Abstract
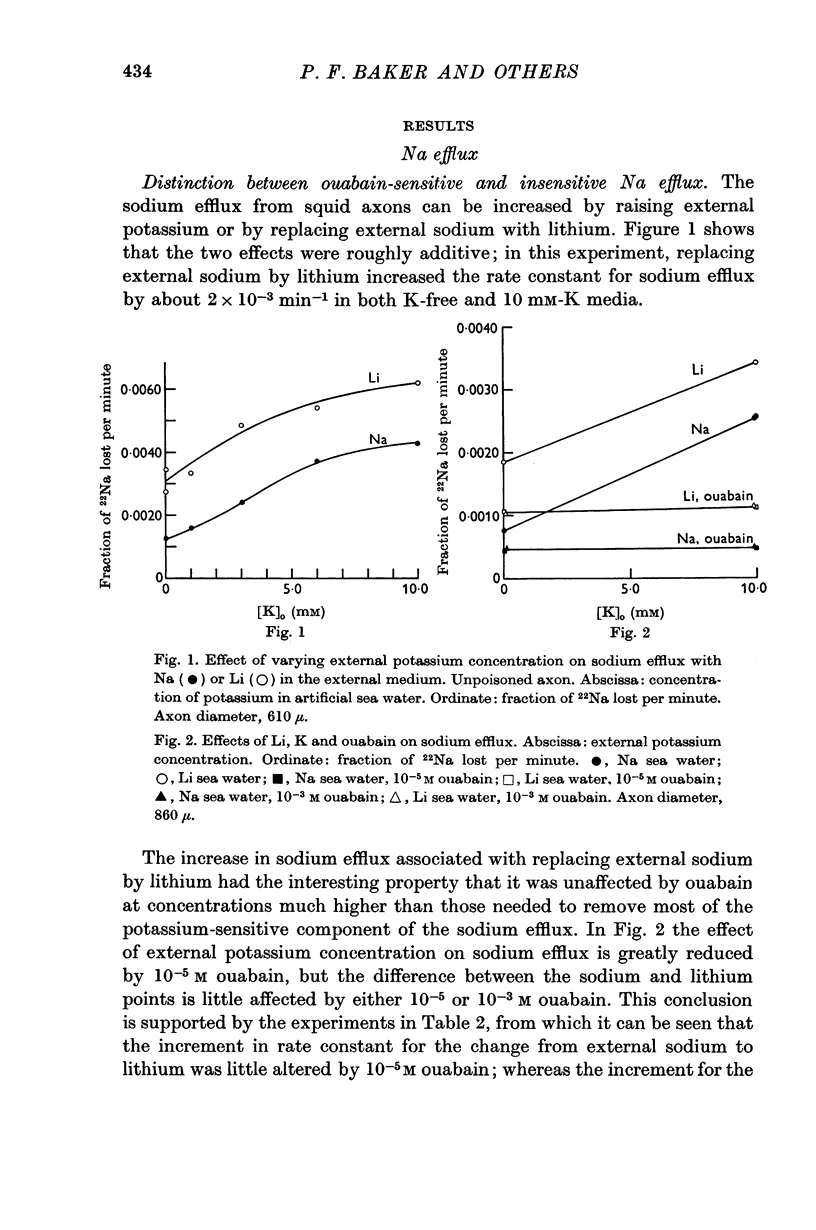
1. Previous work has shown that the sodium efflux from the axons of Loligo forbesi increases when external sodium is replaced by lithium.
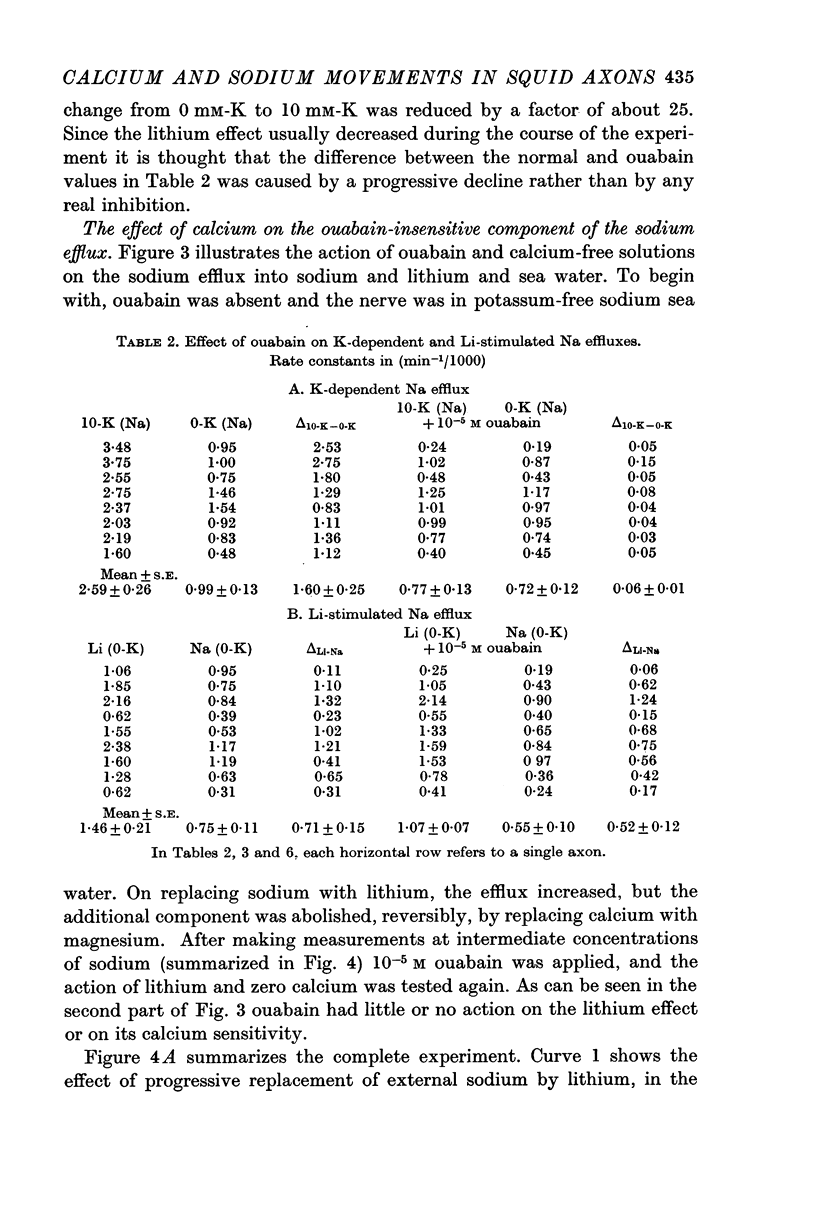
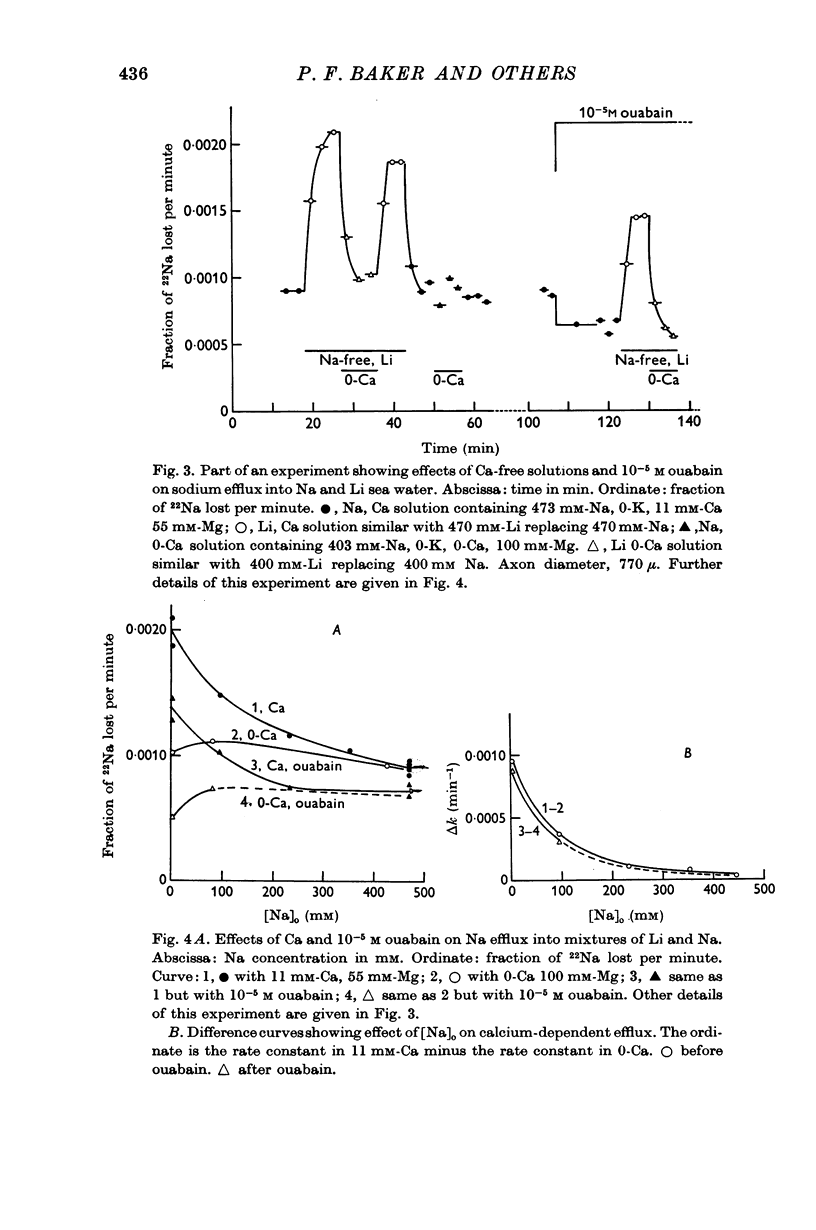
