Abstract
Neisseria meningitidis, the causative agent of meningococcal disease in humans, is likely to be exposed to nitrosative stress during natural colonization and disease. The genome of N. meningitidis includes the genes aniA and norB, predicted to encode nitrite reductase and nitric oxide (NO) reductase, respectively. These gene products should allow the bacterium to denitrify nitrite to nitrous oxide. We show that N. meningitidis can support growth microaerobically by the denitrification of nitrite via NO and that norB is required for anaerobic growth with nitrite. NorB and, to a lesser extent, the cycP gene product cytochrome c′ are able to counteract toxicity due to exogenously added NO. Expression of these genes by N. meningitidis during colonization and disease may confer protection against exogenous or endogenous nitrosative stress.
Neisseria meningitidis is the major cause of bacterial meningitis but is carried asymptomatically in the oronasopharynges of 5 to 10% (8) of the population. Disease results when bacteria spread from the nasopharynx into the bloodstream. Bacteremia may then lead to severe sepsis syndrome, which results in significant morbidity and mortality (7, 31, 32).
Colonization of the nasopharynx is the initial step in the pathogenesis of disease due to N. meningitidis. By deduction, invasive bacteria must avoid being killed in the nasopharynx, a tissue rich in mononuclear phagocytes, including resident macrophages (29). Macrophages kill bacteria by a number of mechanisms, including the oxidative burst, which is associated with release of reactive oxygen species. N. meningitidis expresses gene products to counteract oxidative stress (glutathione peroxidase [26] and possibly superoxide dismutase and catalase [2]). However, there is accumulating evidence that human macrophages also release nitric oxide (NO) in response to microbial products, including N. meningitidis lipopolysaccharide (4). As NO is a freely diffusible molecule, this effect could result in death or inhibition of intracellular and extracellular pathogenic bacteria (34). Despite this, there has been no investigation to date into mechanisms employed by N. meningitidis to counteract NO toxicity.
In addition to exerting toxic effects on invading bacteria, NO has been implicated in injury to the host microvasculature. A good example is meningitis, which can result in neurological deficit due to ischemic or inflammatory damage to the central nervous system (5, 22, 33). NO is also an intermediate in the denitrification pathway from nitrite (NO2−) to nitrous oxide (N2O).
It has been shown that in the closely related organism Neisseria gonorrhoeae, the expression of two genes, aniA, encoding a copper-containing nitrite reductase (9, 25), and norB, encoding a NO reductase (20), allows the gonococcus to grow anaerobically by denitrification. Hence, if the meningococcus, like N. gonorrhoeae (21), is capable of supplementing growth by using denitrification as an alternative to oxygen respiration, then the meningococcus may have to resist internally generated NO in addition to NO synthesized by the host.
We have identified two genetic loci within the N. meningitidis MC58 genome that contain putative genes involved in NO metabolism, which may be implicated in the pathogenesis of N. meningitidis. Cytochrome c′ is encoded by gene NMB0923 (cycP) of the N. meningitidis MC58 genome (31). This gene is predicted to encode a 151-amino-acid polypeptide with similarity to cytochrome c′ from other organisms (1). One distinctive characteristic is that instead of a cleavable signal sequence to direct the protein to the periplasm, it is predicted to contain a noncleavable sequence which directs the protein to the outer membrane, where it is covalently attached to a lipid moiety. As such the meningococcal cytochrome resides within the same cellular compartment as in other organisms but is more restricted in its movement.
Cytochrome c′ contains a single heme, which is covalently attached towards the C terminus of the polypeptide (1). The function of cytochrome c′ in Rhodobacter capsulatus is to bind and remove NO, hence lowering the toxicity due to this free radical (12, 13). This cytochrome may have a similar role in the meningococcus and possibly a role in evading the immune response of the host during infection.
The meningococcus also contains the genes necessary for the respiratory reduction of nitrite to nitrous oxide. The aniA gene product (NMB1623 of N. meningitidis MC58) (31) is expected to catalyze the reduction of nitrite to NO via a copper-type nitrite reductase as in the gonococcus (9, 25). Like cytochrome c′, this protein is also predicted to be covalently attached to the outer membrane and to reside within the periplasm. In the gonococcus, aniA is induced by anaerobiosis, and this induction is enhanced by the presence of nitrite (19). Control of gene expression is regulated via FNR and NarP/Q (23). Adjacent to aniA and divergently transcribed is norB (NMB1622), which encodes a putative NO reductase responsible for reducing NO to nitrous oxide. The predicted NO reductase in N. meningitidis is very similar to those of the gonococcus (N. gonorrhoeae) (20) and Ralstonia eutropha (11). Unlike the NO reductases of other organisms, for example, Paracoccus denitrificans (14), these enzymes lack a c-heme-containing subunit that donates electrons to the subunit containing the active site, but there is an N-terminal extension which contains two putative membrane spans and probably acts as a quinol oxidase, which provides two electrons to the active site per quinol oxidized. Like those of N. gonorrhoeae, the gene products AniA and NorB of N. meningitidis are predicted to constitute a pathway that may enable the organism to grow under conditions of low oxygen in the presence of nitrite. NorB may also have the additional role of helping the organism evade the immune response of the host during infection by acting as an environmental buffer, thus keeping NO concentrations low.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains, plasmids, and primers used are shown in Table 1. For growth on plates, Columbia agar was supplemented with 5% horse blood and GC agar was supplemented with 2% Vitox. Plates were incubated aerobically in an atmosphere of 5% CO2 at 37°C or anaerobically in a gas jar with an atmosphere made anaerobic with an anaerobic gas-generating kit (Oxoid). Liquid cultures were grown in Mueller-Hinton broth (MHB; Oxoid) in the presence of 10 mM NaHCO3 or 5% CO2 at 37°C, and where stated 5 mM NaNO2 was added. For aerobic growth, 5-ml cultures were grown in 25-ml universals shaken at 120 rpm, or 20-ml cultures were grown in 250-ml conical flasks shaken at 120 rpm. For microaerobic growth, 25-ml universals containing 20 ml of medium were incubated while stationary at 37°C. All cultures were inoculated with colonies taken from freshly grown aerobic or anaerobic plates. Viable counts were determined by serial dilution of cultures in phosphate-buffered saline and plating on prewarmed blood agar plates or GC agar plates accordingly. The plates were incubated overnight at 37°C, and the mean number of CFU per milliliter from three determinations was calculated. All results were confirmed in at least three independent experiments. When required, strains were supplemented with the following antibiotics: spectinomycin (50 μg ml−1), kanamycin (50 μg ml−1), ampicillin (50 μg ml−1), and chloramphenicol (2 μg ml−1).
