Abstract
1. Spontaneous spike activity and action potentials evoked by external field stimulation were recorded, intracellularly and with the double sucrose gap method, from the smooth muscle of guinea-pig taenia coli.
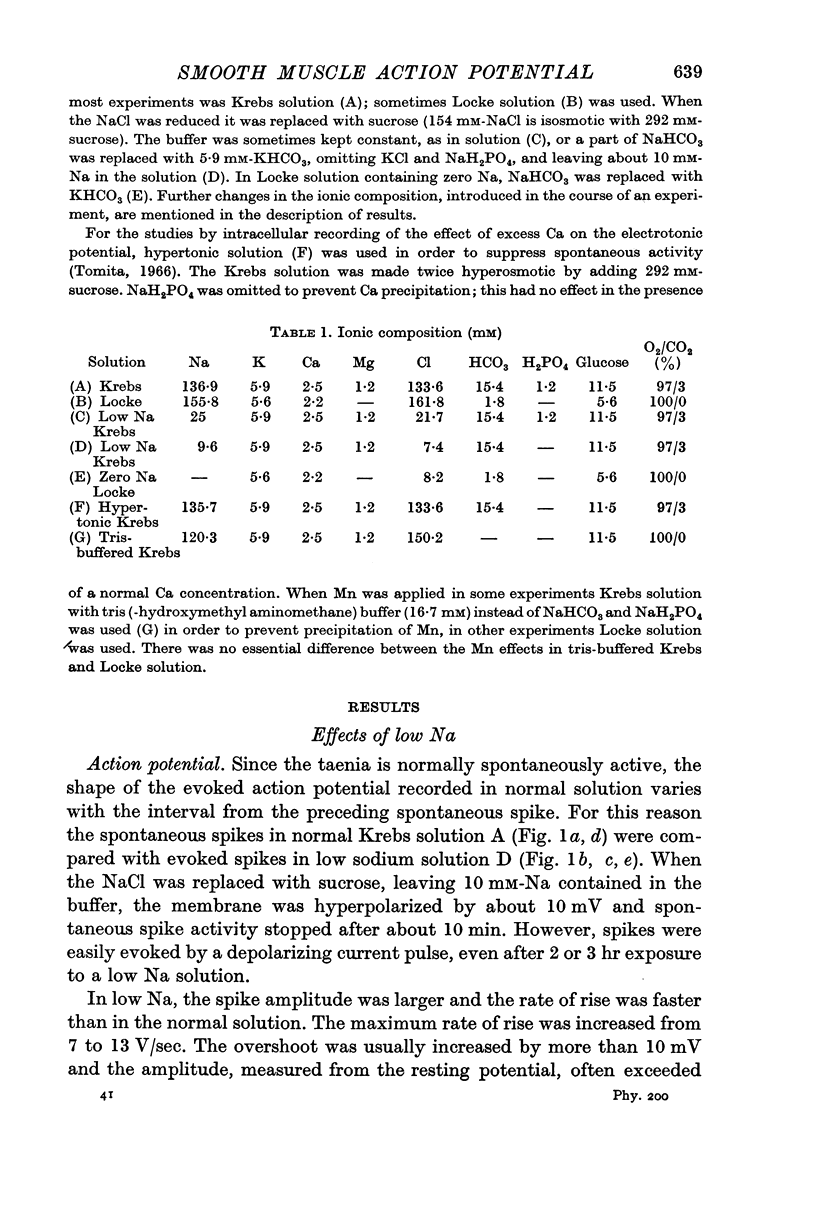