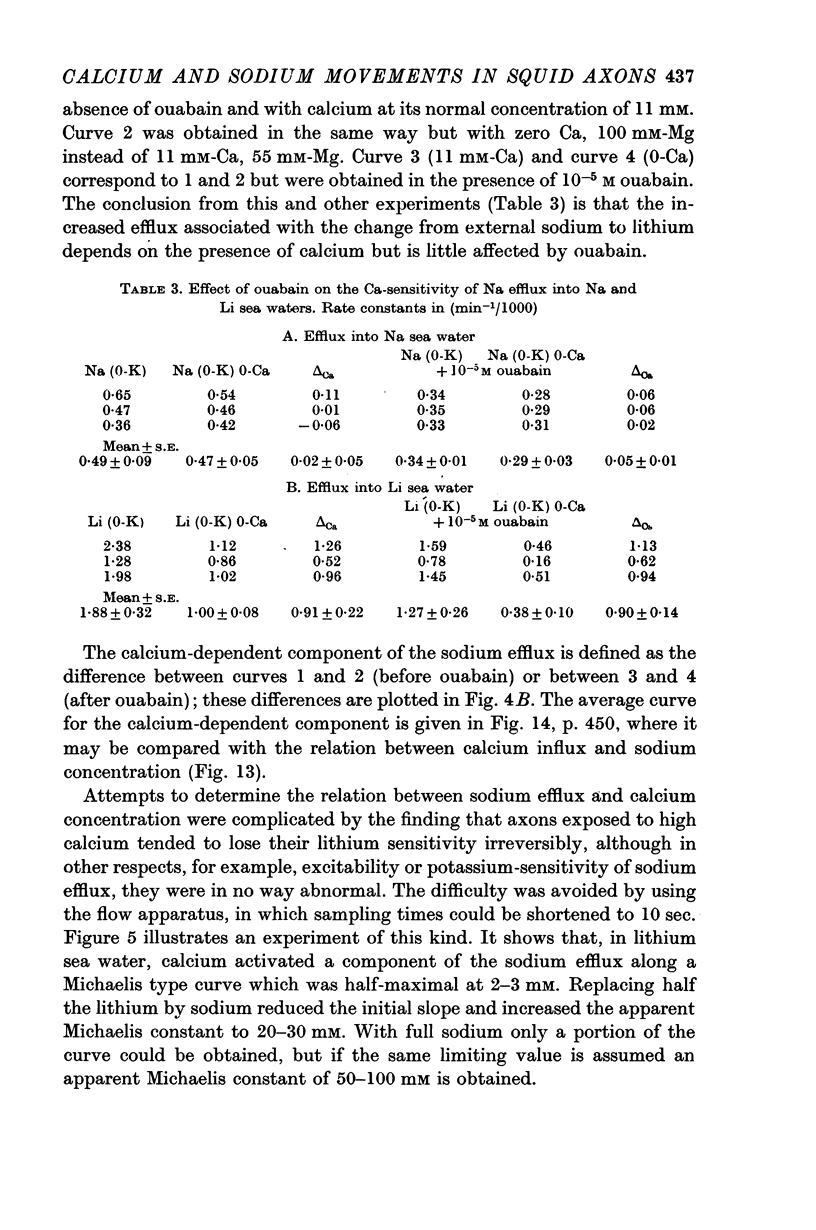
2. The increase in efflux in lithium was unaffected by ouabain but was abolished by removal of external calcium; in these respects it differed from the potassium-dependent sodium efflux which was abolished by ouabain but not reduced by removal of external calcium.
