Abstract
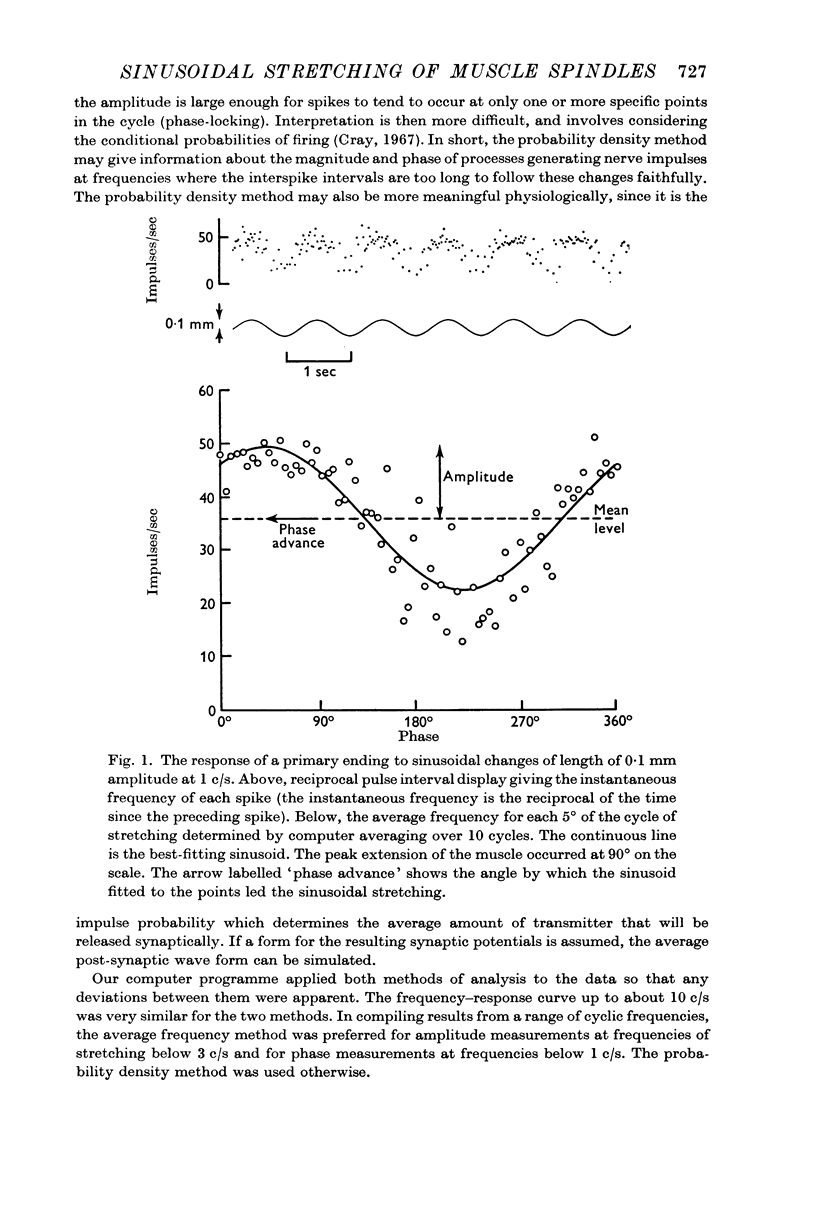
1. Nerve impulses were recorded from spindle afferents of the soleus muscle during sinusoidal changes in muscle length at frequencies from 0·03 to 300 c/s. This was done in the decerebrate cat with intact motor outflow and `spontaneous' fusimotor activity. Computer averaging over a number of cycles was used to measure the response, in impulses/sec, at different phases of the cycle.