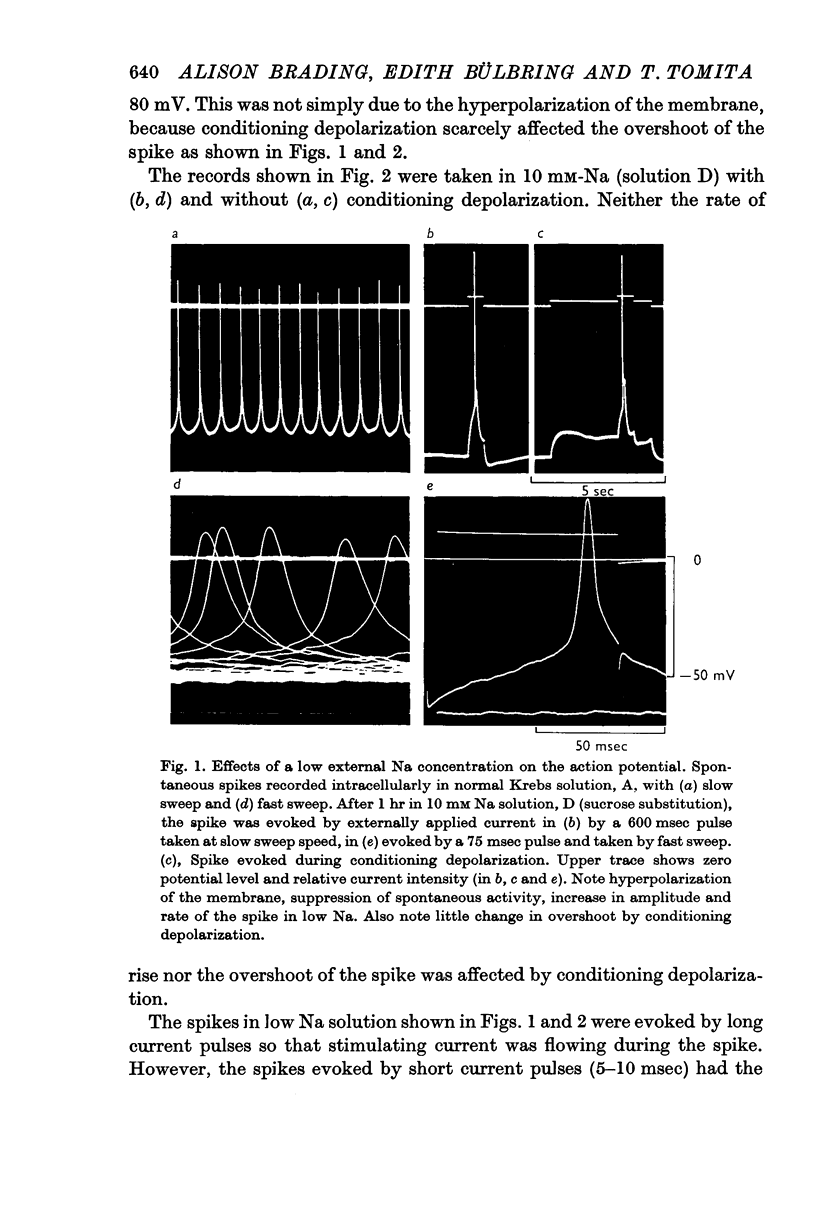
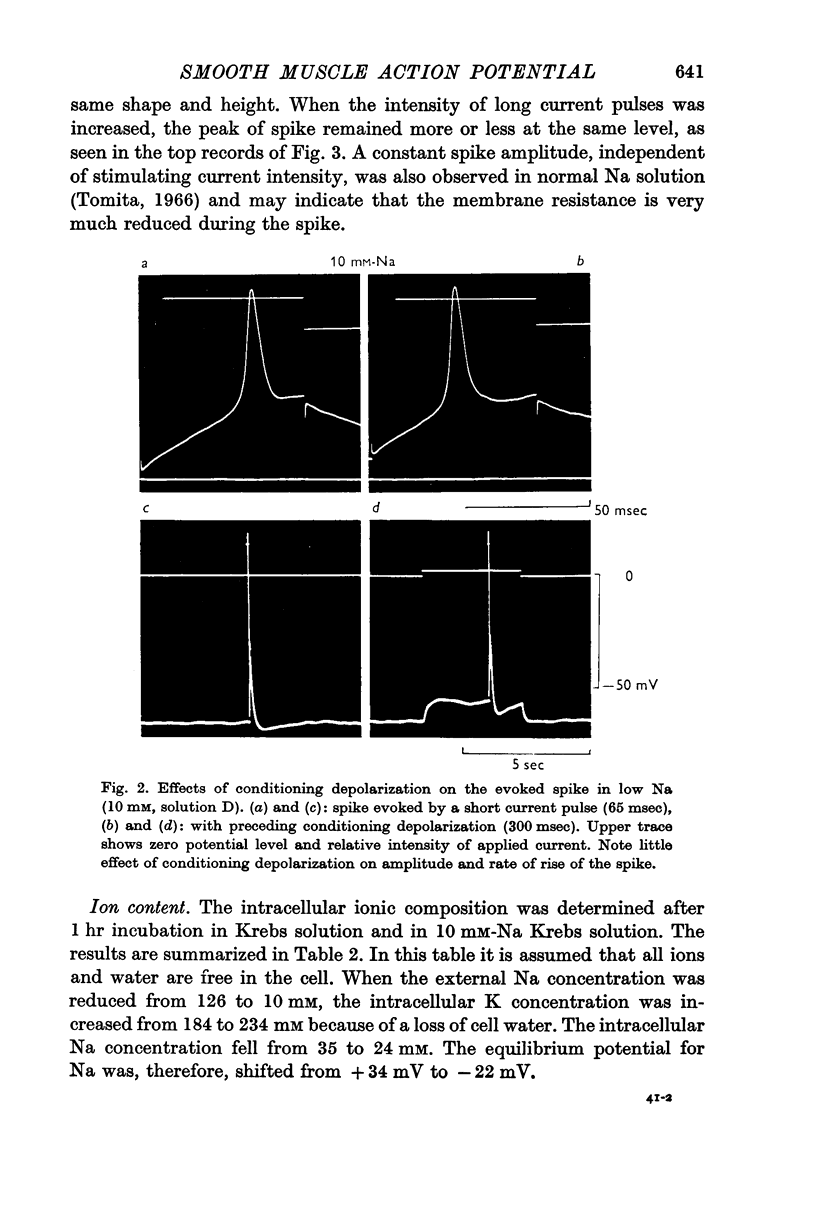
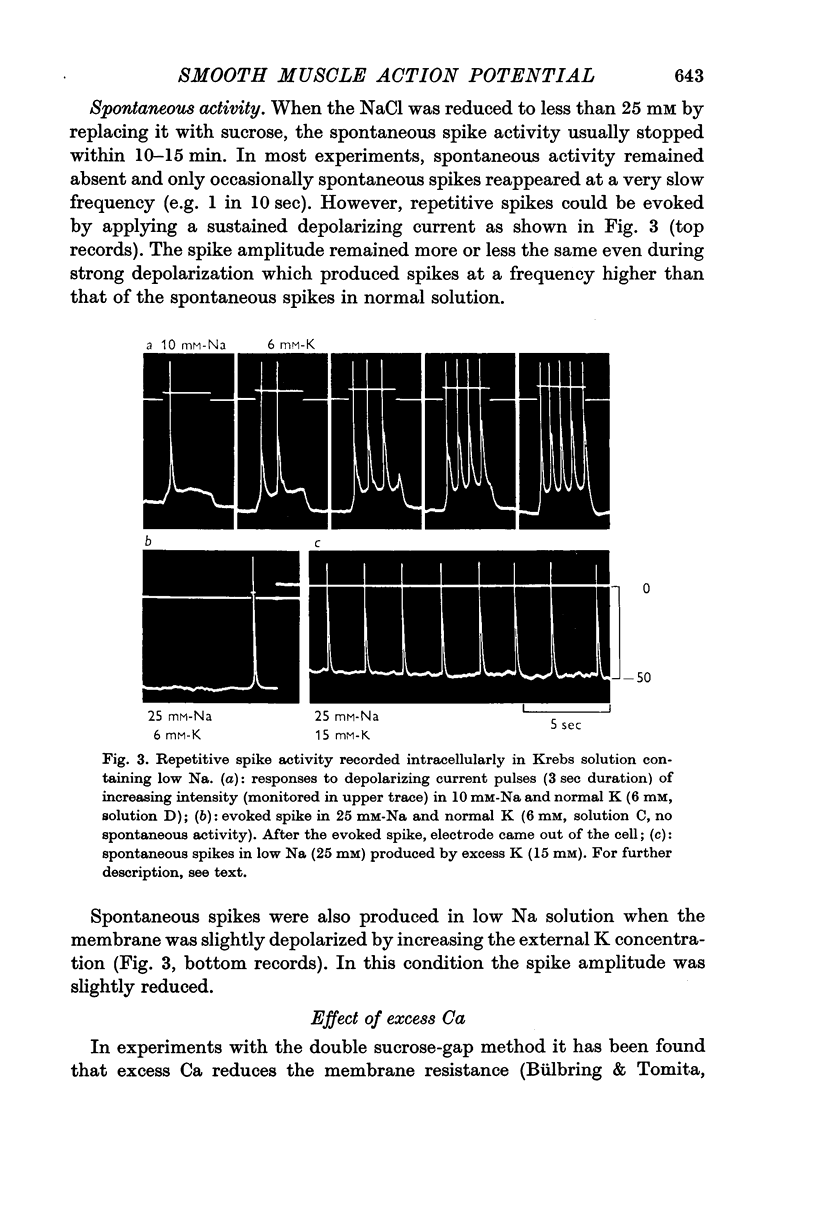
2. Replacement of external NaCl with sucrose (leaving 10 mM-Na in the buffer) caused hyperpolarization and stopped spontaneous activity within 10 min. Spikes could, however, be