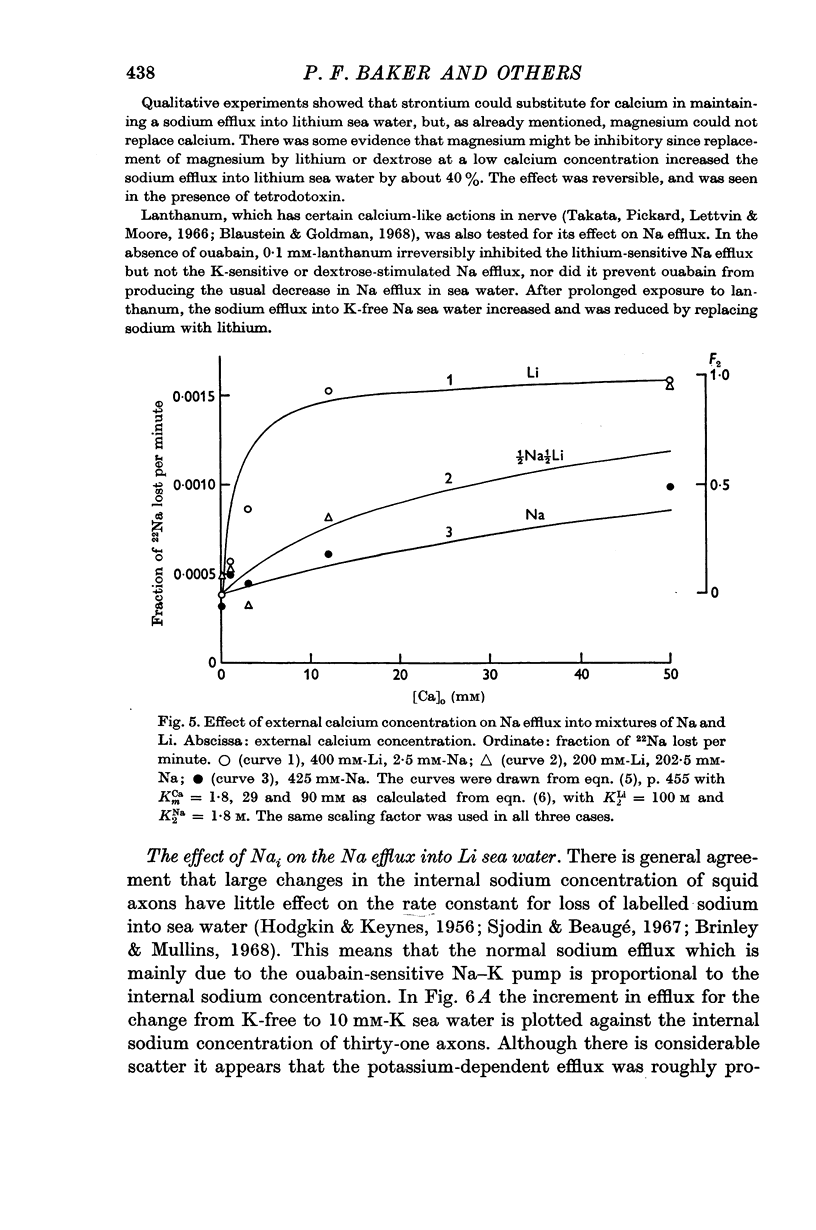
3. Strontium but not magnesium could replace calcium in activating the ouabain-insensitive sodium efflux; lanthanum had an inhibitory effect.
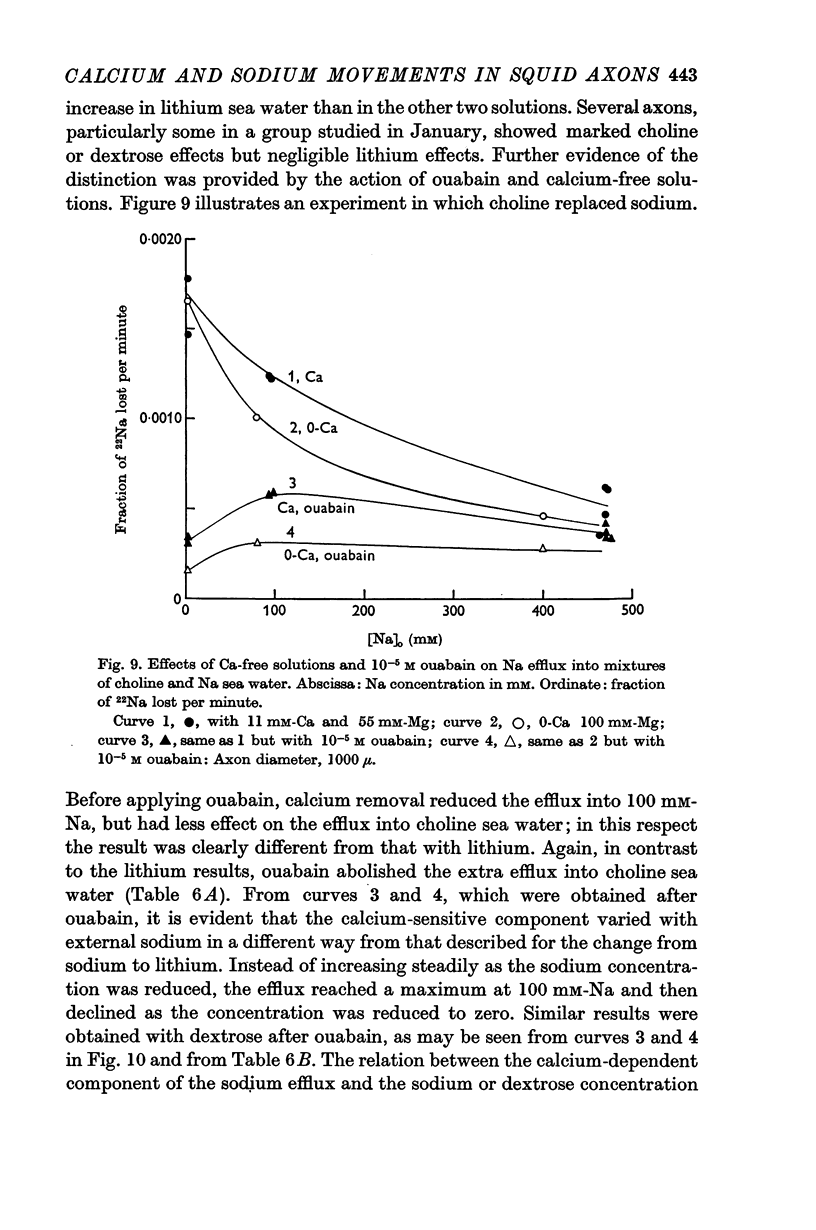
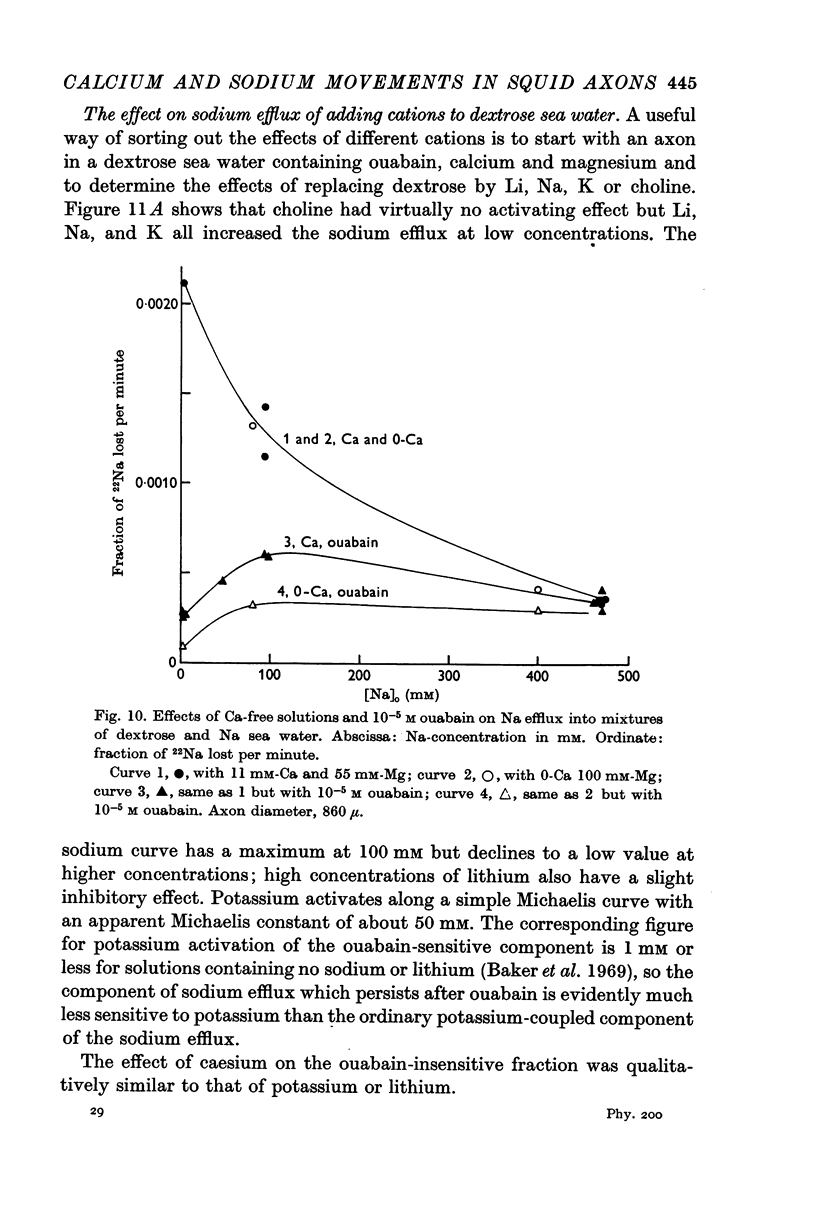
4. Replacing all the external NaCl by choline chloride or dextrose gave a rise in Na efflux