TABLE 1.
Strains, plasmids, and primers
| Strain, plasmid, or primer | Genotype or sequence |
|---|---|
| MC58 | Wild-type group B N. meningitidis |
| MA1 | MC58 cycP::Ω |
| MA2 | MC58 norB::Ω |
| pMA1 | 2,094-bp PCR fragment generated by using primers MFA1 and MFA2 was cloned into EcoRV site in pZErO (Invitrogen); Kanr |
| pMA2 | XmnI-digested pMA1, which resulted in release of a 162-bp fragment, was ligated with a 2-kb SmaI-digested Ω cassette from pHP45Ω; Kanr Spr |
| pMA3 | 1,944-bp PCR fragment generated by using primers MFA3 and MFA4 was cloned into SmaI site in pUC18; Ampr |
| pMA4 | StuI-digested pMA3 was ligated with 2-kb SmaI-digested Ω cassette from pHP45Ω; Ampr Spr |
| MFA1 | 5′TTTAATTCAAAGCAGCTATTTGGGCG3′ |
| MFA2 | 5′CTTCATTCCTGCAAAAGTCTGCGCCAC3′ |
| MFA3 | 5′TATCAGGCTCCGGACTGGACGGC3′ |
| MFA4 | 5′CGTACGTACCCAACGCAAGGTA3′ |
Molecular biological manipulations and construction of strains.
The cycP gene with flanking DNA was PCR amplified by using Pwo polymerase and primers MFA1 and MFA2. The resulting 2,094-bp fragment was cloned into the EcoRV restriction site in pZErO to generate pMA1. The plasmid was subsequently digested with XmnI to release a 162-bp fragment from between the two XmnI sites within the cycP coding sequence. The resulting sites were ligated with the 2-kb SmaI-digested Ω cassette, encoding spectinomycin resistance, from pHP45Ω (28). Primers OMN and OMC, which are complementary to sequences within the Ω cassette, were used to sequence the junction for verification of the correct insertion of the Ω cassette into cycP. The resulting plasmid (pMA2) was used for construction of a cycP chromosomal mutant in MC58.
A similar approach was used to create a construct for insertional mutagenesis of norB. Primers MFA3 and MFA4 were used to amplify norB, which was subsequently cloned into pUC18 which had been linearized with SmaI, yielding pMA3. The Ω cassette was inserted into a StuI site within norB. The resulting plasmid (pMA4) was used for construction of a norB chromosomal mutant in MC58.
N. meningitidis was transformed according to the method of Seifert and coworkers (30). N. meningitidis MC58 was suspended in prewarmed MHB containing 10 mM MgCl2, at a final optical density at 600 nm of 0.2 to 0.3. Approximately 1 μg of plasmid DNA, typically 1/10 of the total volume, was then added to an aliquot of the colony suspension. The mixture was incubated at 37°C for 30 min, diluted (1 in 10) into fresh MHB containing 10 mM MgCl2 and 10 mM NaHCO3, and finally outgrown for 4 to 6 h at 37°C with gentle shaking (90 rpm). Aliquots of 100 μl of the transformation mixture were selected on blood agar containing spectinomycin and grown for 18 h. Resulting colonies were replica plated onto blood agar plates containing kanamycin (for cycP strains) or ampicillin (for norB strains). The presence of a chromosomal mutation in spectinomycin-resistant and kanamycin- and ampicillin-sensitive colonies was verified by colony PCR with primers MFA2 and OMN for N. meningitidis MA1 (cycP::Ω) and primers MFA4 and OMN for N. meningitidis MA2 (norB::Ω).
Analytical techniques.
Cell extracts were prepared by repeated freeze-thaw treatments. Liquid culture (15 ml) was harvested, the pellet was resuspended in 0.5 ml of 100 mM Tris-HCl (pH 8), and a few grains of DNase I were added. The suspension was frozen at −20°C and thawed at room temperature. This procedure was repeated three times. Samples (25 μg) were run on sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE) and stained for heme (15). Purified cytochrome c′ (1 μg) from R. capsulatus was used as a control.