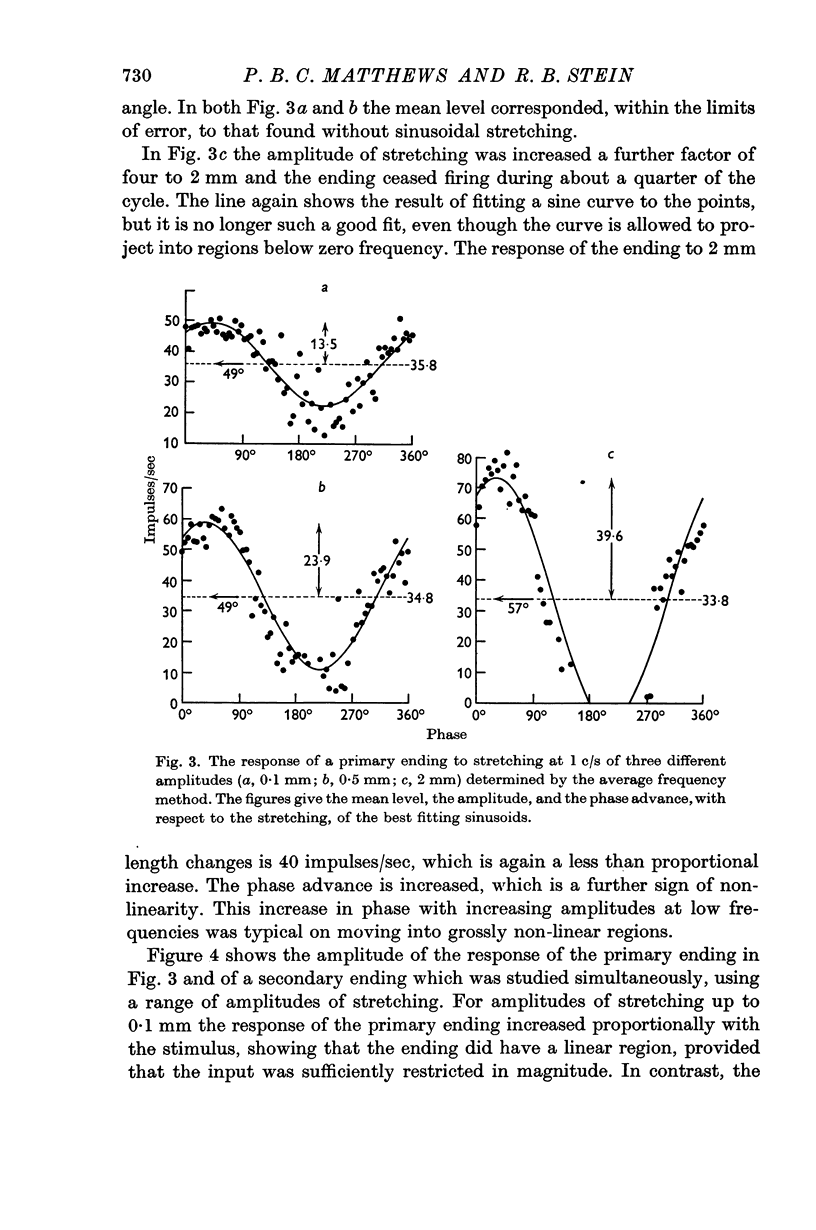
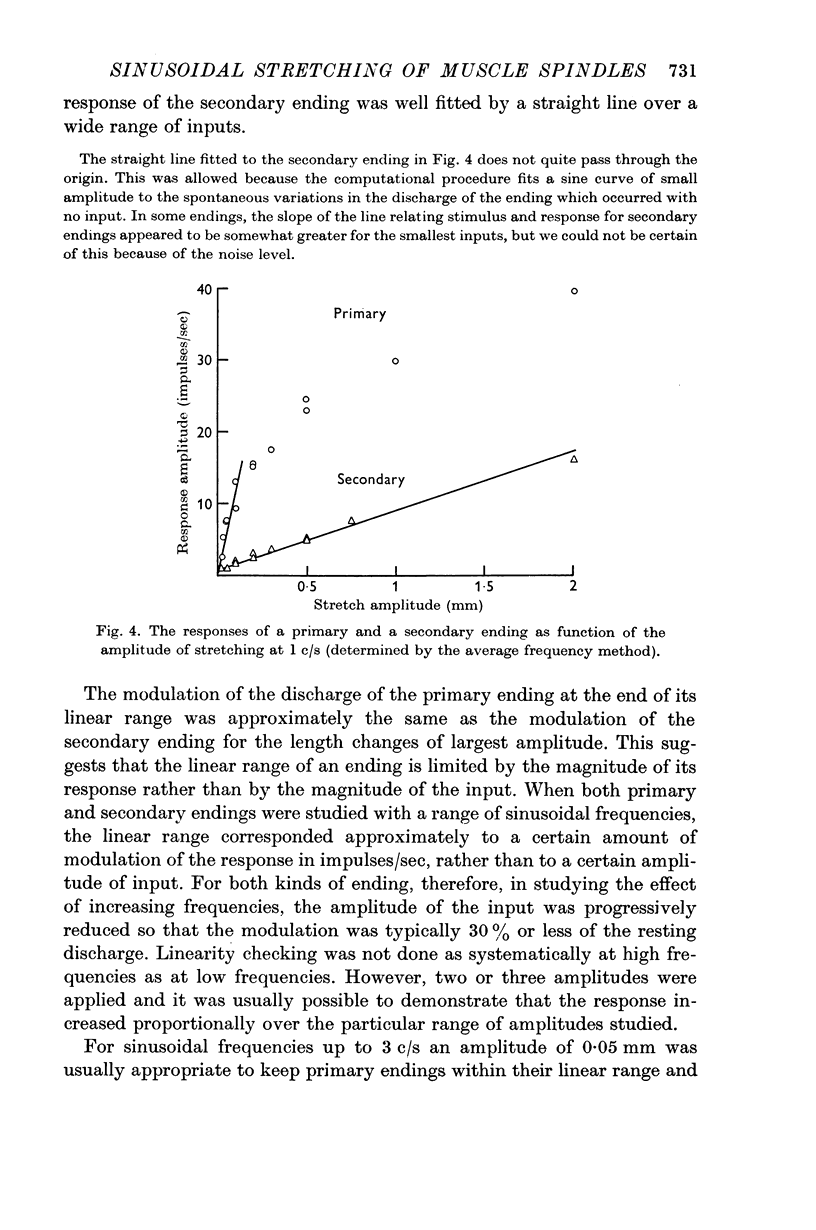
2. A linear range was described in which the discharges of the endings were approximately sinusoidally modulated, and in which increasing the amplitude of the stretching produced a proportional increase in the response. At 1 c/s the linear range extended up to only about 0·1 mm for primary endings, but was greater than 1 mm for secondary endings.
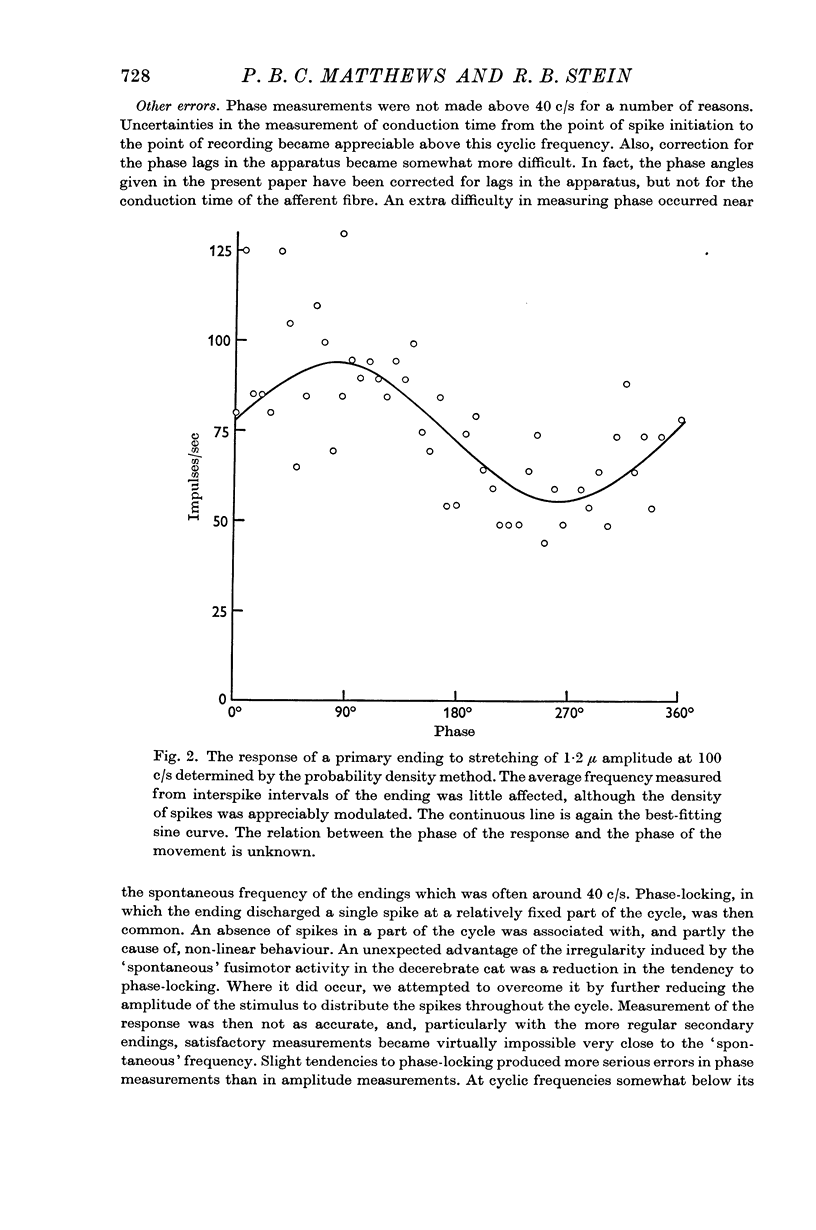
3. The sensitivity of an ending in its linear range was defined as the amplitude of its response divided by the amplitude of the length change. The sensitivity of both primary and secondary endings increased progressively on increasing the frequency of stretching above 1 c/s. The experimental observations relating the sensitivity to the sinusoidal frequency were fitted over much of the range by a curve given by the vector sum of components proportional to the length and to the velocity of stretching. This curve has two parameters, a sensitivity at low frequencies (S), and a corner frequency (F) at which the length and velocity contributions are equal. The value of F was about 1·5 c/s for both primary and secondary muscle spindle endings. The value of S was very much greater for primary endings (median value 95 impulses/sec/mm) than for secondary endings (median value 7 impulses/sec/mm). The increasing sensitivity of the endings at higher frequencies caused a progressive reduction in the linear range when it was expressed as an amplitude of stretching, but it remained approximately constant when it was expressed as a modulation of the frequency of discharge.
4. The primary endings were also extremely sensitive to maintained changes in length provided they were of sufficiently small amplitude.