which was abolished by ouabain but not by removal of external calcium.
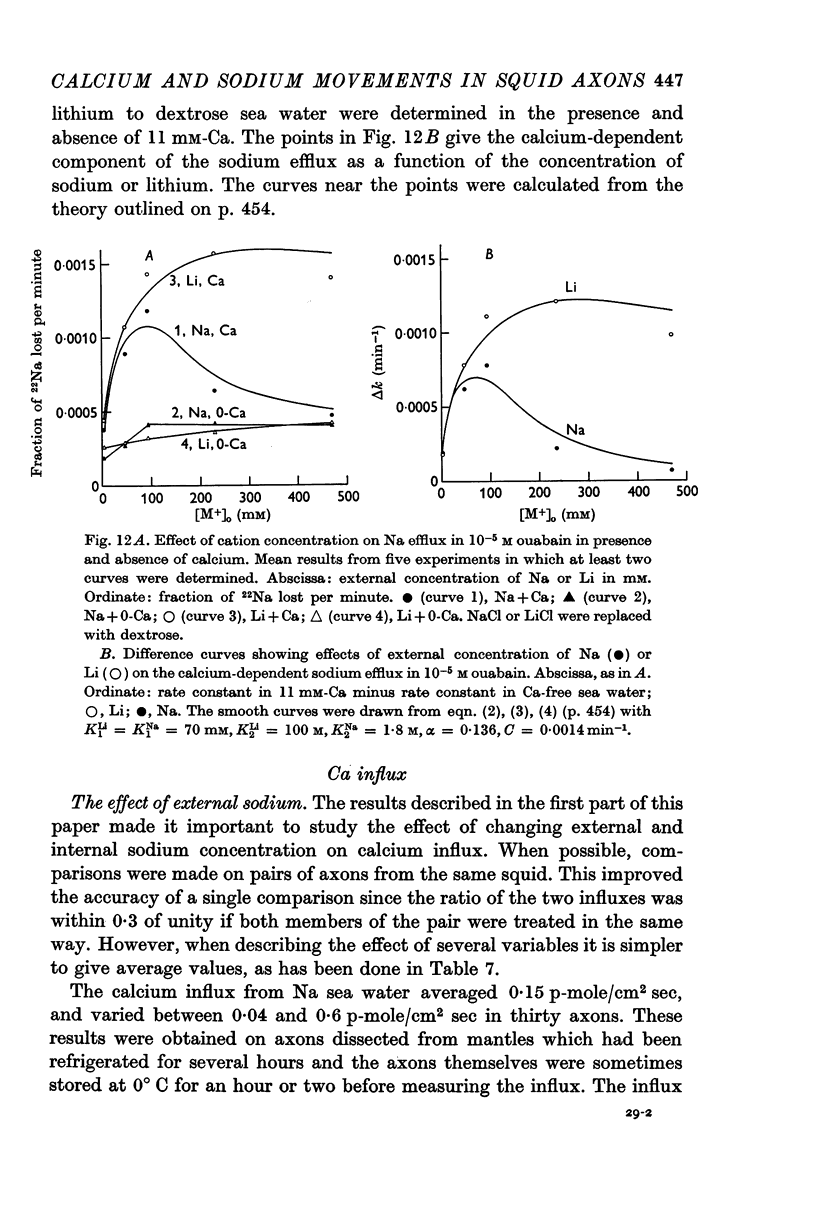
5. The rise in Na efflux resulting from partial replacement of NaCl by dextrose or choline chloride consisted of two components one of which was ouabain-insensitive and calcium-dependent and the other was inhibited by ouabain but calcium-insensitive.
