Abstract
1. Isometric force was measured in skinned segments of frog semitendinosus muscle fibres exposed to solutions in which the calcium ion concentration was controlled with EGTA.
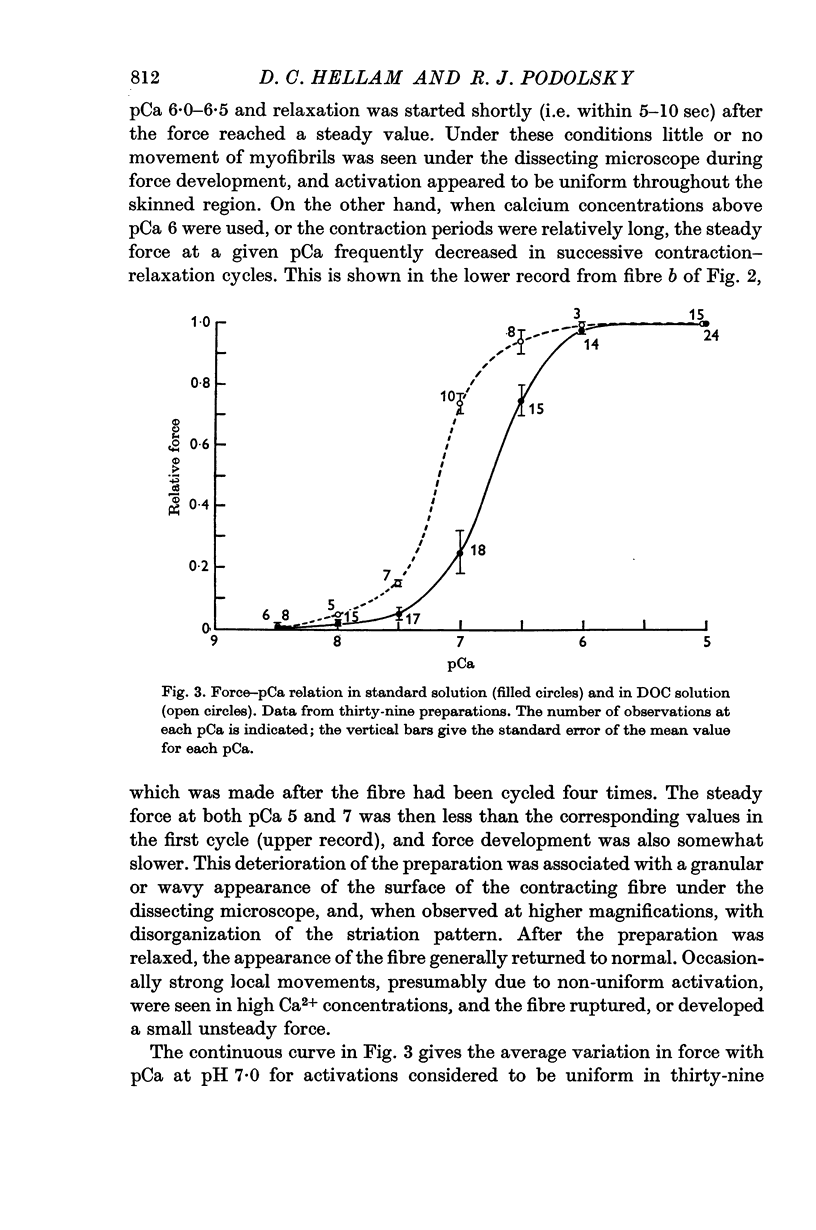
2. The threshold for force development, calculated from an apparent stability constant for the CaEGTA complex of 106.69 M-1 at pH 7·0, was generally close to pCa 7·5. Maximum force was reached at about pCa 6·0.