evoked for 2-3 hr. The amplitude, the overshoot and rate of rise of the spike were increased.
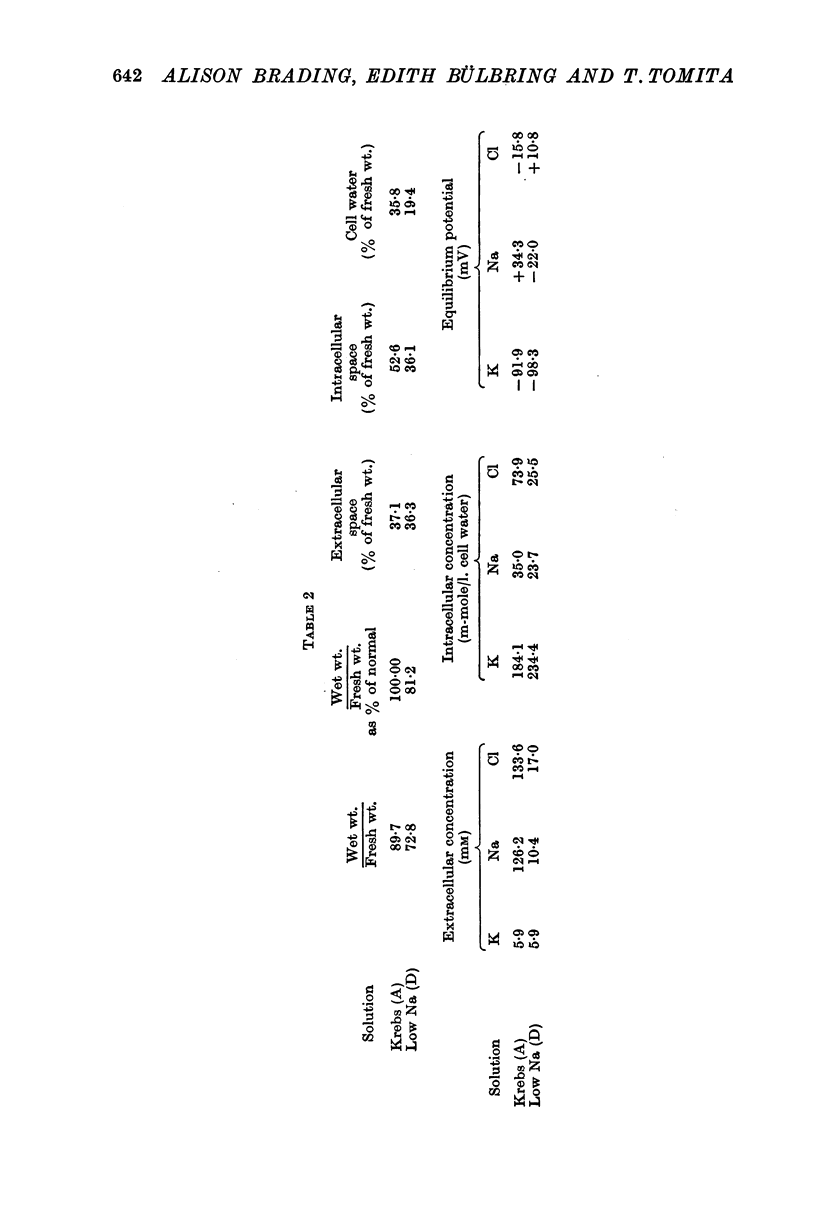
3. In 10 mM-[Na]o the intracellular Na concentration was reduced from 35 to 24 mM, shifting the Na-equilibrium potential from +34 to -22 mV.