Nitrite was assayed colorimetrically with the Griess reagent (16), in 1-ml reaction mixtures containing 5 μl of cell suspension and 895 μl of a 1% sulfanilamide solution. The reaction was started with the addition of 100 μl of a 0.02% solution of N-naphthylenediamine. Absorbance was measured at 540 nm.
Inhibition of oxygen respiration by NO was monitored with a Clark-type oxygen electrode (Rank Brothers, Bottisham, United Kingdom) as described previously (12). NO was assayed with an iso-NO electrode (World Precision Instruments). Saturated solutions of NO in water were generated by sparging anaerobic water (in a 10-ml Bijou container fitted with a rubber septum) with NO gas (Aldrich, Poole, United Kingdom).
The effects of the NO donors sodium nitroprusside (SNP), S-nitrosoglutathione (GSNO), and S-nitrosopenicillamine (SNAP) were assessed. Dilutions of freshly grown liquid cultures of N. meningitidis were spread onto GC agar plates containing 2% Vitox and the NO donors. Plates were incubated in an atmosphere of 5% CO2 at 37°C.
RESULTS
Microaerobic growth and denitrification.
Although we have identified genes necessary for denitrification in the meningococcus, and the closely related gonococcus is a known denitrifier (9, 20), it was nevertheless important to determine whether the meningococcus is a functioning denitrifier under physiologically relevant conditions. This may have implications for the survival of the organism inside human host tissue and therefore its pathogenicity.
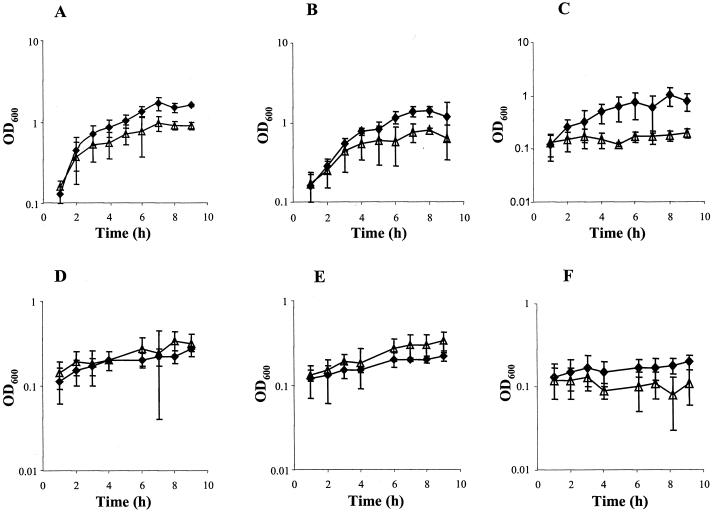
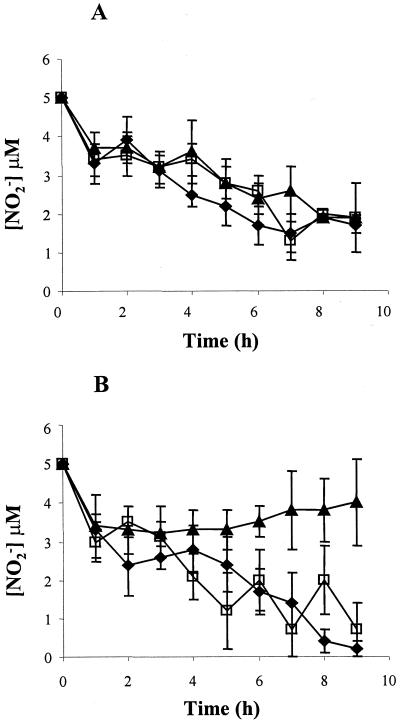
Cultures were set up for aerobic and microaerobic growth in both the presence and absence of 5 mM nitrite, which may be able to supplement growth by acting as an alternative electron acceptor to oxygen. The results of growth of N. meningitidis strains MC58 (wild type), MA1 (cycP), and MA2 (norB) under each condition are presented in Fig. 1. The growth rates and growth yields of MC58 and MA1 were greater in the presence of nitrite than in its absence. Conversely, the norB strain was unable to grow microaerobically in the presence of nitrite. As expected, nitrite became depleted during microaerobic growth in cultures of MC58 and MA1 supplemented with 5 mM nitrite (Fig. 2), but this was not observed during growth of MA2. From these data we can conclude that under microaerobic conditions (i) nitrite enhances growth of N. meningitidis and (ii) in the absence of a NO reductase N. meningitidis has impaired growth in the presence of nitrite. Under aerobic growth conditions the presence of nitrite impairs growth of all the N. meningitidis strains (Fig. 1), most markedly the norB strain, which is incapable of aerobic growth in the presence of nitrite. Householder et al. (20) demonstrated that exogenous addition of 2 mM nitrite is more toxic to the gonococcus than endogenously produced NO, although they did not show how much NO is actually produced by reduction of nitrite by aniA. Therefore, it may be assumed that, as in the gonococcus, it is the presence of nitrite which inhibits growth of aerobic cultures in the meningococcus. However, we cannot at present rule out the possibility of sufficient NO being synthesized from nitrite under aerobic conditions (via nitrite reductase) to inhibit aerobic growth of all the N. meningitidis strains. That nitrite is reduced to NO is supported by the data in Fig. 2, which shows removal of nitrite from aerobic cultures over the growth period. The NO produced from nitrite disappeared in all the strains, presumably by reaction with oxygen. However, the steady-state NO concentration in N. meningitidis MA2 is likely to be much higher than in the other strains due to the absence of NorB. The other N. meningitidis strains also grow poorly aerobically in the presence of nitrite, likely because the activity of NO reductase is low even in these strains. Competition for electrons by the oxidases active under aerobic conditions probably prevents enough of the electrons necessary for NO reduction from reaching the NO reductase. Nitrate at 5 mM does not affect the growth of N. meningitidis strains either aerobically or anaerobically (data not shown).
