Abstract
The Treponema denticola cheA gene, encoding the central kinase of the general chemotaxis pathway, was analyzed for its role in chemotaxis and tissue penetration. The cheA gene was interrupted by insertion of an ermF-ermAM gene cassette. Reverse transcription-PCR confirmed that the other downstream chemotaxis genes within the same operon (cheW, cheX, and cheY) were still expressed in the cheA mutant strain. Lack of cheA resulted in decreased swarming on soft-agar swarm plates and failure to respond chemotactically to a mixture of nutrients. Behavioral analyses using video microscopy revealed that the cheA mutant exhibited coordinated cell movement. The cellular reversal frequency, however, was severely reduced, indicating that CheA in T. denticola mainly controls cellular reversal and that active chemotaxis signaling input is not required for coordination of flagellar rotation at both cell poles.
Chemotaxis is the ability of motile organisms to navigate through their environment in response to gradients of chemicals. This feature allows bacteria to migrate towards favorable environments and avoid harmful situations and has also been implicated as an important virulence factor for a variety of pathogens (8, 10-12, 24, 30, 42). Previous findings suggest the requirement of a functional chemotaxis pathway for tissue penetration by T. denticola (36).
The biochemistry and genetics of bacterial chemotaxis have been well characterized in two closely related enteric bacteria, Escherichia coli and Salmonella enterica serovar Typhimurium (1, 38). Chemotactic stimuli are detected by the membrane-spanning (or soluble) methyl-accepting chemotaxis proteins (MCPs) and subsequently transformed into an appropriate motor response by the two-component system CheA/CheY. The histidine kinase CheA forms a membrane-associated complex with the MCPs and CheW and undergoes ATP-dependent autophosphorylation in compliance with the stimulus bound to the respective MCP. The nature of the stimulus is then communicated to the motor via phosphotransfer reactions from CheA to the response regulator CheY, which controls the direction of flagellar motor rotation according to its phosphorylation level. Repellents increase autophosphorylation of CheA, whereas attractants decrease this activity.
Chemotaxis has been demonstrated in various spirochetes, and a number of chemotactic stimuli have been identified. Borrelia burgdorferi recognizes serum as an attractant and H2O2, nonphysiological pH (>8.5 or <6.8), KCl, CaCl2, and small alcohols as repellents (47). Brachyspira hyodysenteriae responds to mucin, fucose, galactose, lactose, serine, cysteine, and blood (29, 39), while Leptospira interrogans responds to hemoglobin (25, 51). Spirochaeta aurantia performs chemotaxis towards glucose, xylose, and various other carbohydrates (21), whereas for Treponema denticola glucose, rabbit serum, albumin, and growth medium are all attractants (32, 48).
Homologues of many of the known chemotaxis genes are present in spirochetes (22, 28, 33; www.tigr.org/tdb/mdb/mdbinprogress.html). Therefore, the general strategy of transforming an environmental stimulus into a motor response is presumably very similar to that in other bacteria (37). However, there are some very distinctive features of the motility and chemotaxis of spirochetes that do not occur in other bacteria (31). The flagella are inserted in a subpolar location near both ends of the bacterium and rotate within the periplasm. Models that have been developed for translation of Leptospira illini and B. burgdorferi suggest that the periplasmic flagella rotate counterclockwise at the anterior end and in the opposite direction at the posterior end of the cell, promoting clockwise movement of the cell body (3, 4, 14-16, 31). If both motors spin in the same direction, the movement becomes noncoordinated and results in a jerky movement called flexing (21). However, little is known about how the direction of flagellar rotation at both ends is coordinated and modulated by the chemotaxis proteins.
The cell body of spirochetes is particularly long: the cell poles are about 10 to 15 μm apart in T. denticola and even further in the larger spirochetes, such as some members of the genera Cristispira (30 to 180 μm) and Spirochaeta (5 to 250 μm) (26). Therefore, the typical strategy of signal transduction via simple diffusion to the motors is calculated to be too slow to achieve the observed transmission of chemosensory information between the motors located at the distant poles of the cell. A limited signaling range of only a few micrometers for diffusible internal signals, such as phosphorylated CheY, has been demonstrated in filamentous E. coli (27, 46). Consequently, the change in membrane potential that has been observed in S. aurantia upon addition of chemotactic stimuli has been suggested to be involved in chemotactic signal transduction of spirochetes (17-20). These unusual features of spirochete motility and chemotaxis raise the interesting question of how chemotactic signal processing and coordination between the flagellar motors occur.