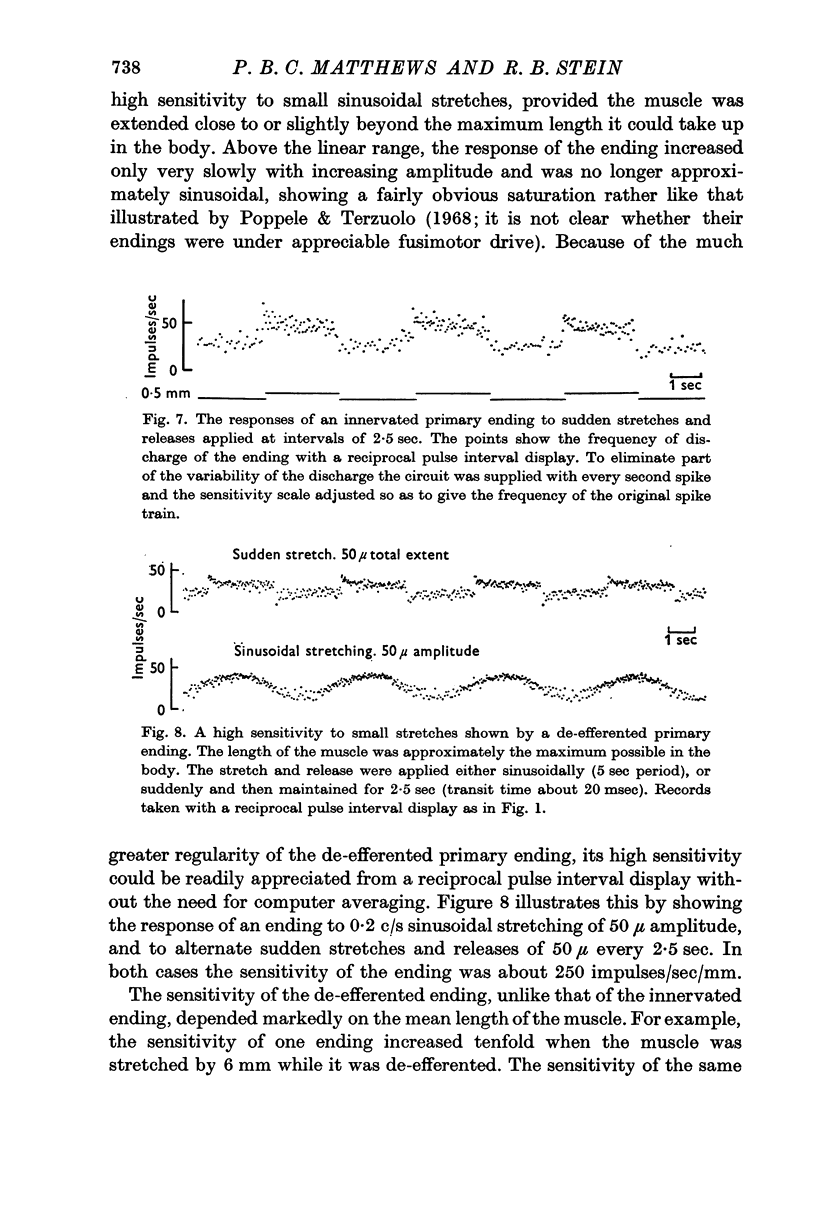
5. In the presence of fusimotor activity a high sensitivity of small changes was found over a wide range of muscle lengths. De-efferented endings had a comparable high sensitivity when the muscle was at or beyond physiological full extension, but not when the muscle was shorter.
6. The results are contrasted with those obtained previously using stretches of large amplitude, and the physiological significance of the high sensitivity of primary endings to small stretches is discussed in relation to the reflex control of movement.