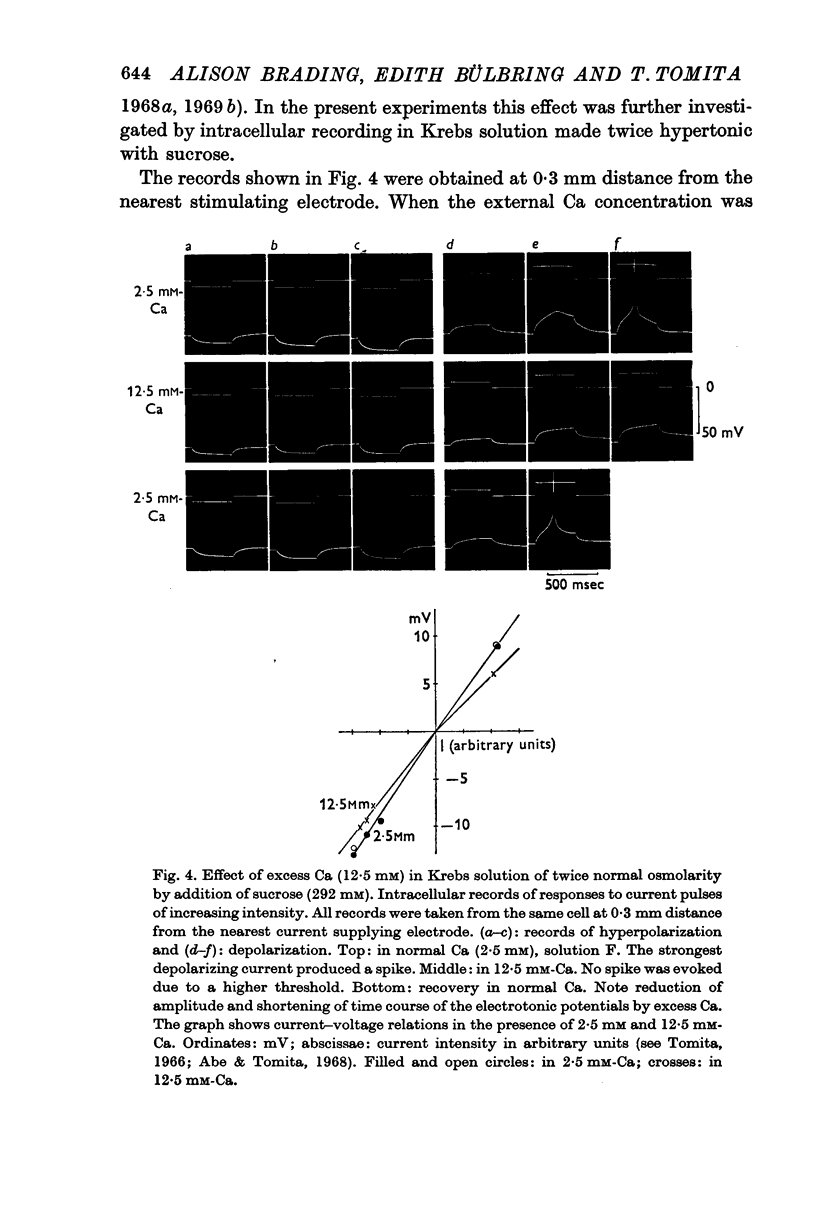
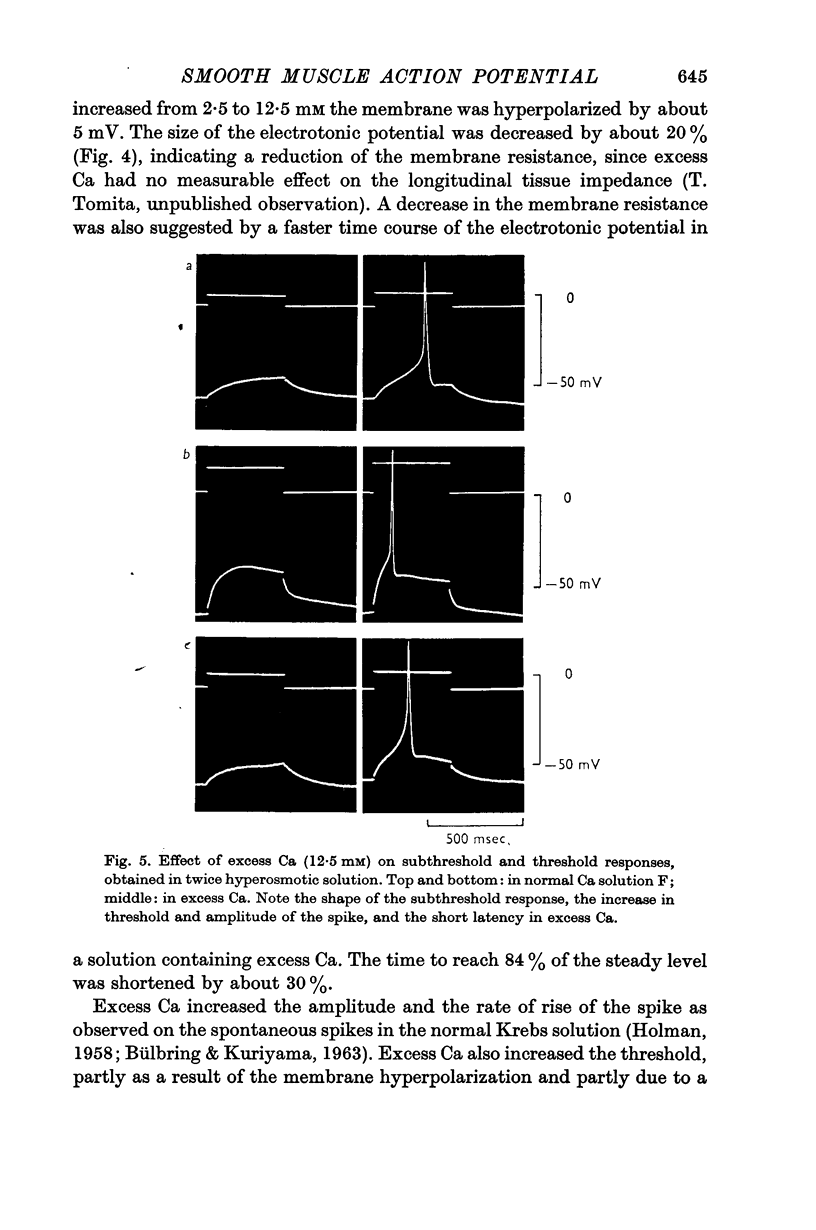
4. Excess Ca (12·5 mM) caused hyperpolarization and increased membrane conductance. The amplitude and the rate of rise of the spike were increased, the threshold