In this study we constructed a cheA gene inactivation mutant of T. denticola ATCC 35405 to address some of the above questions by interrupting the general chemotaxis pathway and its interaction with the flagellar motor. This mutant allows examination of the unstimulated direction of motor rotation and supports the idea that cheA is essential for chemotaxis in T. denticola as in other bacterial species (1).
Construction of a cheA mutant of T. denticola.
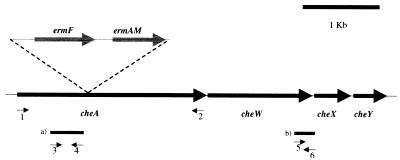
CheA is the central kinase of the general chemotaxis pathway, and it has been shown for other bacteria that its inactivation abolishes all chemotaxis responses (7, 8, 13, 23, 44, 45, 50). Some bacterial species, including the pathogenic spirochete B. burgdorferi, contain multiple copies of the general chemotaxis genes, such as cheA (9). Genome and Southern blot analysis of the T. denticola chromosome revealed the presence of only one copy of cheA in this oral spirochete (22, 32; www.tigr.org/tdb/mdb/mdbinprogress.html). In T. denticola, cheA is organized in an operon with the other general chemotaxis genes, cheW and cheY, as well as with cheX, a putative spirochete chemotaxis gene of unknown function (Fig. 1). In this study, a cheA mutant was constructed by disrupting the gene through introduction of an ermF-ermAM gene cassette that confers resistance to erythromycin. This approach has previously been used to successfully create a variety of gene inactivation mutants of T. denticola (28, 33-35).
FIG. 1.
Schematic of the general chemotaxis operon of T. denticola containing cheA, cheW, cheX, and cheY. The insertion site of the ermF-ermAM gene cassette is shown as well as the primer pairs that were used for amplification of the entire cheA gene (1, bamf; 2, bamr) and for RT-PCR (3, BamCheAF; 4, LSCheA; 5, BamHIF; 6, X-BglR). The resulting RT-PCR products upstream (350 bp) (a) and downstream (260 bp) (b) of the ermF-ermAM insertion site are indicated.
Cultures of T. denticola ATCC 35405 (5) were grown anaerobically (85% N2, 10% H2, 5% CO2) at 35°C in TYGVS broth (41), and chromosomal DNA was isolated as described previously (40). This chromosomal DNA served as a template for amplification of T. denticola cheA using the following primers, which were designed according to published sequences (22; www.tigr.org/tdb/mdb/mdbinprogress.html) and introduce a BamHI recognition site (underlined) at either end of the gene: 5′ CGGGATCCATGAGTGATTATCTTGATATC 3′ (bamf) and 5′ CGGGATCCCTACCAAATTGAAGCCTCG 3′ (bamr) (Fig. 1). The resulting PCR product was digested with BamHI and cloned into the BamHI site that is present in the multiple cloning site of the pUC18 vector (49) to yield pJT9. A fragment encoding ermF-ermAM including the respective promoter regions was equally PCR amplified from plasmid pKMR4PE (6) using the following primer pair, which introduces NheI recognition sites (underlined) at both ends of the fragment: 5′ CTAGCTAGCCCGATAGCTTCCGCTATT 3′ and 5′ CTAGCTAGCGAAGCTGTCAGTAGTATAC 3′. This fragment was NheI digested and cloned into a naturally occurring NheI site located at position 885 of the approximately 2.4-kb cheA. A plasmid with insertion of ermF-ermAM in the direction of cheA expression was chosen and called pJT10. pJT10 was linearized with AatII, an enzyme that cuts only once in the pUC18 backbone. Different amounts (1, 2, and 5 μg) of the linearized plasmid were electroporated into T. denticola ATCC 35405 as described previously (34) with the modifications developed by Limberger and coworkers (35). The gene inactivation mutants were selected on TYGVS plates (0.5% agar) containing erythromycin (25 μg/ml). About 1,000 Ermr colonies/μg of DNA were obtained. Similar high yields for gene inactivation mutants were observed by other investigators (35). These results, however, vary from experiment to experiment (J. Izard, personal communication). Ten Ermr colonies were chosen, and insertion of the Ermr cassette into cheA was confirmed by colony PCR. Two of these mutants were selected for further studies.