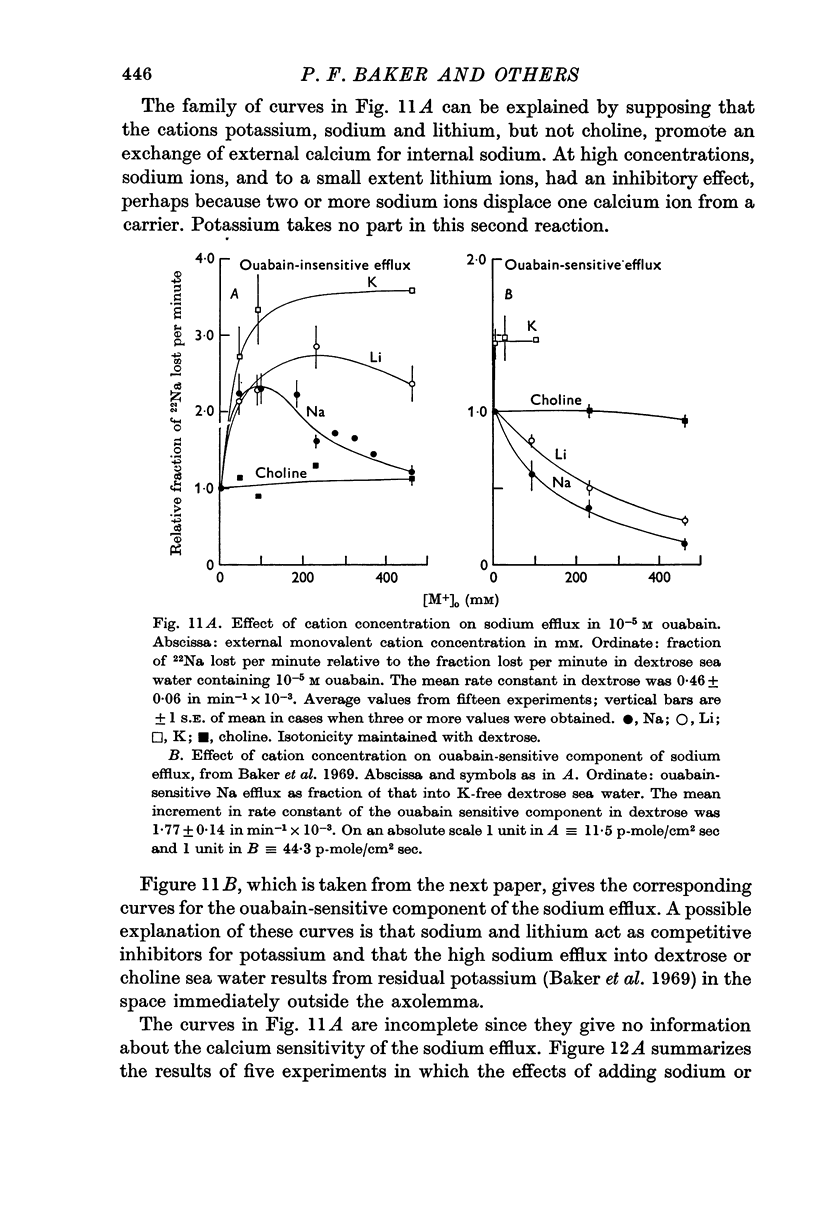
6. The ouabain-insensitive component of the Na efflux was activated by low concentrations of Na, Li or K but inhibited by high concentrations of Na and to a lesser extent Li. The inhibiting effect of high Na was of the kind expected if these ions displace calcium from an external site.
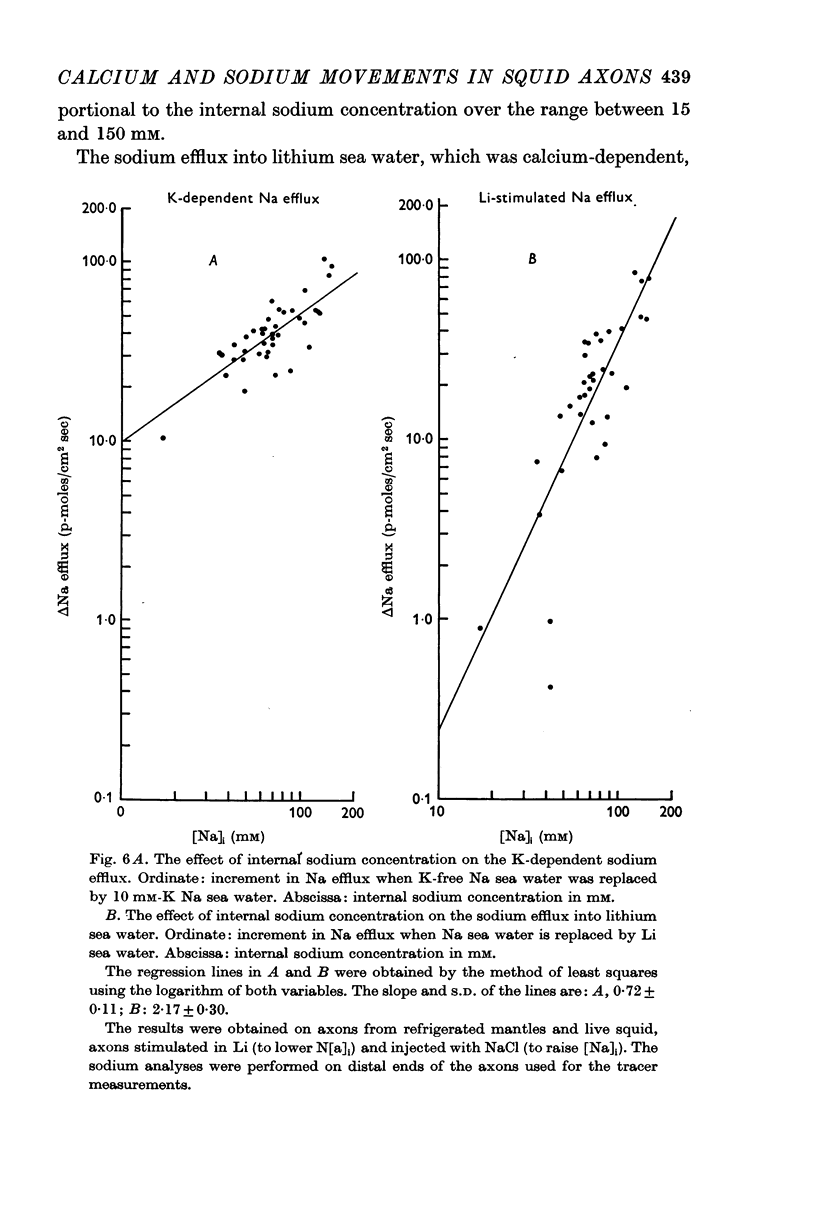