was raised and the latency of the spike evoked by threshold stimulation became shorter.
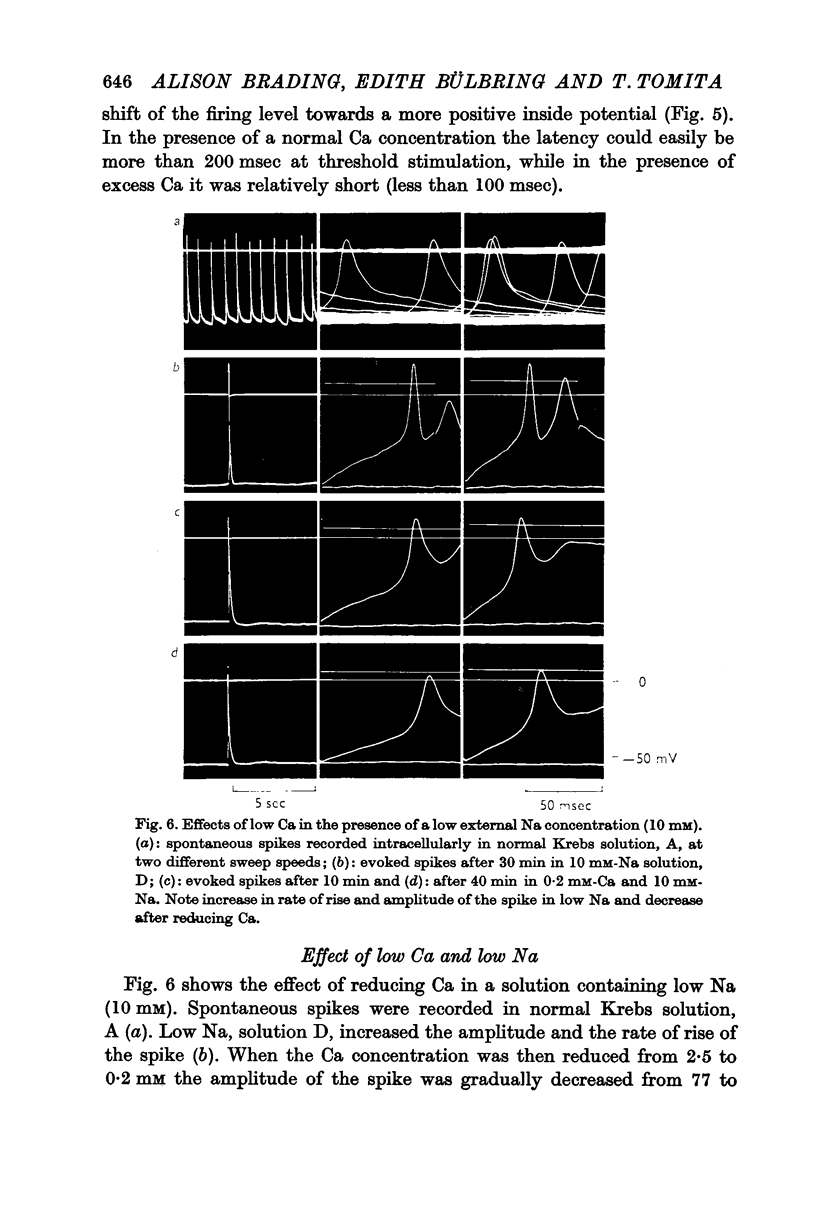
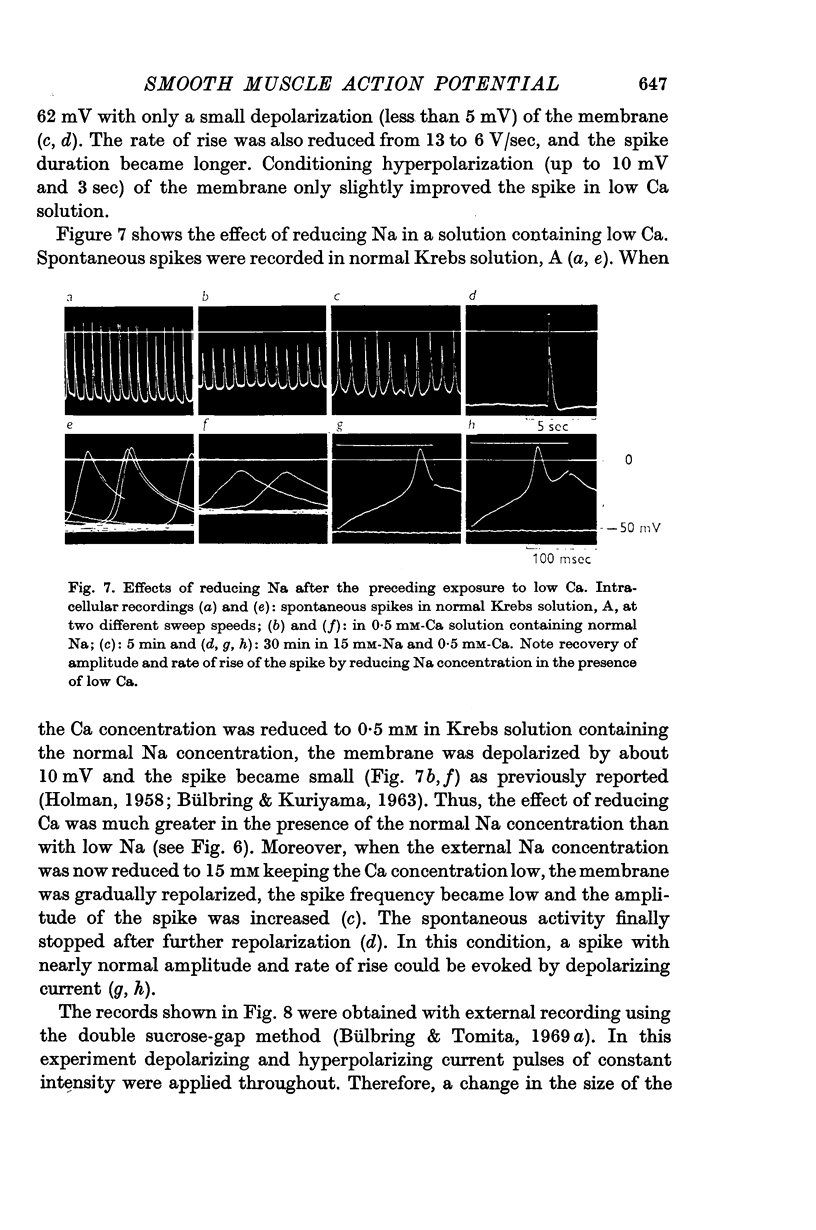
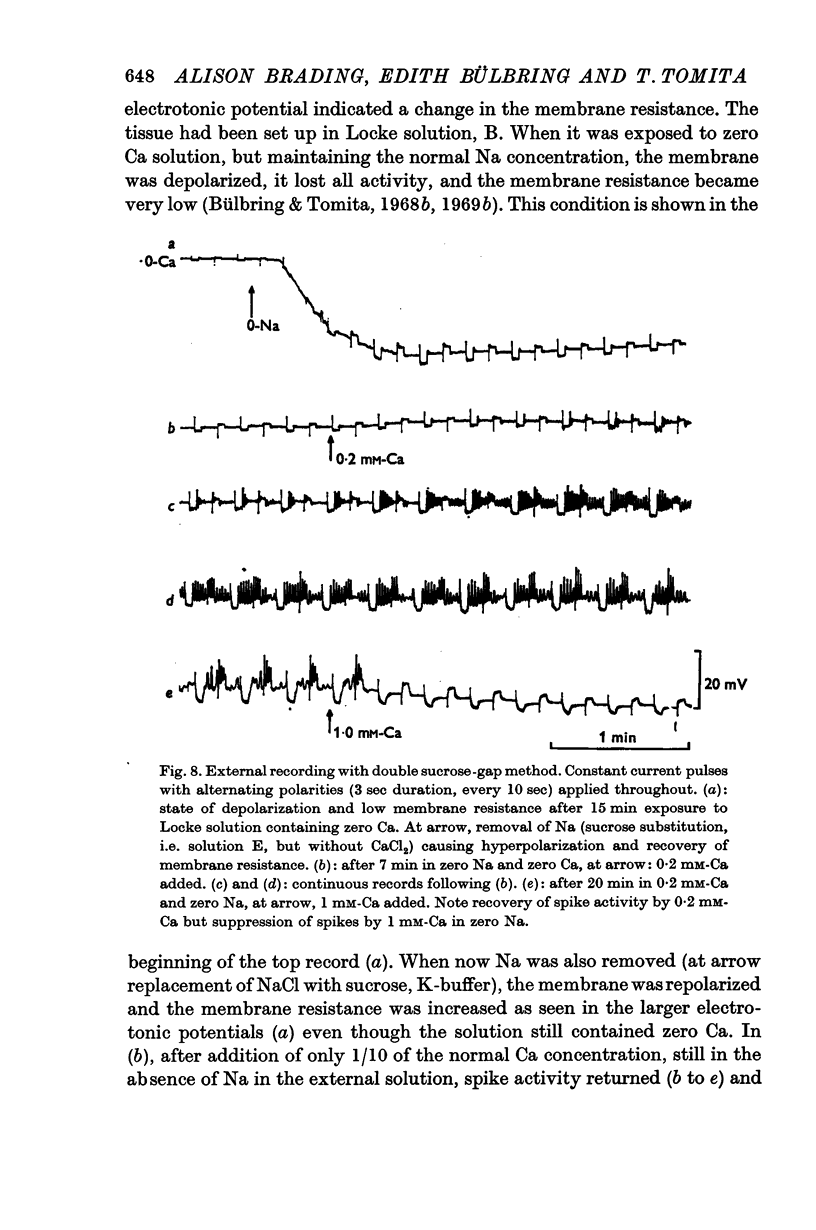