Full text
PDF







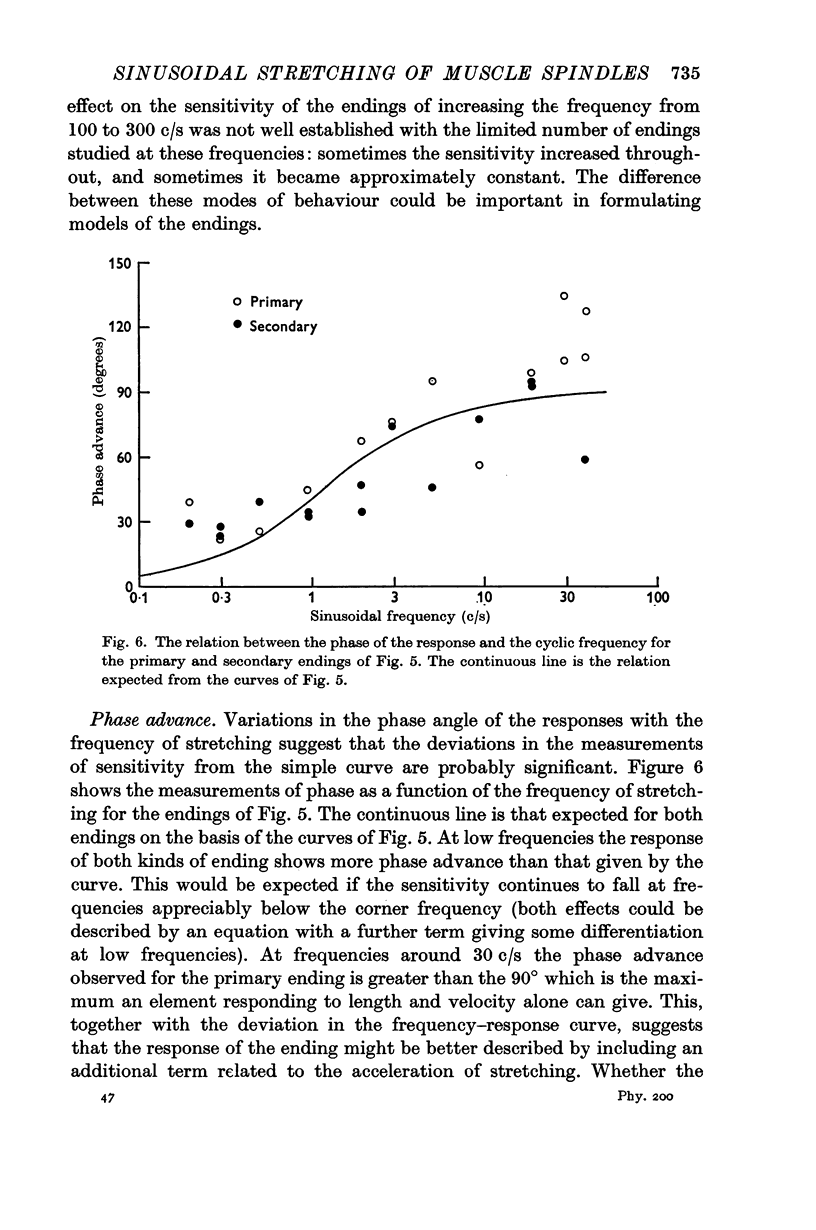







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B. F., Lennerstrand G., Thoden U. Response characteristics of muscle spindle endings at constant length to variations in fusimotor activation. Acta Physiol Scand. 1968 Nov;74(3):301–318. doi: 10.1111/j.1748-1716.1968.tb04238.x. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Engberg I., Matthews P. B. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967 Oct;192(3):773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. FURTHER STUDIES OF STATIC AND DYNAMIC FUSIMOTOR FIBRES. J Physiol. 1964 Oct;174:132–151. doi: 10.1113/jphysiol.1964.sp007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. THE EFFECTS OF STIMULATION OF STATIC AND DYNAMIC FUSIMOTOR FIBRES ON THE RESPONSE TO STRETCHING OF THE PRIMARY ENDINGS OF MUSCLE SPINDLES. J Physiol. 1964 Oct;174:109–131. doi: 10.1113/jphysiol.1964.sp007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELDRED E., GRANIT R., MERTON P. A. Supraspinal control of the muscle spindles and its significance. J Physiol. 1953 Dec 29;122(3):498–523. doi: 10.1113/jphysiol.1953.sp005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R. Neuromuscular interaction in postural tone of the cat's isometric soleus muscle. J Physiol. 1958 Oct 31;143(3):387–402. doi: 10.1113/jphysiol.1958.sp006067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. R. Conditional probability analyses of the spike activity of single neurons. Biophys J. 2008 Dec 31;7(6):759–777. doi: 10.1016/S0006-3495(67)86621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüsser O. J., Thiele B. Reaktionen primärer und sekundärer Muskelspindelafferenzen auf sinusförmige mechanische Reizung. I. Variation der Sinusfrequenz. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968 Apr 23;300(3):161–184. [PubMed] [Google Scholar]
- HAMMOND P. H., MERTON P. A., SUTTON G. G. Nervous gradation of muscular contraction. Br Med Bull. 1956 Sep;12(3):214–218. doi: 10.1093/oxfordjournals.bmb.a069553. [DOI] [PubMed] [Google Scholar]
- JANSEN J. K., MATTHEWS P. B. The effects of fusimotor activity on the static responsiveness of primary and secondary endings of muscle spindles in the decerebrate cat. Acta Physiol Scand. 1962 Aug;55:376–386. doi: 10.1111/j.1748-1716.1962.tb02451.x. [DOI] [PubMed] [Google Scholar]
- Jansen J. K., Poppele R. E., Terzuolo C. A. Transmission of proprioceptive information via the dorsal spinocerebellar tract. Brain Res. 1967 Oct;6(2):382–384. doi: 10.1016/0006-8993(67)90206-5. [DOI] [PubMed] [Google Scholar]