FIG. 1.
Effect of nitrite on growth of N. meningitidis. Growth curves for wild-type (A and D), cycP (B and E), and norB (C and F) strains grown aerobically (A, B, and C) and microaerobically (D, E, and F) in the presence (triangles) and absence (squares) of 5 mM nitrite are shown. Results are means from at least four experiments ± standard deviations. OD600, optical density at 600 nm
FIG. 2.
Nitrite utilization by N. meningitidis. Time courses of nitrite concentration in wild-type (filled squares), cycP (open squares), and norB (filled triangles) cultures grown aerobically (A) or microaerobically (B) in the presence of 5 mM nitrite are shown. Results are means from at least four experiments ± standard deviations.
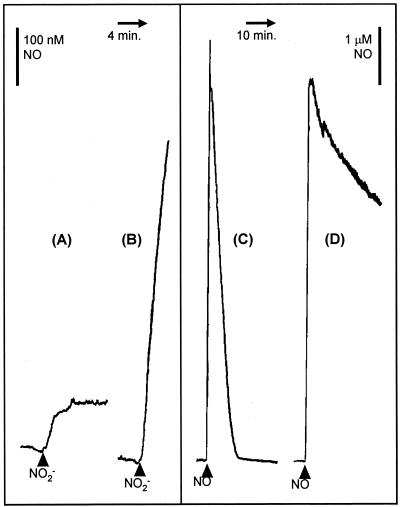
The growth experiments described above provided evidence that N. meningitidis can supplement microaerobic growth by respiring nitrite and that this process probably occurs via a NO intermediate. It was expected that anaerobic growth would also similarly be supplemented by nitrite respiration. However, it was necessary to demonstrate directly that nitrite is metabolized via NO and that the norB gene product is involved in NO metabolism. Suspensions of N. meningitidis MC58 (wild type) and MA2 (norB) were sparged with nitrogen gas until anaerobic, and the concentration of NO was monitored with a NO electrode. On addition of 1 mM nitrite, approximately 100 nM NO accumulated, whereupon the culture reached a new steady state (Fig. 3A). In a strain lacking norB, however, the addition of 1 mM nitrite caused NO to accumulate continuously, at an initial rate of approximately 2.9 nmol min−1 mg of protein−1 (Fig. 3B), presumably because AniA reduces nitrite to NO but there is no further reduction. This suggests that nitrite is reduced to NO by N. meningitidis. To confirm that norB is required for NO metabolism, the disappearance of NO from N. meningitidis suspensions treated as described above was monitored with a NO electrode. N. meningitidis MC58 removes NO at a rate of approximately 12 nmol min−1mg of protein−1 (Fig. 3C), whereas NO removal is slow in suspensions of the norB mutant strain (Fig. 3D) and the low rate of removal was similar to that seen in a growth medium control (i.e., the NO depletion was due to diffusion of the NO out of the reaction chamber). These experiments show conclusively that N. meningitidis possesses nitrite-reducing and NO-reducing activities; i.e., the organism is capable of the minimum requirement for denitrification, which is the production of gaseous products from nitrogen oxyanions, presumably nitrous oxide (35), and that in the absence of a functional norB, enough NO may accumulate to inhibit respiration of the norB strain (see Fig. 5). N. meningitidis MA1 behaved essentially identically to N. meningitidis MC58 in experiments described in this section (data not shown).
FIG. 3.
Metabolism of nitrite and NO by N. meningitidis. NO concentration was measured with an iso-NO electrode (World Precision Instruments). Suspensions containing 3 ml of N. meningitidis at a concentration of 0.1 mg of protein ml−1 in MHB were sparged with nitrogen gas until anaerobic in a 7-ml water-jacketed chamber kept at 30°C. The cell suspension was stirred with a magnetic flea, and the chamber was kept anaerobic with a rubber septum through which the NO electrode probe was inserted. Traces show the effects of 1 mM nitrite on wild-type (A) and norB (B) strains and the effect of 8 μM NO on cycP (C) and norB (D) strains.
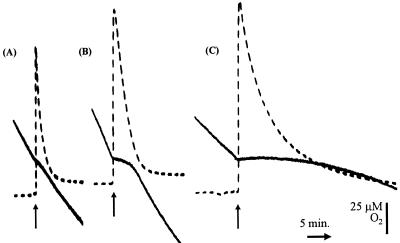
FIG. 5.