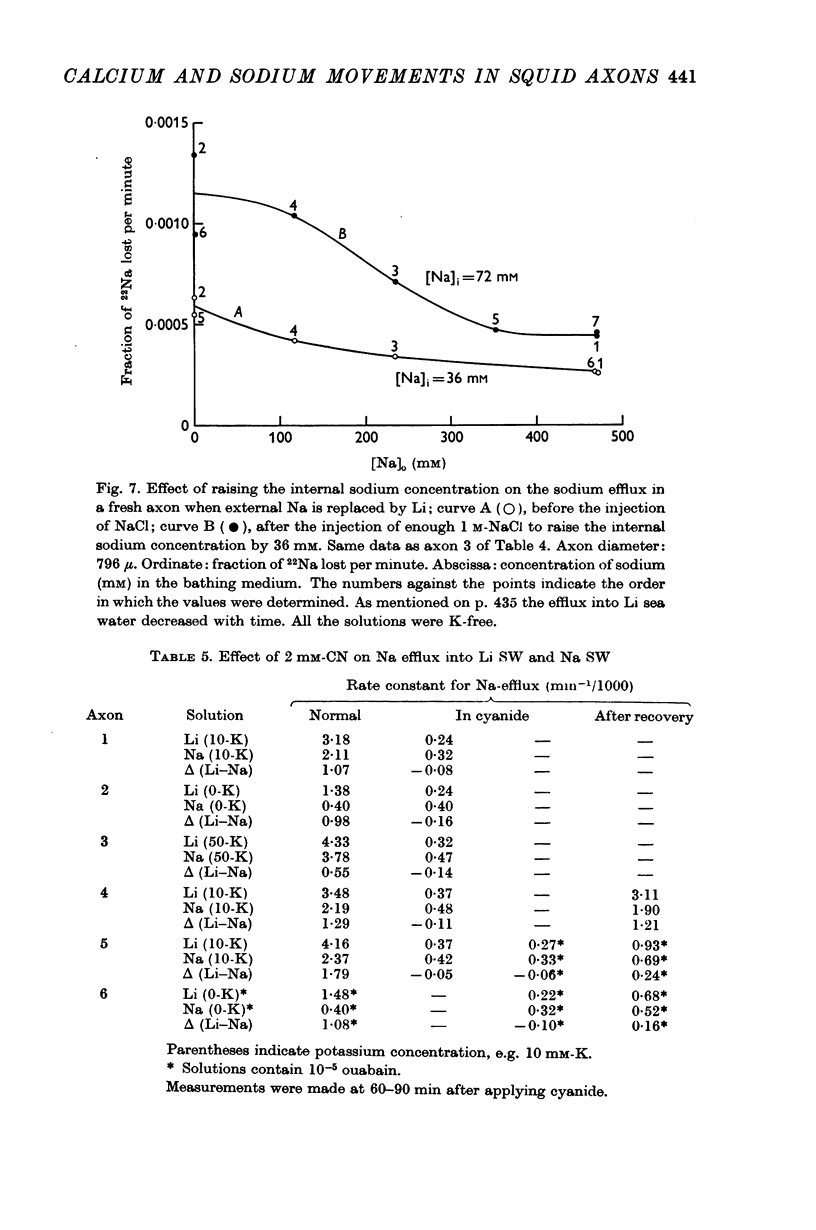
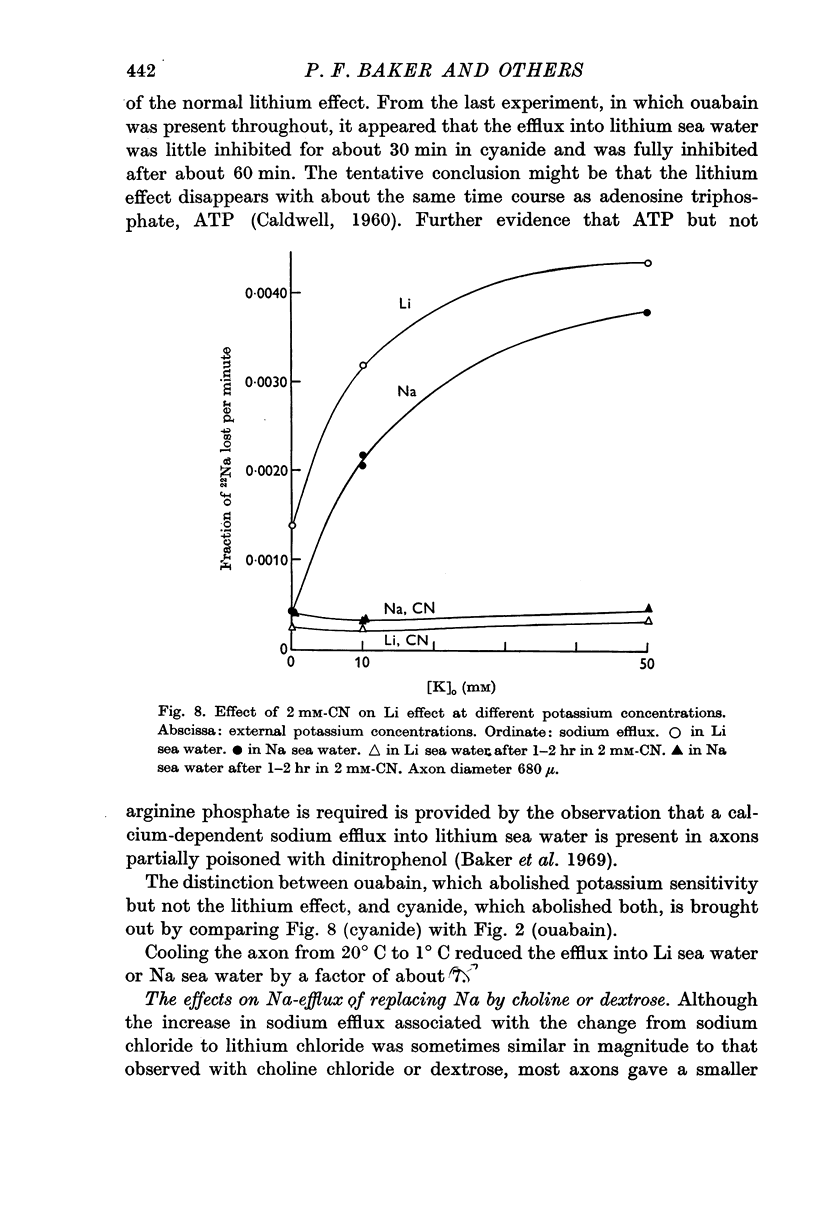
7. The ouabain-insensitive component of the Na efflux was abolished by cyanide, had a Q10 of 2·7; and was roughly proportional to [Na]i2. It was much more variable in magnitude than the ouabain-sensitive, potassium-dependent component of the sodium efflux.
