1492(199709)10:6<271::aid-nbm473>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Kambouris NG, Nuss HB, Johns DC, Tomaselli GF, Marban E, Balser JR. Phenotypic characterization of a novel long-QT syndrome mutation (R1623Q) in the cardiac sodium channel. Circulation. 1998;97:640–644. doi: 10.1161/01.cir.97.7.640. [DOI] [PubMed] [Google Scholar]
- 19.Yang T, Roden DM. Regulation of sodium current development in cultured atrial tumor myocytes (AT-1 cells) Am J Physiol. 1996;271:H541–H547. doi: 10.1152/ajpheart.1996.271.2.H541. [DOI] [PubMed] [Google Scholar]
- 20.Berk M. Lamotrigine and the treatment of mania in bipolar disorder. Eur Neuropsychopharmacol. 1999;9:S119–S123. doi: 10.1016/s0924-977x(99)00025-5. [DOI] [PubMed] [Google Scholar]
- 21.Fish JM, Antzelevitch C. Role of sodium and calcium channel block in unmasking the Brugada syndrome. Heart Rhythm. 2004;1:210–217. doi: 10.1016/j.hrthm.2004.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, Lemarec H, Nademanee K, Perez Riera AR, Shimizu W, Schulze-Bahr E, Tan H, Wilde A. Brugada syndrome: report of the Second Consensus Conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell JE, Mackenzie TB. Cardiac effects of lithium therapy in man: a review. J Clin Psychiatry. 1982;43:47–51. [PubMed] [Google Scholar]
- 24.Mateer JR, Clark MR. Lithium toxicity with rarely reported ECG manifestations. Ann Emerg Med. 1982;11:208–211. doi: 10.1016/s0196-0644(82)80500-3. [DOI] [PubMed] [Google Scholar]
- 25.Klumpers UM, Boom K, Janssen FM, Tulen JH, Loonen AJ. Cardiovascular risk factors in outpatients with bipolar disorder. Pharmacopsychiatry. 2004;37:211–216. doi: 10.1055/s-2004-832594. [DOI] [PubMed] [Google Scholar]
- 26.Frassati D, Tabib A, Lachaux B, Giloux N, Dalery J, Vittori F, Charvet D, Barel C, Bui-Xuan B, Megard R, Jenoudet LP, Descotes J, Vial T, Timour Q. Hidden cardiac lesions and psychotropic drugs as a possible cause of sudden death in psychiatric patients: a report of 14 cases and review of the literature. Can J Psychiatry. 2004;49:100–105. doi: 10.1177/070674370404900204. [DOI] [PubMed] [Google Scholar]