- Koeze T. H., Phillips C. G., Sheridan J. D. Thresholds of cortical activation of muslce spindles and alpha motoneurones of the baboon's hand. J Physiol. 1968 Mar;195(2):419–449. doi: 10.1113/jphysiol.1968.sp008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIPPOLD O. C., REDFEARN J. W., VUCO J. The effect of sinusoidal stretching upon the activity of stretch receptors in voluntary muscle and their reflex responses. J Physiol. 1958 Dec 30;144(3):373–386. doi: 10.1113/jphysiol.1958.sp006108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennerstrand G., Thoden U. Dynamic analysis of muscle spindle endings in the cat using length changes of different length-time relations. Acta Physiol Scand. 1968 May-Jun;73(1):234–250. doi: 10.1111/j.1748-1716.1968.tb04100.x. [DOI] [PubMed] [Google Scholar]
- Lennerstrand G., Thoden U. Muscle spindle responses to concomitant variations in lenght and in fusimotor activation. Acta Physiol Scand. 1968 Sep-Oct;74(1):153–165. doi: 10.1111/j.1748-1716.1968.tb04224.x. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. MUSCLE SPINDLES AND THEIR MOTOR CONTROL. Physiol Rev. 1964 Apr;44:219–288. doi: 10.1152/physrev.1964.44.2.219. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. THE RESPONSE OF DE-EFFERENTED MUSCLE SPINDLE RECEPTORS TO STRETCHING AT DIFFERENT VELOCITIES. J Physiol. 1963 Oct;168:660–678. doi: 10.1113/jphysiol.1963.sp007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. The reflex excitation of the soleus muscle of the decerebrate cat caused by vibbration applied to its tendon. J Physiol. 1966 May;184(2):450–472. doi: 10.1113/jphysiol.1966.sp007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppele R. E., Terzuolo C. A. Myotatic reflex: its input-output relation. Science. 1968 Feb 16;159(3816):743–745. doi: 10.1126/science.159.3816.743. [DOI] [PubMed] [Google Scholar]
- STUART D., OTT K., ISHIKAWA K., ELDRED E. MUSCLE RECEPTOR RESPONSES TO SINUSOIDAL STRETCH. Exp Neurol. 1965 Sep;13:82–95. doi: 10.1016/0014-4886(65)90007-5. [DOI] [PubMed] [Google Scholar]
- Schäfer S. S., Henatsch H. D. Dehnungsantworten der primären Muskelspindel-Afferenz bei elektrischer Reizung und natürlicher Innervation der beiden fusimotorischen Fasertypen. Exp Brain Res. 1968;4(4):275–291. doi: 10.1007/BF00235696. [DOI] [PubMed] [Google Scholar]
- Talbot W. H., Darian-Smith I., Kornhuber H. H., Mountcastle V. B. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]