Confirmation of the insertion of the ermF-ermAM cassette into cheA.
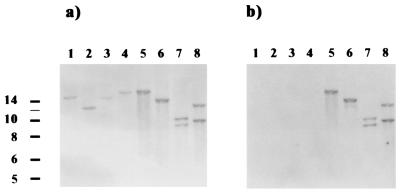
We used Southern blotting to verify the correct insertion of ermF-ermAM into the cheA gene. Chromosomal DNA of ATCC 35405 and the two putative cheA mutants was isolated, digested, and separated on a 1% agarose gel. The DNA was transferred onto a nylon membrane (Bio-Rad) and hybridized with DNA probes (described below) for Southern blot analysis. The respective signals were detected by using the DNA labeling and detection kit from Boehringer (Mannheim, Germany). The Southern blot results confirmed the correct integration of the Ermr cassette into the mutant strains. The blot was first hybridized with a 2.4-kb cheA-specific probe that reacted with specific fragments of the wild-type DNA and with different-size fragments in the mutant strains as expected (Fig. 2a). Since the mutant strains yielded identical results, only one is shown. The fragments generated by EagI or SacII digestion in the mutant run higher than those in the wild type, as these enzymes do not cut within the ermF-ermAM genes. SacI and BstXI both have recognition sites within these genes, resulting in two fragments for the mutant strain. The probe was then stripped off the blot according to the instructions for the DNA labeling and detection kit and rehybridized with an ermF-ermAM-specific probe (Fig. 2b). This probe did not bind to the wild-type DNA and reacted in the mutant strains with the same fragments that were detected earlier with the cheA probe. One of the cheA mutant strains was named RL101 and used throughout this study for further analysis.
FIG. 2.
Southern blot analysis of cheA gene inactivation mutants. Lanes: 1 to 4, wild-type strain ATCC 35405; 5 to 8, Ermr cheA gene inactivation mutant; 1 and 5, EagI-digested DNA; 2 and 6, SacII-digested DNA; 3 and 7, SacI-digested DNA; 4 and 8, BstXI-digested DNA. The probes were a 2.4-kb fragment containing the entire cheA gene, derived by BamHI digestion of pJT9 (a), and a 2.1-kb NheI fragment containing ermF-ermAM from pJT10 (b). Molecular sizes (kilobases) are on the left.
Expression of downstream genes in the cheA gene inactivation mutant.
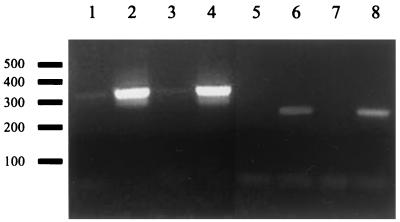
As shown in Fig. 1, cheA is the first gene in an operon that encodes other central functions of the chemotaxis pathway. Accordingly, there was concern regarding possible polar effects due to ermF-ermAM insertion. It has been reported that insertion of the ermF-ermAM gene cassette does not have significant polar effects on the expression of downstream genes in an operon (35). To examine if this was also the case in the cheA mutant strain, transcription of the genes located downstream of cheA was analyzed by reverse transcription-PCR (RT-PCR) (Fig. 3). RNA was extracted by using the RNeasy kit (Qiagen) according to the manufacturer's instructions and treated with RNase-free DNase for 1 h at 37°C. About 1 μg of RNA was incubated with reverse transcriptase and random primers to generate cDNA of wild-type T. denticola ATCC 35405 and the mutant RL101. Similar quantities of these cDNAs and the respective control reaction mixtures without addition of reverse transcriptase were used as templates for PCRs with primer pairs that specifically amplify regions upstream and downstream of the ermF-ermAM insertion site (Fig. 1). The primer pair BamCheAF (5′ TCCCGAGGATCGTAACTCCAT 3′) and LSCheAR (5′ GGAGAAACTGATGCAGGAATA 3′) is complementary to bp 111 to 460 of cheA and amplifies a 350-bp fragment upstream of the ermF-ermAM insertion site. Another primer pair, BamHIF (5′ TTGAGTACCATGATTGTACT 3′) and X-BglR (5′ TTATTTCTCCTTATCCTTTTA 3′), was used to examine relative transcription levels downstream of the resistance cassette insertion site. This primer pair amplifies a 256-bp fragment located at bp 1102 of cheW and bp 1 of cheX. No significant difference between the wild type and the mutant strain was apparent (Fig. 3), indicating that significant expression of downstream genes still occurs in the cheA mutant strain. Therefore, chemotaxis and motility phenotypes of RL101 should be a result of lack of cheA only.