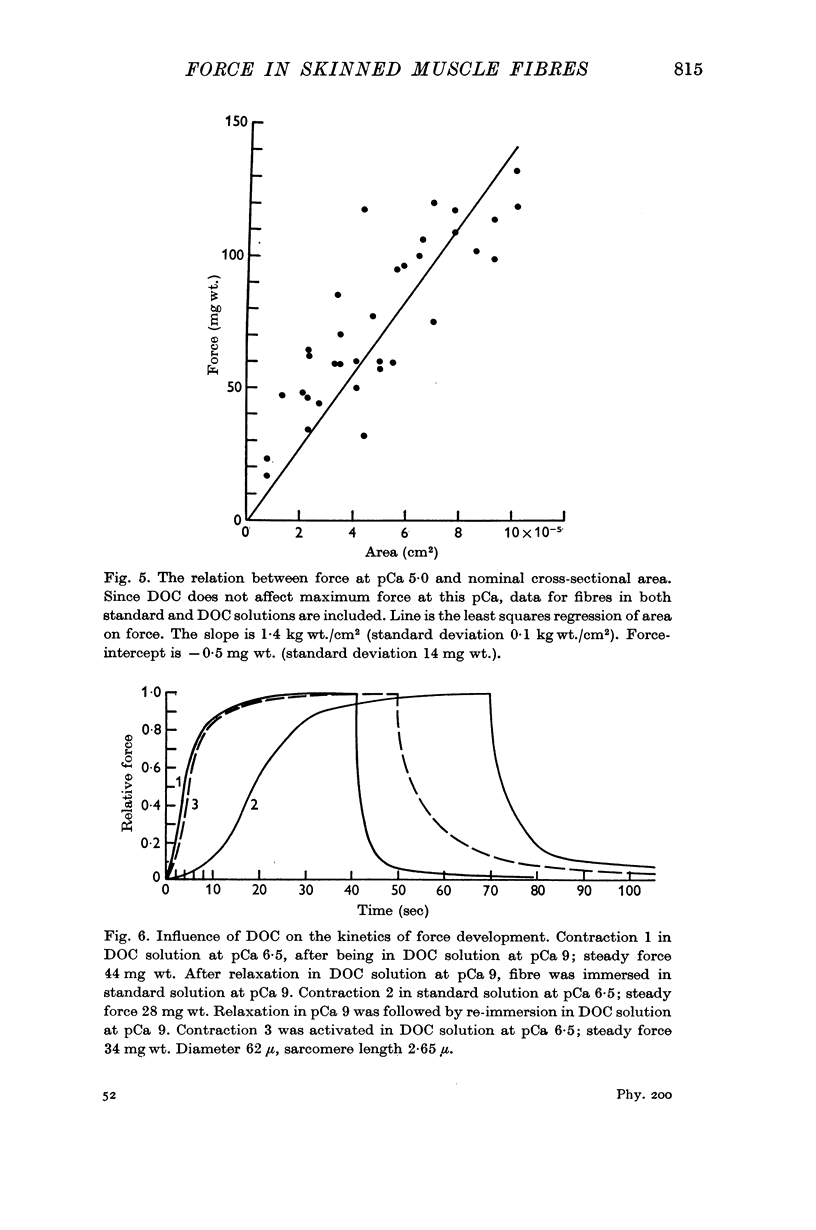
3. Maximum force is proportional to the cross-sectional area of the fibres.
4. The rate of force development was slower than that expected from simple diffusion of a substance from the bathing solution into the fibre. The delay appears to be due to slow equilibration of the EGTA buffer system during calcium uptake by the sarcoplasmic reticulum.
5. Addition of deoxycholate (DOC) to the bathing solution produced a reversible increase in the rate of force development. The steady force was also increased for values of pCa that gave less than maximum force, which shifted the force—pCa relation toward lower calcium concentrations by about 0·5 pCa unit.