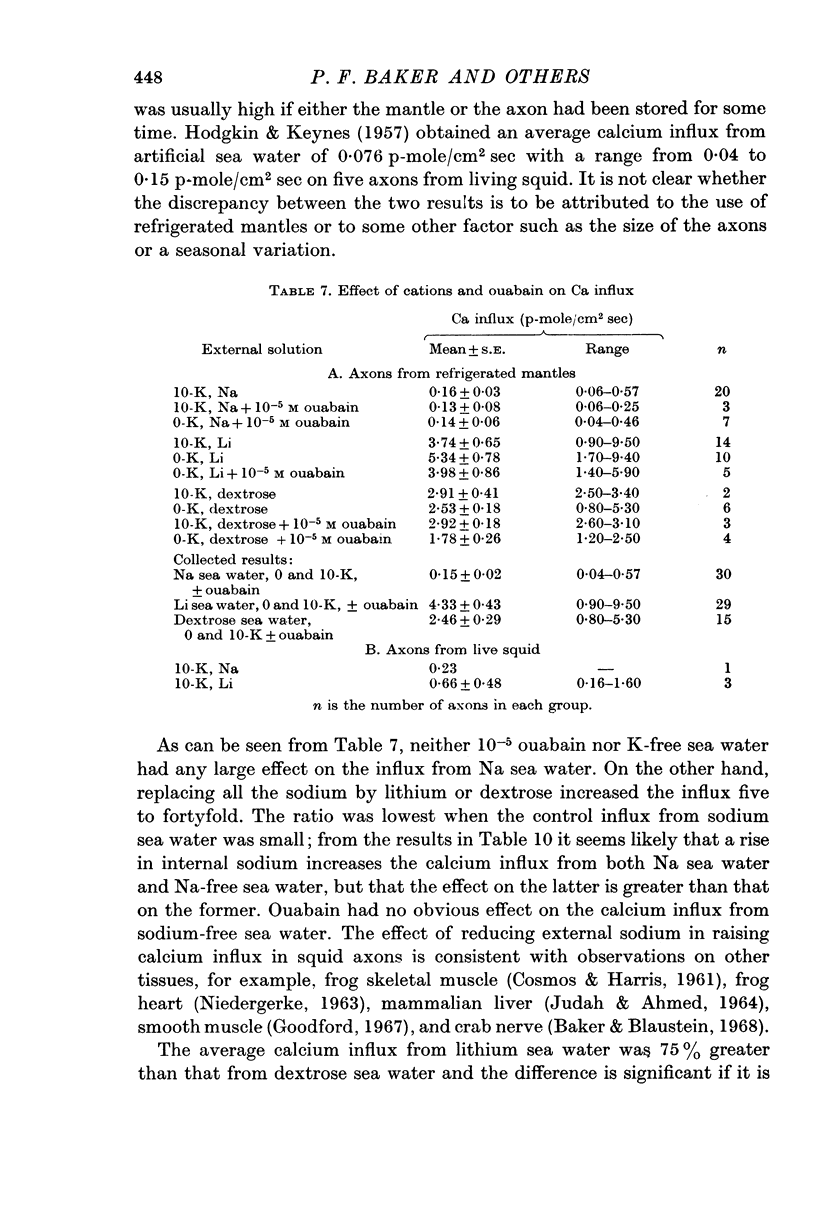
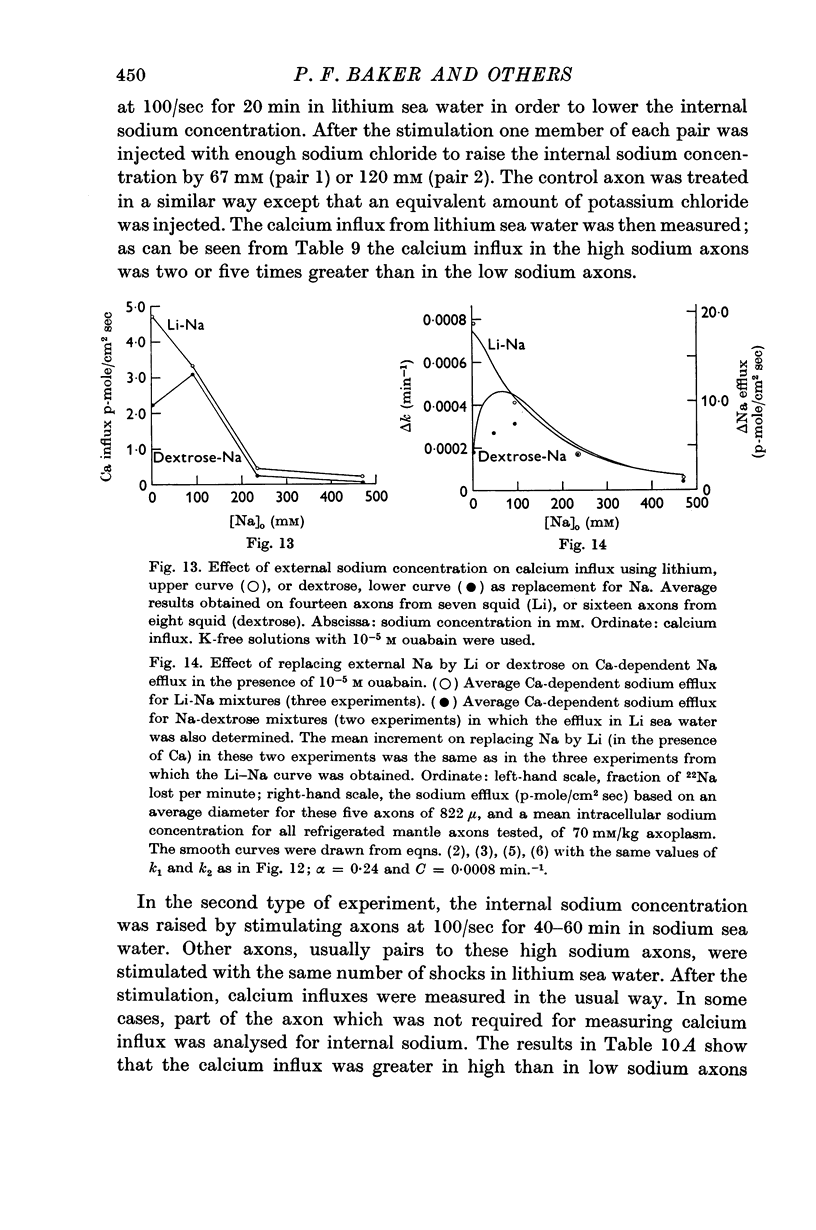
8. The calcium influx increased five to fortyfold when external NaCl was replaced by LiCl or dextrose, the increase for Li being larger than the increase for dextrose.