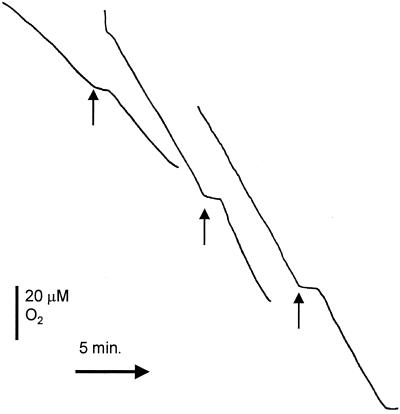
Oxygen and NO measurements in MC58, MA1, and MA2. Oxygen disappearance was measured with a Clark electrode (solid lines) while NO was measured simultaneously (dashed lines). The chamber of the oxygen electrode contained 3 ml of wild-type (A), cycP (B), or norB (C) N. meningitidis strains at a concentration of 0.5 mg protein ml−1 in MHB. The NO electrode was inserted into the chamber stopper such that there was no headspace within the reaction vessel. Arrows mark the additions of 9.3 μM NO at an oxygen concentration of 120 μM.
Genes functioning in protection against NO toxicity.
The data presented here demonstrate that the activity of NO reductase is dependent upon the presence of the gene norB and hence its active product. In contrast, mutation in the cycP gene did not affect NO reductase activity. To confirm that cytochrome c′ is expressed as a protein in N. meningitidis, the soluble extracts of N. meningitidis MC58 and the cycP-deficient strain were run on a SDS-10% PAGE gel which was heme stained (Fig. 4). There is a band of approximately 17 kDa (the predicted size of cytochrome c′ bound to outer membrane lipid) in MC58 which is absent in MA1. This suggests that the band is due to holocytochrome c′, demonstrating the presence of CycP in N. meningitidis.
FIG. 4.
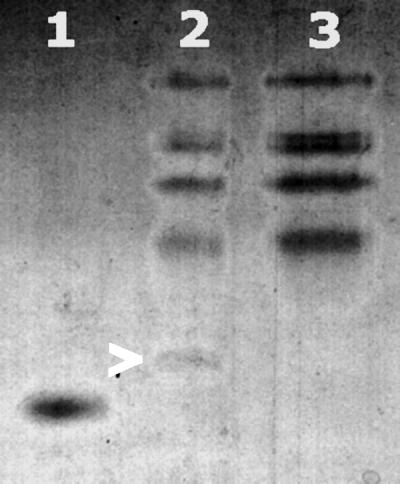
Evidence for expression of holocytochrome c′. Samples were run on SDS-10% PAGE and stained for proteins containing covalently bound hemes. Lane 1, 1 μg of cytochrome c′ purified from R. capsulatus used as a control hemoprotein; lane 2, 25 μg of total soluble extract from wild-type N. meningitidis (cytochrome c′ is marked by an arrow); lane 3, 25 μg of total soluble extract from N. meningitidis cycP.
To investigate the role of NorB and cytochrome c′ in protection against NO, we examined the impact of the nitrosative stress-generating compounds SNP, GSNO, and SNAP on the growth of N. meningitidis MC58, MA1, and MA2 (Table 2). SNP contains an NO group in the oxidation state NO+ and can act as a nitrosating agent at neutral pH values (24), whereas GSNO and SNAP are S-nitrosothiols often used by researchers as NO donors due to their spontaneous decomposition to NO (17, 18). The effect on the norB strain was very marked; concentrations of SNP, GSNO, and SNAP which allowed rapid growth of the wild-type strain severely attenuated growth of N. meningitidis MA2. Clearly, as well as having a role in growth by denitrification, the norB gene product is able to protect cells against nitrosative stress. The cycP-deficient strain grew poorly in the presence of SNP and SNAP compared to MC58, indicating that cytochrome c′ also has a role in protecting against toxicity due to nitrosative stress, albeit to a lesser degree than NorB.
TABLE 2.
Sensitivity of meningococci to NO donors
| Inhibitor concn (mM) | Strain | Extent of growtha
|
||
|---|---|---|---|---|
| SNP | GSNO | SNAP | ||
| 0 | MC58 | +++ | +++ | +++ |
| MA1 | +++ | +++ | +++ | |
| MA2 | +++ | +++ | +++ | |
| 0.1 | MC58 | +++ | +++ | +++ |
| MA1 | + | +++ | +++ | |
| MA2 | − | +++ | +++ | |
| 0.25 | MC58 | +++ | +++ | +++ |
| MA1 | + | +++ | +++ | |
| MA2 | − | − | − | |
| 1.0 | MC58 | − | +++ | +++ |
| MA1 | − | +++ | +++ | |
| MA2 | − | − | − | |
| 1.5 | MC58 | − | +++ | +++ |
| MA1 | − | +++ | +++ | |
| MA2 | − | − | − | |
| 2.0 | MC58 | − | +++ | +++ |
| MA1 | − | +++ | + | |
| MA2 | − | − | − | |
| 3.0 | MC58 | − | +++ | +++ |
| MA1 | − | +++ | − | |
| MA2 | − | − | − | |
+++, Growth of colonies to a diameter of >2 mm after 18 h of incubation; +, poor growth, i.e., small colonies (<1 mm in diameter) after 24 or 48 h; −, no growth observed after 48 h.