FIG. 3.
RT-PCR analysis of RL101. Lanes 1 to 4, amplification of a ∼350-bp fragment upstream of the Ermr insertion site using primer pair BamCheAF and JSCheA; lanes 5 to 8, amplification of an ∼260-bp fragment downstream of the Ermr insertion site using primer pair BamHIF and X-BglR. Lanes 1, 2, 5, and 6, wild-type samples; lanes 3, 4, 7, and 8, cheA mutant RNA. Samples in lanes 2, 4, 6, and 8 were treated with reverse transcriptase, whereas samples in lanes 1, 3, 5, and 7 were not.
The cheA mutant is defective in chemotaxis.
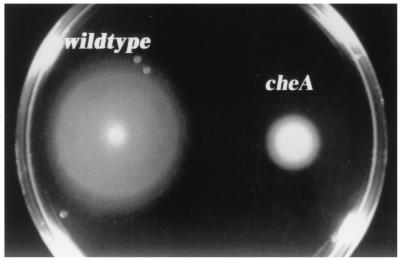
Swarming on soft agar is a useful assay for examining chemotaxis, since chemotaxis mutants exhibit a poor swarming phenotype (2). To compare the swarming behavior of RL101 to that of its parent, ATCC 35045, about 5 × 108 exponentially growing motile cells were harvested by low-speed centrifugation (1,000 × g) for 6 min and resuspended in 20 μl of TYGVS. This bacterial suspension (2 μl; about 5 × 107 bacteria) was placed in a TYGVS swarm plate (0.4% agarose). The plates were incubated anaerobically at 35°C for 3 days, and swarming diameters of 20 swarms for each strain were measured. Wild-type swarms were about three times larger than the ones produced by the mutant strain (Fig. 4), even though the mutant and its wild-type parent contained the same number of motile cells (>95%) as determined by dark-field microscopy (magnification, ×400; Nikon) (data not shown). To ensure that growth impairment of the mutant strain was not the reason for this difference in swarming, the amount of cells that had been placed on the swarm plates was inoculated into 5 ml of TYGVS medium. Growth curves of wild-type and mutant strains were practically identical (data not shown).
FIG. 4.
Wild-type and cheA mutant behavior on TYGVS swarm plates containing 0.4% agarose. Shown are typical swarms formed by an inoculum of about 5 × 107 motile bacteria after 3 days of anaerobic incubation at 35°C.
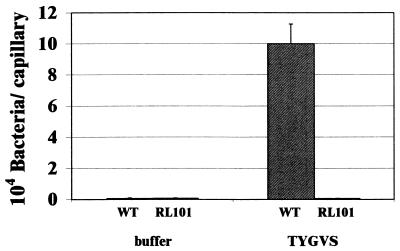
The chemotactic properties of the cheA mutant strain were further analyzed in capillary assays. Capillary assays were performed as described previously for B. burgdorferi (47) but with prereduced reagents under anaerobic conditions. Capillaries were filled with the growth medium TYGVS as an attractant or with chemotaxis buffer as a negative control and prereduced by anaerobic incubation for at least 24 h prior to the experiment. As shown in Fig. 5, the wild type exhibited a strong response to the growth medium whereas the cheA mutant was unable to accumulate in the attractant-filled capillaries in numbers exceeding the negative control.
FIG. 5.
Capillary assay of wild-type ATCC 35405 and cheA mutant strain RL101. Chemotaxis responses towards the spirochete growth medium TYGVS are shown. Capillaries filled with chemotaxis buffer were used as a negative control. Data are averages from two independent experiments that were performed with triplicate capillaries for the different solutions tested.
