Abstract
1. Miniature end-plate potentials (m.e.p.p.s) were intra- and extracellularly recorded from neuromuscular junctions in rat phrenic nerve—diaphragm preparations in vitro.
2. Statistical analysis of the intervals between m.e.p.p.s showed that when the mean number of events in time t was plotted as a function of the variance of the events in time t there was a significant deviation from the straight line relationship expected for a Poisson process. Computer simulation showed that this deviation is explicable if release was generated by the random phasing of the activity of a number of releasing sites.
3. There was no indication that release of one quantum influences the probability of release of remaining quanta (drag, clustering). It is suggested that m.e.p.p.s whose amplitude is larger than the mode result from the release of the contents of vesicles whose volume is also supramodal.
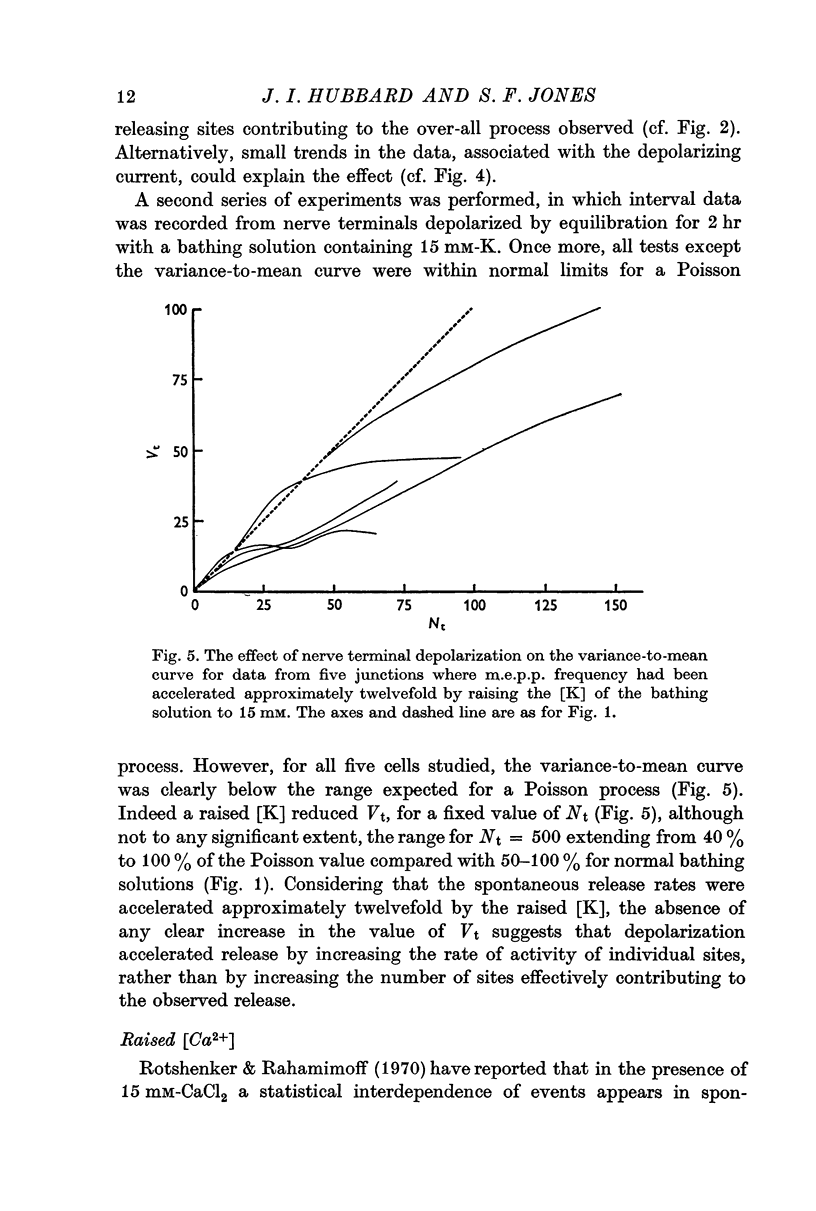
4. The effects of depolarization of nerve terminals upon the variance-mean curve suggest an increase in the activity of sites rather than an increase in their number.