5. The effect of reducing the external Ca concentration depended on the Na concentration present, being greater with higher external [Na]o. When the membrane was depolarized and spikes deteriorated in low Ca (0·2-0·5 mM) reduction of Na to 10 mM caused repolarization and recovery of the action potential.
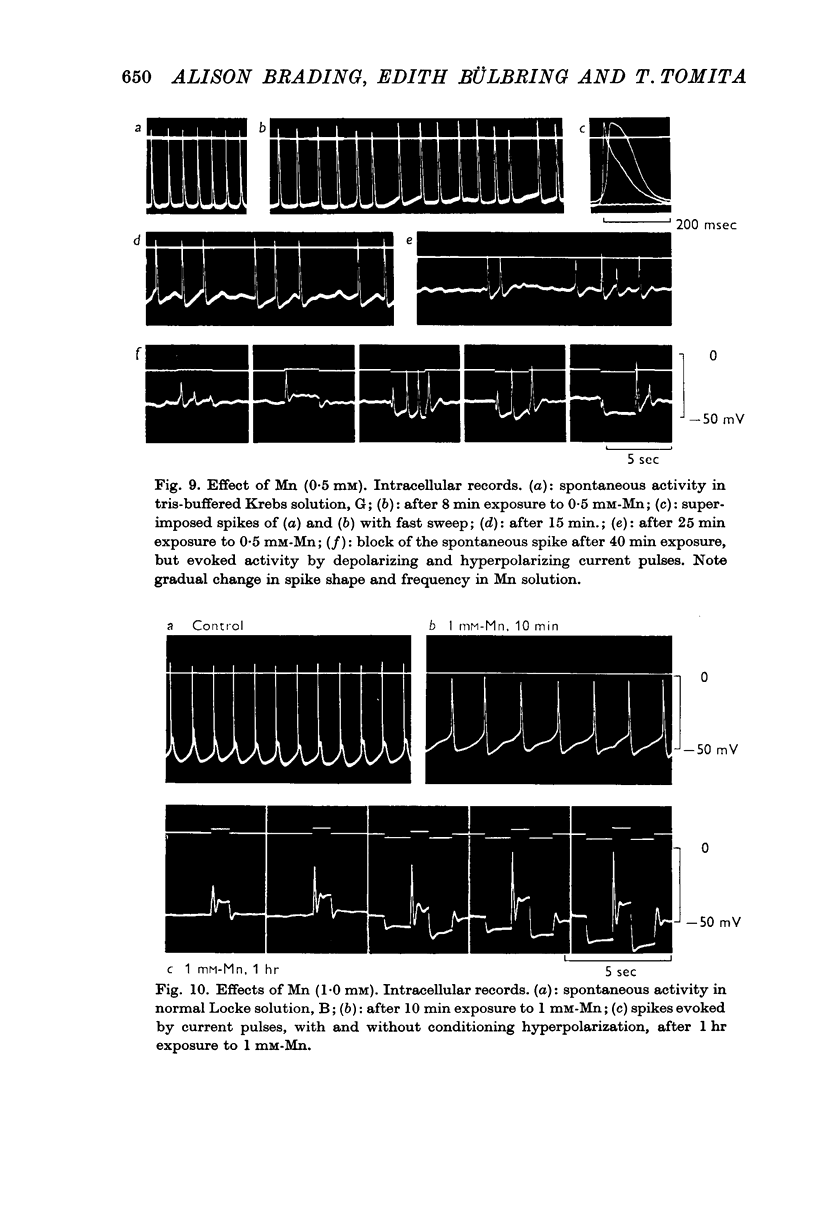
6. Mn (0·5-1·0 mM) blocked spontaneous spike discharge after 20 min. Higher concentrations (more than 2·0 mM) were required to block the evoked action potential.