Video microscopy was used to observe the motility bias of the wild-type and cheA mutant T. denticola strains. Motile cells were grown to mid-log phase in TYGVS, and 2 to 3 μl of this culture was placed on a slide and covered with an 18- by 18-mm cover slide. The cover slide was sealed with petroleum jelly to prevent dehydration. Without addition of viscous substances such as methylcellulose or gelatin, rotational movement in the growth medium alone results in very little or no translational displacement. Under these conditions the bacteria rotate “on the spot,” facilitating long-term observation of bacteria that exhibit a motility bias that greatly favors one direction of movement and consequently rapidly leave the field of view. The bacteria were observed at room temperature through a 60× objective lens using phase-contrast microscopy (Nikon) and recorded with a video camera (HyperHAD/CCD-IRIS/RGB; Sony). The videotapes were analyzed by slow-motion replay. Five independent wild-type and cheA mutant cultures were each observed over a 5-min period, and about 50 individual bacteria were examined. Wild-type cells reversed direction of rotation every 1 to 9 s, with an average of 3.6 ± 1.6 s. In contrast, the cheA mutants failed to change direction of rotation during the 5-min observation period. In B. burgdorferi, inactivation of its similarly organized cheA2 gene also results in a nonreversing phenotype (N. Charon, personal communication).
In summary, we constructed and characterized a cheA mutant strain of T. denticola. Unlike previous T. denticola chemotaxis mutants, such as dmcA and dmcB mutants (28, 33), the cheA mutant is totally defective in chemotaxis. The only copy of cheA in T. denticola was inactivated in this mutant by insertion of an Ermr gene. Expression of the downstream genes cheW, cheX, and cheY was confirmed to occur in the mutant. This facilitates examination of the cheA phenotype specifically, rather than a general deficiency in the chemotaxis operon. Consistent with genome data, there is only one chemotaxis system in T. denticola. Inactivation of the cheA gene abolishes all chemotaxis responses tested. The cheA mutant strain RL101 was unable to exhibit a measurable chemotactic response in capillary assays and showed a diminished swarm size on swarm plates, a phenotype that is found in cheA mutants of other bacteria, such as the spirochete B. burgdorferi (Charon, personal communication). Lack of CheA in flagellated bacteria causes the motor to turn exclusively in one direction, resulting in a constantly running phenotype in most species (1, 43) or a tumbly phenotype, as described for Bacillus subtilis (13). In spirochetes the situation is more complex, as current models for motility suggest that the motors at both ends have to rotate in opposite directions to promote translational movement of the cell. Interestingly, the T. denticola cheA mutant constructed in this study and also the cheA2 mutant of B. burgdorferi exhibit a constantly running phenotype, implying that the default state of the motor at both ends may be rotation in opposite directions and that no active input from the chemotaxis proteins is required for coordination. CheA appears to be involved only in regulating the cellular reversal frequency, so either coordination of motor rotation at either cell end is physical or there is a different system that regulates coordination of flagellar rotation.
With genome information available and the recent development of genetic tools such as gene inactivation, we are now in a position to further our understanding of the special features of spirochete chemotaxis and their implication in virulence.
Acknowledgments
We thank Howard Kuramitsu, Nyles Charon, and James Miller for their support of our work.
This work was partially supported by NIH grants DE12532 and GM54666 to W.S.