5. Statistical analysis indicated at least 200 ± 100 (mean ± 1 S.E.) releasing sites. This number is of the same order as the number of sites of vesicle aggregation and presynaptic membrane density seen in electron micrographs of nerve terminals of this preparation.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., CURTIS D. R. THE EXCITATION OF THALAMIC NEURONES BY ACETYLCHOLINE. Acta Physiol Scand. 1964 May-Jun;61:85–99. doi: 10.1111/j.1748-1716.1964.tb02945.x. [DOI] [PubMed] [Google Scholar]
- BIRKS R., HUXLEY H. E., KATZ B. The fine structure of the neuromuscular junction of the frog. J Physiol. 1960 Jan;150:134–144. doi: 10.1113/jphysiol.1960.sp006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. Spontaneous potential at sympathetic nerve endings in smooth muscle. J Physiol. 1962 Mar;160:446–460. doi: 10.1113/jphysiol.1962.sp006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE W. V. Structural variations of nerve endings in the striated muscles of the rat. J Comp Neurol. 1957 Dec;108(3):445–463. doi: 10.1002/cne.901080306. [DOI] [PubMed] [Google Scholar]
- Clark A. W., Mauro A., Longenecker H. E., Jr, Hurlbut W. P. Effects of black widow spider venom on the frog neuromuscular junction. Effects on the fine structure of the frog neuromuscular junction. Nature. 1970 Feb 21;225(5234):703–705. doi: 10.1038/225703a0. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Biophysical aspects of neuro-muscular transmission. Prog Biophys Biophys Chem. 1956;6:121–170. [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Localization of active spots within the neuromuscular junction of the frog. J Physiol. 1956 Jun 28;132(3):630–649. doi: 10.1113/jphysiol.1956.sp005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELMQVIST D., QUASTEL D. M. PRESYNAPTIC ACTION OF HEMICHOLINIUM AT THE NEUROMUSCULAR JUNCTION. J Physiol. 1965 Apr;177:463–482. doi: 10.1113/jphysiol.1965.sp007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J. The effects of osmotic pressure changes on the spontaneous activity at motor nerve endings. J Physiol. 1956 Dec 28;134(3):689–697. doi: 10.1113/jphysiol.1956.sp005675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hubbard J. I. An investigation of the post-tetanic potentiation of end-plate potentials at a mammalian neuromuscular junction. J Physiol. 1966 May;184(2):353–375. doi: 10.1113/jphysiol.1966.sp007919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hubbard J. I. Evidence for a Poisson distribution of miniature end-plate potentials and some implications. Nature. 1965 Oct 23;208(5008):395–396. doi: 10.1038/208395a0. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Quastel D. M. Competition between sodium and calcium ions in transmitter release at mammalian neuromuscular junctions. J Physiol. 1966 Jul;185(1):95–123. doi: 10.1113/jphysiol.1966.sp007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I. The effect of calcium and magnesium on the spontaneous release of transmitter from mammalian motor nerve endings. J Physiol. 1961 Dec;159:507–517. doi: 10.1113/jphysiol.1961.sp006824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J., Katz B., Miledi R. Structural and functional changes of frog neuromuscular junctions in high calcium solutions. Proc R Soc Lond B Biol Sci. 1971 Sep 28;178(1053):407–415. doi: 10.1098/rspb.1971.0072. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. An examination of the effects of osmotic pressure changes upon transmitter release from mammalian motor nerve terminals. J Physiol. 1968 Aug;197(3):639–657. doi: 10.1113/jphysiol.1968.sp008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Kwanbunbumpen S. Evidence for the vesicle hypothesis. J Physiol. 1968 Feb;194(2):407–420. doi: 10.1113/jphysiol.1968.sp008415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I. Mechanism of transmitter release. Prog Biophys Mol Biol. 1970;21:33–124. [PubMed] [Google Scholar]