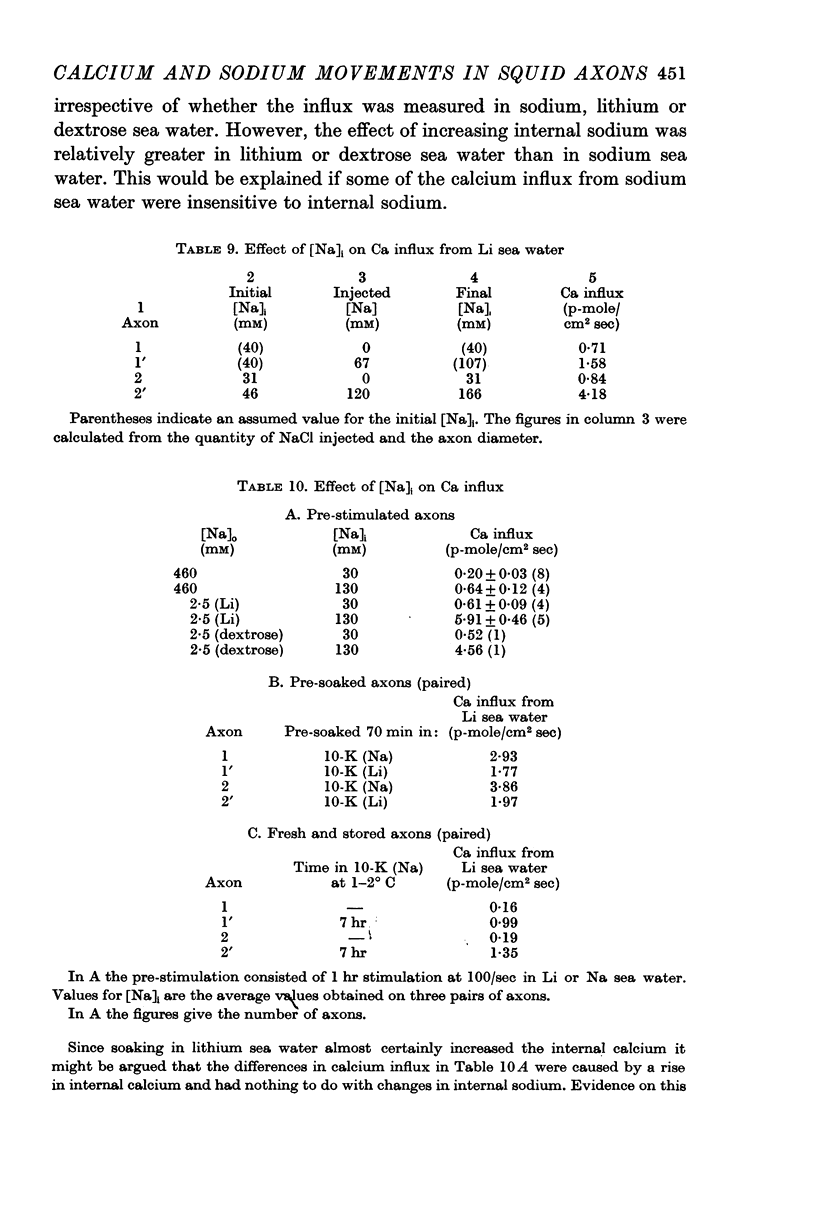
9. The calcium influx from Na, Li or dextrose sea water was increased three to tenfold by increasing the internal Na about fourfold.
10. The experiments provide evidence for a coupling between an inward movement of calcium and an outward movement of sodium.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Keynes R. D., Manil J., Shaw T. I., Steinhardt R. A. The ouabain-sensitive fluxes of sodium and potassium in squid giant axons. J Physiol. 1969 Feb;200(2):459–496. doi: 10.1113/jphysiol.1969.sp008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Manil J., Steinhardt R. A. A ouabain-insensitive, calcium-sensitive sodium efflux from giant axons of Loligo. J Physiol. 1967 Jul;191(2):100P–102P. [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P. Sodium-dependent uptake of calcium by crab nerve. Biochim Biophys Acta. 1968 Jan 3;150(1):167–170. doi: 10.1016/0005-2736(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Banks P. The effect of ouabain on the secretion of catecholamines and on the intracellular concentration of potassium. J Physiol. 1967 Dec;193(3):631–637. doi: 10.1113/jphysiol.1967.sp008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The action of sodium pump inhibitors on neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):381–399. doi: 10.1098/rspb.1968.0046. [DOI] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The influence of internal sodium on the behaviour of motor nerve endings. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):401–421. doi: 10.1098/rspb.1968.0047. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Goldman D. E. The action of certain polyvalent cations on the voltage-clamped lobster axon. J Gen Physiol. 1968 Mar;51(3):279–291. doi: 10.1085/jgp.51.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Mullins L. J. Sodium fluxes in internally dialyzed squid axons. J Gen Physiol. 1968 Aug;52(2):181–211. doi: 10.1085/jgp.52.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr Sodium and potassium fluxes in isolated barnacle muscle fibers. J Gen Physiol. 1968 Apr;51(4):445–477. doi: 10.1085/jgp.51.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. I. Partial inhibition of the active transport of cations in the giant axons of Loligo. J Physiol. 1960 Jul;152:591–600. doi: 10.1113/jphysiol.1960.sp006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. L. The effects of injecting 'energy-rich' phosphate compounds on the active transport of ions in the giant axons of Loligo. J Physiol. 1960 Jul;152:561–590. doi: 10.1113/jphysiol.1960.sp006509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. The phosphorus metabolism of squid axons and its relationship to the active transport of sodium. J Physiol. 1960 Jul;152:545–560. doi: 10.1113/jphysiol.1960.sp006508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSMOS E., HARRIS E. J. In vitro studies of the gain and exchange of calcium in frog skeletal muscle. J Gen Physiol. 1961 Jul;44:1121–1130. doi: 10.1085/jgp.44.6.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy A. A. A net gain of sodium ions and a net loss of potassium ions accompanying the uptake of glycine by mouse ascites-tumour cells in the presence of sodium cyanide. Biochem J. 1968 Jun;108(2):195–206. doi: 10.1042/bj1080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRUMENTO A. S., MULLINS L. J. POTASSIUM-FREE EFFECT IN SQUID AXONS. Nature. 1964 Dec 26;204:1312–1313. doi: 10.1038/2041312b0. [DOI] [PubMed] [Google Scholar]
- Forbes W. H., Roughton F. J. The equilibrium between oxygen and haemoglobin: I. The oxygen dissociation curve of dilute blood solutions. J Physiol. 1931 Mar 23;71(3):229–260. doi: 10.1113/jphysiol.1931.sp002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. THE ACTION OF CARDIAC GLYCOSIDES ON ION MOVEMENTS. Pharmacol Rev. 1964 Dec;16:381–407. [PubMed] [Google Scholar]
- Goodford P. J. The calcium content of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1967 Sep;192(1):145–157. doi: 10.1113/jphysiol.1967.sp008293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Experiments on the injection of substances into squid giant axons by means of a microsyringe. J Physiol. 1956 Mar 28;131(3):592–616. doi: 10.1113/jphysiol.1956.sp005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C. N., Milner R. D. Cations and the secretion of insulin from rabbit pancreas in vitro. J Physiol. 1968 Nov;199(1):177–187. doi: 10.1113/jphysiol.1968.sp008647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C. N., Milner R. D. The role of sodium and potassium in insulin secretion from rabbit pancreas. J Physiol. 1968 Feb;194(3):725–743. doi: 10.1113/jphysiol.1968.sp008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUDAH J. D., AHMED K. THE BIOCHEMISTRY OF SODIUM TRANSPORT. Biol Rev Camb Philos Soc. 1964 May;39:160–193. doi: 10.1111/j.1469-185x.1964.tb00953.x. [DOI] [PubMed] [Google Scholar]
- NIEDERGERKE R. MOVEMENTS OF CA IN FROG HEART VENTRICLES AT REST AND DURING CONTRACTURES. J Physiol. 1963 Jul;167:515–550. doi: 10.1113/jphysiol.1963.sp007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin R. A., Beaugé L. A. The ion selectivity and concentration dependence of cation coupled active sodium transport in squid giant axons. Curr Mod Biol. 1967 May;1(2):105–115. doi: 10.1016/0303-2647(67)90022-6. [DOI] [PubMed] [Google Scholar]
- Takata M., Pickard W. F., Lettvin J. Y., Moore J. W. Ionic conductance changes in lobster axon membrane when lanthanum is substituted for calcium. J Gen Physiol. 1966 Nov;50(2):461–471. doi: 10.1085/jgp.50.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]