We also assessed the effect of NO on oxygen respiration in the three N. meningitidis strains. Oxygen uptake is inhibited more markedly in the norB-deficient strain (MA2) than either the wild-type (MC58) or the cycP-deficient strain (MA1) (Fig. 5). Inhibition of MA2 respiration occurred in response to concentrations of NO lower than those required to inhibit respiration of MC58. Even after 30 min there was still significant inhibition of oxygen uptake from the norB strain, whereas respiration of the wild-type and cycP strains completely recovered after approximately 1 and 3 min, respectively, when 9.3 μM NO was added to the respiring suspensions (Fig. 5), suggesting that inhibition of respiration is transient in MC58 but is likely to be permanent in the norB-deficient strain (Fig. 5A and C). In fact, oxygen respiration in strain MA2 appears to be permanently inhibited by concentrations of NO as low as 0.3 μM (using cell suspensions containing 0.1 mg of protein ml−1) whereas NO concentrations greater than 3 μM were required to achieve inhibition of respiration in strains MC58 and MA1 (data not shown). The cycP-deficient strain (MA1) exhibited a pattern of inhibition by NO similar to that of the wild-type (compare Fig. 5A and B) except that the inhibition by NO consistently lasted longer in MA1 (mean inhibition by 9.3 μM NO in suspensions containing 0.1 mg of protein ml−1, 64 ± 18 s for MC58 and 103 ± 20 s for MA1), as would be expected for a strain lacking an NO detoxification system. The inhibition of respiration by NO in the wild-type strain was independent of oxygen concentration (Fig. 6). This contrasts with Escherichia coli and Salmonella enterica serovar Typhimurium, in which the main supposed NO detoxification system operating is the flavohemoglobin (which is an NO oxygenase) (27). The lack of dependence on oxygen concentration in N. meningitidis MC58 is probably due to the activity of NorB, which is not an oxygen-dependent enzyme. Cytochrome c′ is also independent of oxygen (13).
FIG. 6.
Lack of effect of oxygen concentration on the period of inhibition by NO. The effects of NO on respiration rates of wild-type N. meningitidis are shown. Arrows indicate the point at which NO (9.3 μM) was added to the oxygen electrode chamber. The reaction chamber contained 3 ml of wild-type N. meningitidis at a concentration of 0.5 mg of protein ml−1 in MHB. Oxygen concentration had no effect on the period of inhibition by NO.
DISCUSSION
Denitrification is the reduction of nitrogen oxyanions to gaseous products. This process is linked to the respiratory electron transport chain in denitrifying bacteria, so that nitrate or nitrite can act as an electron acceptor to support respiratory growth. We have shown that the meningococcus can denitrify nitrite (but not nitrate) and that this process supports growth of the organism.
Complete denitrification is the reduction of nitrate to dinitrogen gas, catalyzed by four distinct enzymes: nitrate reductase (nitrate to nitrite), nitrite reductase (nitrite to NO), NO reductase (NO to nitrous oxide), and nitrous oxide reductase (nitrous oxide to dinitrogen) (reviewed in reference 3). It was important to determine how much denitrification is possible in N. meningitidis. Databases containing the complete genome sequences of N. meningitidis MC58 (a group B strain) and N. meningitidis Z2491 (a group A strain) were searched using BlastP and TBlastN at http://tigrblast.tigr.org/cmr-blast/. There are two genetically distinct types of dissimilatory nitrate reductases, a membrane-bound enzyme, NAR, and a periplasmic enzyme, NAP. Searches with narG (encoding the large subunit of NAR) and napA (encoding the large subunit of NAP) from E. coli yielded no significant hits. We concluded that N. meningitidis does not contain any gene similar to those for known dissimilatory nitrate reductases. This is in keeping with our finding that N. meningitidis cannot use nitrate to promote respiratory growth under anaerobic conditions. There are two types of nitrite reductase involved in denitrification, a copper-containing enzyme (Cu-NiR) and a heme-containing enzyme (cd1-NiR). Searches using cd1-NiR from Pseudomonas stutzeri and Cu-NiR from Achromobacter cycloclastes revealed that N. meningitidis possesses the gene for Cu-NiR only (aniA). N. meningitidis also contains a gene for NO reductase (norB). Searching with nosZ (the structural gene for N2O reductase) from R. eutropha showed no evidence for N2O reductase in N. meningitidis. We also searched for a multiheme nitrite reductase (nrfA) using the gene from E. coli. The multiheme nitrite reductase reduces nitrite to ammonia and can help support respiratory growth under anaerobic conditions. However, Blast searches revealed no genes with significant similarity in N. meningitidis. Other proteins associated with denitrification are the cupredoxins azurin and pseudoazurin, encoded by azu and paz, respectively. N. meningitidis contains a gene with significant similarity to azu from Pseudomonas aeruginosa but no paz-like gene. azu in N. meningitidis encodes a protein predicted to be attached to the outer membrane.