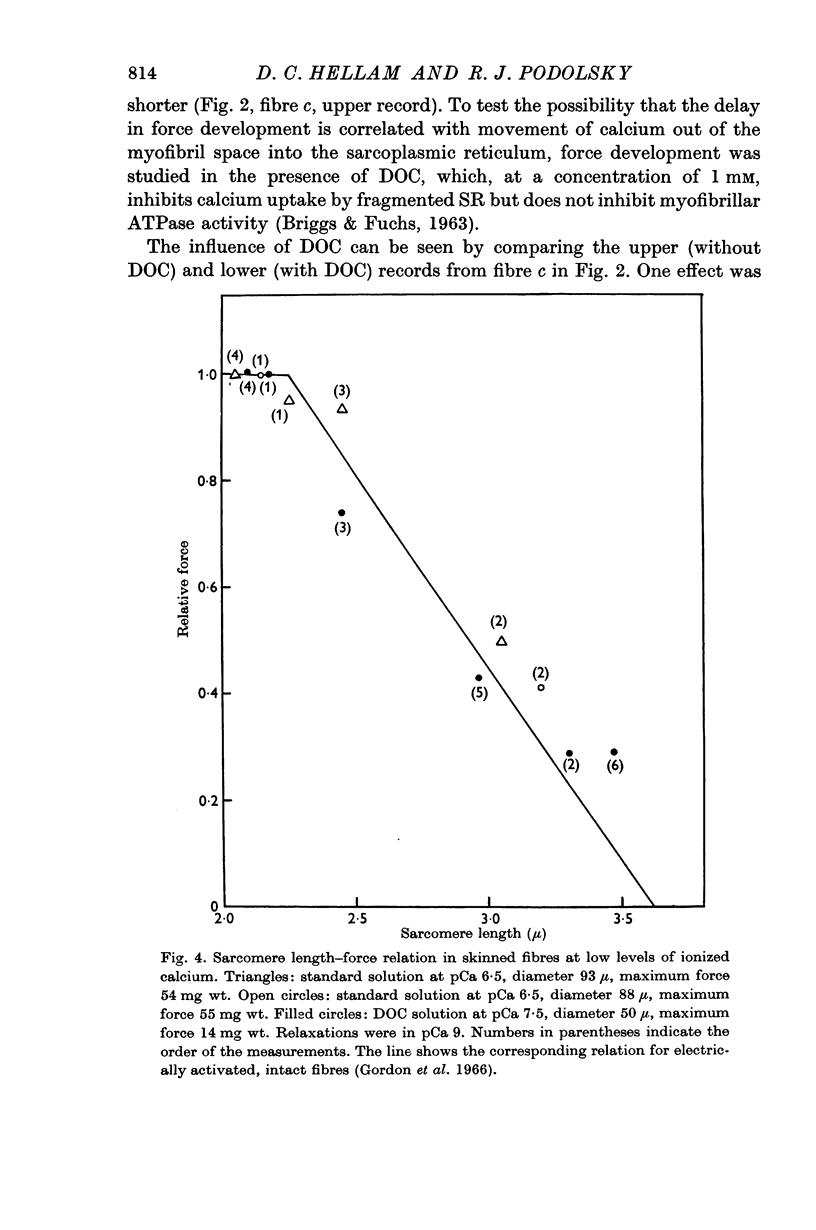
6. The length—force relation in partially activated preparations is similar to that reported for electrically activated intact fibres. This result suggests that in the region of myofilament overlap the affinity of the binding sites for calcium is uniform along the length of the calciumbinding myofilament.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIGGS F. N., FUCHS F. The nature of the muscle-relaxing factor. I. An improved assay system. J Gen Physiol. 1963 May;46:883–891. doi: 10.1085/jgp.46.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSTANTIN L. L., FRANZINI-ARMSTRONG C., PODOLSKY R. J. LOCALIZATION OF CALCIUM-ACCUMULATING STRUCTURES IN STRIATED MUSCLE FIBERS. Science. 1965 Jan 8;147(3654):158–160. doi: 10.1126/science.147.3654.158. [DOI] [PubMed] [Google Scholar]
- Costantin L. L., Podolsky R. J. Depolarization of the internal membrane system in the activation of frog skeletal muscle. J Gen Physiol. 1967 May;50(5):1101–1124. doi: 10.1085/jgp.50.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Lipmann F. ADENOSINE TRIPHOSPHATE-LINKED CONCENTRATION OF CALCIUM IONS IN A PARTICULATE FRACTION OF RABBIT MUSCLE. J Cell Biol. 1962 Sep 1;14(3):389–400. doi: 10.1083/jcb.14.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILO R. S., BOHR D. F., RUEGG J. C. GLYCERINATED SKELETAL AND SMOOTH MUSCLE: CALCIUM AND MAGNESIUM DEPENDENCE. Science. 1965 Mar 26;147(3665):1581–1583. doi: 10.1126/science.147.3665.1581. [DOI] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PODOLSKY R. J., COSTANTIN L. L. REGULATION BY CALCIUM OF THE CONTRACTION AND RELAXATION OF MUSCLE FIBERS. Fed Proc. 1964 Sep-Oct;23:933–939. [PubMed] [Google Scholar]
- WEBER A., HERZ R., REISS I. On the mechanism of the relaxing effect of fragmented sarcoplasmic reticulum. J Gen Physiol. 1963 Mar;46:679–702. doi: 10.1085/jgp.46.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER A., HERZ R. The binding of calcium to actomyosin systems in relation to their biological activity. J Biol Chem. 1963 Feb;238:599–605. [PubMed] [Google Scholar]