- Hubbard J. I., Willis W. D. The effects of depolarization of motor nerve terminals upon the release of transmitter by nerve impulses. J Physiol. 1968 Feb;194(2):381–405. doi: 10.1113/jphysiol.1968.sp008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Willis W. D. The effects of depolarization of motor nerve terminals upon the release of transmitter by nerve impulses. J Physiol. 1968 Feb;194(2):381–405. doi: 10.1113/jphysiol.1968.sp008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel M., Gautron J., Lesbats B. Isolement des vésicules synaptiques de l'organe électrique de la Torpille et localisation de l'acétylcholine à leur niveau. C R Acad Sci Hebd Seances Acad Sci D. 1968 Jan 15;266(3):273–275. [PubMed] [Google Scholar]
- Jones S. F., Kwanbunbumpen S. Some effects of nerve stimulation andhemicholinium on quantal transmitter release at the mammalian neuromuscular junction. J Physiol. 1970 Mar;207(1):51–61. doi: 10.1113/jphysiol.1970.sp009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. F., Kwanbunbumpen S. The effects of nerve stimulation and hemicholinium on synaptic vesicles at the mammalian euromuscular junction. J Physiol. 1970 Mar;207(1):31–50. doi: 10.1113/jphysiol.1970.sp009046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. Spontaneous release of transmitter substance in multiquantal units. J Physiol. 1957 May 23;136(3):595–605. doi: 10.1113/jphysiol.1957.sp005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker H. E., Jr, Hurlbut W. P., Mauro A., Clark A. W. Effects of black widow spider venom on the frog neuromuscular junction. Effects on end-plate potential, miniature end-plate potential and nerve terminal spike. Nature. 1970 Feb 21;225(5234):701–703. doi: 10.1038/225701a0. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. QUANTAL COMPONENTS OF THE SYNAPTIC POTENTIAL IN THE CILIARY GANGLION OF THE CHICK. J Physiol. 1964 Dec;175:1–16. doi: 10.1113/jphysiol.1964.sp007499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel E., Potter L. T. Synaptic vesicles in freeze-etched electric tissue of Torpedo. Brain Res. 1970 Sep 29;23(1):95–100. doi: 10.1016/0006-8993(70)90352-5. [DOI] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. I. The single spike train. Biophys J. 1967 Jul;7(4):391–418. doi: 10.1016/S0006-3495(67)86596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter L. T. Synthesis, storage and release of [14C]acetylcholine in isolated rat diaphragm muscles. J Physiol. 1970 Jan;206(1):145–166. doi: 10.1113/jphysiol.1970.sp009003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R., Colomo F. Inhibitory action of sodium ions on transmitter release at the motor end-plate. Nature. 1967 Sep 9;215(5106):1174–1176. doi: 10.1038/2151174a0. [DOI] [PubMed] [Google Scholar]
- Rotshenker S., Rahamimoff R. Neuromuscular synapse: stochastic properties of spontaneous release of transmitter. Science. 1970 Nov 6;170(3958):648–649. doi: 10.1126/science.170.3958.648. [DOI] [PubMed] [Google Scholar]
- Sheridan M. N., Whittaker V. P., Israël M. The subcellular fractionation of the electric organ of Torpedo. Z Zellforsch Mikrosk Anat. 1966;74(3):293–307. [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Changes in potassium concentration around motor nerve terminals, produced by current flow, and their effects on neuromuscular transmission. J Physiol. 1961 Jan;155:46–58. doi: 10.1113/jphysiol.1961.sp006612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker V. P. The application of subcellular fractionation techniques to the study of brain function. Prog Biophys Mol Biol. 1965;15:39–96. doi: 10.1016/0079-6107(65)90004-0. [DOI] [PubMed] [Google Scholar]