Dedicated denitrifiers such as Paracoccus denitrificans and the denitrifying pseudomonads contain upwards of 40 genes which are involved in the synthesis of the apparatus necessary for denitrification (structural genes for the enzymes are supplemented by many genes required for assembly of nitrate reductase, nitrous oxide reductase, and cd1-NiR and many regulatory genes). Our analysis of the N. meningitidis genomes indicates that it possesses only a few genes (aniA, norB, fnr, narP, narQ, and azu) which are likely to be involved in denitrification (in other organisms azu is not strictly required for denitrification but it can transfer electrons to the denitrifying reductases). N. meningitidis as such represents a minimal denitrifier; it can carry out the two central reactions of the pathway and use these activities to support its growth, but the cost of maintaining this capability is a very small amount of genome space.
It is possible that the capacity of N. meningitidis to grow by denitrification may be physiologically relevant to its lifestyle in vivo. During mucosal invasion the organism is likely to encounter microenvironments of reduced oxygen concentration in which meningococcal survival and growth will be enhanced by the capacity to denitrify. During meningococcal sepsis, severely impaired tissues may be inadequately perfused with oxygenated blood, yet the denitrifying bacteria may continue to proliferate. In the related organism N. gonorrhoeae, the expression of anaerobically induced aniA is high during the disease state (10), suggesting that, at least in that case, the organism adapts to anaerobic conditions within genital mucosa.
In addition to its role in denitrification, NorB has been shown to have a role in protection of N. meningitidis against exogenously added NO and NO-related compounds. A second gene product, CycP (cytochrome c′), also appears to afford some protection against this toxic free radical. This protection against NO may be physiologically relevant, since the macrophages of the human host may produce NO in order to kill invading microorganisms. Resistance of N. meningitidis against NO may thus have implications for the ability of this organism to survive and cause disease. As well as protecting the organism from the toxic effects of NO, the NO removal systems may have further implications for the normal physiology of the infected human host, as NO is a potent vasodilatory agent (6). If N. meningitidis depletes tissues of NO, then the blood flow may decrease and tissues may fail to be perfused with oxygen. Meningococcal disease causes localized tissue hypoxia, and perhaps this is, in part, due to dysregulation of vasomotor tone as a result of decrease in local NO concentrations in peripheral tissues via microbial NO metabolism. Further detailed studies of both control of gene expression by global regulators and analysis of the promoter region will be required to determine the molecular basis of the differences in regulation between the closely related meningococcus and gonococcus. This may lead to further understanding of how two closely related organisms adapt to differing habitats and are associated with such distinctive disease phenotypes in humans.
Acknowledgments
This work was supported by a grant from the Wellcome Trust awarded to J.W.B.M. and R.C.R.
REFERENCES
- 1.Ambler, R. P., R. G. Bartsch, M. Daniel, M. D. Kamen, L. McLellan, T. E. Meyer, and B. Van. 1981. Amino acid sequences of bacterial cytochromes c′ and c-556. Proc. Natl. Acad. Sci. USA 78:6854-6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archibald, F. S., and M. N. Duong. 1986. Superoxide dismutase and oxygen toxicity defenses in the genus Neisseria. Infect. Immun. 51:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berks, B. C., S. J. Ferguson, J. W. Moir, and D. J. Richardson. 1995. Enzymes and associated electron transport systems that catalyse the respiratory reduction of nitrogen oxides and oxyanions. Biochim. Biophys. Acta 1232:97-173. [DOI] [PubMed] [Google Scholar]
- 4.Blondiau, C., P. Lagadec, P. Lejeune, N. Onier, J. M. Cavaillon, and J. F. Jeannin. 1994. Correlation between the capacity to activate macrophages in vitro and the antitumor activity in vivo of lipopolysaccharides from different bacterial species. Immunobiology 190:243-254. [DOI] [PubMed] [Google Scholar]
- 5.Boje, K. M. 1996. Inhibition of nitric oxide synthase attenuates blood-brain barrier disruption during experimental meningitis. Brain Res. 720:75-83. [DOI] [PubMed] [Google Scholar]
- 6.Bredt, D. S., and S. H. Snyder. 1994. Nitric oxide: a physiologic messenger molecule. Annu. Rev. Biochem. 63:175-195. [DOI] [PubMed] [Google Scholar]
- 7.Broome, C. V. 1986. The carrier state: Neisseria meningitidis. J. Antimicrob. Chemother. 18(Suppl. A):25-34. [DOI] [PubMed] [Google Scholar]
- 8.Cartwright, K. 1995. Meningococcal disease, p. 1-19. John Wiley & Sons Ltd, Chichester, United Kingdom.