7. The results indicate that the smooth muscle spike in taenia is due to Ca-entry and that Na influences spike activity indirectly by competing with Ca in controlling the membrane potential.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E., KURIYAMA H. Effects of changes in the external sodium and calcium concentrations on spontaneous electrical activity in smooth muscle of guinea-pig taenia coli. J Physiol. 1963 Apr;166:29–58. doi: 10.1113/jphysiol.1963.sp007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R. The effect of cations on the electrical properties of the smooth muscle cells of the guinea-pig vas deferens. J Physiol. 1967 Jun;190(3):465–479. doi: 10.1113/jphysiol.1967.sp008222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Effect of calcium, barium and manganese on the action of adrenaline in the smooth muscle of the guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):121–136. doi: 10.1098/rspb.1969.0015. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Increase of membrane conductance by adrenaline in the smooth muscle of guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):89–102. doi: 10.1098/rspb.1969.0013. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Properties of the inhibitory potential of smooth muscle as observed in the response to field stimulation of the guinea-pig taenia coli. J Physiol. 1967 Apr;189(2):299–315. doi: 10.1113/jphysiol.1967.sp008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Suppression of spontaneous spike generation by catecholamines in the smooth muscle of the guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):103–119. doi: 10.1098/rspb.1969.0014. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. The effect of catecholamines on the membrane resistance and spike generation in the smooth muscle of guinea-pig taenia coli. J Physiol. 1968 Feb;194(2):74–6P. [PubMed] [Google Scholar]
- CASTEELS R., KURIYAMA H. MEMBRANE POTENTIAL AND IONIC CONTENT IN PREGNANT AND NON-PREGNANT RAT MYOMETRIUM. J Physiol. 1965 Mar;177:263–287. doi: 10.1113/jphysiol.1965.sp007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell P. C. Factors governing movement and distribution of inorganic ions in nerve and muscle. Physiol Rev. 1968 Jan;48(1):1–64. doi: 10.1152/physrev.1968.48.1.1. [DOI] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., NAKA K. I. THE INITIATION OF SPIKE POTENTIAL IN BARNACLE MUSCLE FIBERS UNDER LOW INTRACELLULAR CA++. J Gen Physiol. 1964 Sep;48:141–162. doi: 10.1085/jgp.48.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMAN M. E. Membrane potentials recorded with high-resistance micro-electrodes; and the effects of changes in ionic environment on the electrical and mechanical activity of the smooth muscle of the taenia coli of the guineapig. J Physiol. 1958 May 28;141(3):464–488. doi: 10.1113/jphysiol.1958.sp005989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J Gen Physiol. 1966 Mar;49(4):793–806. doi: 10.1085/jgp.49.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y., Tsukui R. Effect on the guinea-pig taenia coli of the substitution of strontium or barium ions for calcium ions. Nature. 1968 Mar 2;217(5131):867–869. doi: 10.1038/217867b0. [DOI] [PubMed] [Google Scholar]
- KURIYAMA H. The influence of potassium, sodium and chloride on the membrane potential of the smooth muscle of taenia coli. J Physiol. 1963 Apr;166:15–28. doi: 10.1113/jphysiol.1963.sp007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Osa T., Toida N. Effect of tetrodotoxin on smooth muscle cells of the guinea-pig taenia coli. Br J Pharmacol Chemother. 1966 Aug;27(2):366–376. doi: 10.1111/j.1476-5381.1966.tb01668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUTTGAU H. C., NIEDERGERKE R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958 Oct 31;143(3):486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedergerke R., Orkand R. K. The dependence of the action potential of the frog's heart on the external and intracellular sodium concentration. J Physiol. 1966 May;184(2):312–334. doi: 10.1113/jphysiol.1966.sp007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrical responses of smooth muscle to external stimulation in hypertonic solution. J Physiol. 1966 Mar;183(2):450–468. doi: 10.1113/jphysiol.1966.sp007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Spike propagation in the smooth muscle of the guinea-pig taenia coli. J Physiol. 1967 Aug;191(3):517–527. doi: 10.1113/jphysiol.1967.sp008265. [DOI] [PMC free article] [PubMed] [Google Scholar]