- 9.Clark, V. L., L. A. Campbell, D. A. Palermo, T. M. Evans, and K. W. Klimpel. 1987. Induction and repression of outer membrane proteins by anaerobic growth of Neisseria gonorrhoeae. Infect. Immun. 55:1359-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark, V. L., J. S. Knapp, S. Thompson, and K. W. Klimpel. 1988. Presence of antibodies to the major anaerobically induced gonococcal outer membrane protein in sera from patients with gonococcal infections. Microb. Pathog. 5:381-390. [DOI] [PubMed] [Google Scholar]
- 11.Cramm, R., R. A. Siddiqui, and B. Friedrich. 1997. Two isofunctional nitric oxide reductases in Alcaligenes eutrophus H16. J. Bacteriol. 179:6769-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cross, R., J. Aish, S. J. Paston, R. K. Poole, and J. W. Moir. 2000. Cytochrome c′ from Rhodobacter capsulatus confers increased resistance to nitric oxide. J. Bacteriol. 182:1442-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross, R., D. Lloyd, R. K. Poole, and J. W. Moir. 2001. Enzymatic removal of nitric oxide catalyzed by cytochrome c′ in Rhodobacter capsulatus. J. Bacteriol. 183:3050-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Boer, A. P., J. van der Oost, W. N. Reijnders, H. V. Westerhoff, A. H. Stouthamer, and R. J. van Spanning. 1996. Mutational analysis of the nor gene cluster which encodes nitric-oxide reductase from Paracoccus denitrificans. Eur. J. Biochem. 242:592-600. [DOI] [PubMed] [Google Scholar]
- 15.Gilmour, R., C. F. Goodhew, and G. W. Pettigrew. 1991. Cytochrome c′ of Paracoccus denitrificans. Biochim. Biophys. Acta 1059:233-238. [DOI] [PubMed] [Google Scholar]
- 16.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126:131-138. [DOI] [PubMed] [Google Scholar]
- 17.Hou, Y., Z. Guo, J. Li, and P. G. Wang. 1996. Seleno compounds and glutathione peroxidase catalyzed decomposition of S-nitrosothiols. Biochem. Biophys. Res. Commun. 228:88-93. [DOI] [PubMed] [Google Scholar]
- 18.Hou, Y., J. Wang, F. Arias, L. Echegoyen, and P. G. Wang. 1998. Electrochemical studies of S-nitrosothiols. Bioorg. Med. Chem. Lett. 8:3065-3070. [DOI] [PubMed] [Google Scholar]
- 19.Householder, T. C., W. A. Belli, S. Lissenden, J. A. Cole, and V. L. Clark. 1999. cis- and trans-acting elements involved in regulation of aniA, the gene encoding the major anaerobically induced outer membrane protein in Neisseria gonorrhoeae. J. Bacteriol. 181:541-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Householder, T. C., E. M. Fozo, J. A. Cardinale, and V. L. Clark. 2000. Gonococcal nitric oxide reductase is encoded by a single gene, norB, which is required for anaerobic growth and is induced by nitric oxide. Infect. Immun. 68:5241-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knapp, J. S., and V. L. Clark. 1984. Anaerobic growth of Neisseria gonorrhoeae coupled to nitrite reduction. Infect. Immun. 46:176-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koedel, U., A. Bernatowicz, R. Paul, K. Frei, A. Fontana, and H. W. Pfister. 1995. Experimental pneumococcal meningitis: cerebrovascular alterations, brain edema, and meningeal inflammation are linked to the production of nitric oxide. Ann. Neurol. 37:313-323. [DOI] [PubMed] [Google Scholar]
- 23.Lissenden, S., S. Mohan, T. Overton, T. Regan, H. Crooke, J. A. Cardinale, T. C. Householder, P. Adams, C. D. Conner, V. L. Clark, H. Smith, and J. A. Cole. 2000. Identification of transcription activators that regulate gonococcal adaptation from aerobic to anaerobic or oxygen-limited growth. Mol. Microbiol. 37:839-855. [DOI] [PubMed] [Google Scholar]
- 24.Maraj, S. R., S. Khan, X.-Y. Cui, R. Cammack, C. L. Joannou, and M. N. Hughes. 1995. Interaction of nitric oxide and redox-related species with biological targets. Arch. Biochim. Biophys. 316:327-334. [Google Scholar]
- 25.Mellies, J., J. Jose, and T. F. Meyer. 1997. The Neisseria gonorrhoeae gene aniA encodes an inducible nitrite reductase. Mol. Gen. Genet. 256:525-532. [DOI] [PubMed] [Google Scholar]
- 26.Moore, T. D., and P. F. Sparling. 1996. Interruption of the gpxA gene increases the sensitivity of Neisseria meningitidis to paraquat. J. Bacteriol. 178:4301-4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poole, R. K., and M. N. Hughes. 2000. New functions for the ancient globin family: bacterial responses to nitric oxide and nitrosative stress. Mol. Microbiol. 36:775-783. [DOI] [PubMed] [Google Scholar]
- 28.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 29.Read, R. C., S. Zimmerli, C. Broaddus, D. A. Sanan, D. S. Stephens, and J. D. Ernst. 1996. The (α2→8)-linked polysialic acid capsule of group B Neisseria meningitidis modifies multiple steps during interaction with human macrophages. Infect. Immun. 64:3210-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seifert, H. S., R. S. Ajioka, D. Paruchuri, F. Heffron, and M. So. 1990. Shuttle mutagenesis of Neisseria gonorrhoeae: pilin null mutations lower DNA transformation competence. J. Bacteriol. 172:40-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 32.van Deuren, M., P. Brandtzaeg, and J. W. M. van der Meer. 2000. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin. Microbiol. Rev. 13:144-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Visser, J. J., R. J. Scholten, and K. Hoekman. 1994. Nitric oxide synthesis in meningococcal meningitis. Ann. Intern. Med. 120:345-346. [DOI] [PubMed] [Google Scholar]
- 34.Webb, J. L., M. W. Harvey, D. W. Holden, and T. J. Evans. 2001. Macrophage nitric oxide synthase associates with cortical actin but is not recruited to phagosomes. Infect. Immun. 69:6391-6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]