REFERENCES
- 1.Armitage, J. P. 1999. Bacterial tactic responses. Adv. Microb. Physiol. 41:229-289. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, J. B., J. Adler, and M. M. Dahl. 1967. Nonchemotactic mutants of Escherichia coli. J. Bacteriol. 93:390-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg, H. C. 1976. How spirochetes may swim. J. Theor. Biol. 56:269-273. [DOI] [PubMed] [Google Scholar]
- 4.Charon, N. W., G. R. Daughtry, R. S. McCuskey, and G. N. Franz. 1984. Microcinematographic analysis of tethered Leptospira illini. J. Bacteriol. 160:1067-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng, S. L., R. Siboo, T. C. Quee, J. L. Johnson, W. R. Mayberry, and E. C. Chan. 1985. Comparative study of six random oral spirochete isolates. Serological heterogeneity of Treponema denticola. J. Periodontal Res. 20:602-612. [DOI] [PubMed] [Google Scholar]
- 6.Chi, B., S. Chauhan, and H. Kuramitsu. 1999. Development of a system for expressing heterologous genes in the oral spirochete Treponema denticola and its use in expression of the Treponema pallidum flaA gene. Infect. Immun. 67:3653-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flanary, P. L., R. D. Allen, L. Dons, and S. Kathariou. 1999. Insertional inactivation of the Listeria monocytogenes cheYA operon abolishes response to oxygen gradients and reduces the number of flagella. Can. J. Microbiol. 45:646-652. [PubMed] [Google Scholar]
- 8.Foynes, S., N. Dorrell, S. J. Ward, R. A. Stabler, A. A. McColm, A. N. Rycroft, and B. W. Wren. 2000. Helicobacter pylori possesses two CheY response regulators and a histidine kinase sensor, CheA, which are essential for chemotaxis and colonization of the gastric mucosa. Infect. Immun. 68:2016-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. C. Venter, et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 10.Freter, R., and P. C. O'Brien. 1981. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: fitness and virulence of nonchemotactic Vibrio cholerae mutants in infant mice. Infect. Immun. 34:222-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freter, R., P. C. O'Brien, and M. S. Macsai. 1979. Effect of chemotaxis on the interaction of cholera vibrios with intestinal mucosa. Am. J Clin. Nutr. 32:128-132. [DOI] [PubMed] [Google Scholar]
- 12.Freter, R., P. C. O'Brien, and M. S. Macsai. 1981. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect. Immun. 34:234-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuhrer, D. K., and G. W. Ordal. 1991. Bacillus subtilis CheN, a homolog of CheA, the central regulator of chemotaxis in Escherichia coli. J. Bacteriol. 173:7443-7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein, S. F., K. F. Buttle, and N. W. Charon. 1996. Structural analysis of the Leptospiraceae and Borrelia burgdorferi by high-voltage electron microscopy. J. Bacteriol. 178:6539-6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein, S. F., and N. W. Charon. 1988. Motility of the spirochete Leptospira. Cell Motil. Cytoskelet. 9:101-110. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein, S. F., N. W. Charon, and J. A. Kreiling. 1994. Borrelia burgdorferi swims with a planar waveform similar to that of eukaryotic flagella. Proc. Natl. Acad. Sci. USA 91:3433-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goulbourne, E. A., Jr., and E. P. Greenberg. 1981. Chemotaxis of Spirochaeta aurantia: involvement of membrane potential in chemosensory signal transduction. J. Bacteriol. 148:837-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goulbourne, E. A., Jr., and E. P. Greenberg. 1983. Inhibition of Spirochaeta aurantia chemotaxis by neurotoxins. J. Bacteriol. 155:1443-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goulbourne, E. A., Jr., and E. P. Greenberg. 1980. Relationship between proton motive force and motility in Spirochaeta aurantia. J. Bacteriol. 143:1450-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goulbourne, E. A., Jr., and E. P. Greenberg. 1983. A voltage clamp inhibits chemotaxis of Spirochaeta aurantia. J. Bacteriol. 153:916-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg, E. P., and E. Canale-Parola. 1977. Chemotaxis in Spirochaeta aurantia. J. Bacteriol. 130:485-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greene, S. R., and L. V. Stamm. 1999. Molecular characterization of a chemotaxis operon in the oral spirochete, Treponema denticola. Gene 232:59-68. [DOI] [PubMed] [Google Scholar]
- 23.Hamblin, P. A., B. A. Maguire, R. N. Grishanin, and J. P. Armitage. 1997. Evidence for two chemosensory pathways in Rhodobacter sphaeroides. Mol. Microbiol. 26:1083-1096. [DOI] [PubMed] [Google Scholar]
- 24.Hawes, M. C., and L. Y. Smith. 1989. Requirement for chemotaxis in pathogenicity of Agrobacterium tumefaciens on roots of soil-grown pea plants. J. Bacteriol. 171:5668-5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiramune, T., C. Shiraiwa, N. Kikuchi, and R. Yanagawa. 1990. A basic study of chemotaxis of leptospiras. Zentralbl. Vetmed. B 37:749-752. [DOI] [PubMed] [Google Scholar]
- 26.Holt, J. G. (ed.). 1994. Bergey's manual of determinative bacteriology, 9th ed. Williams & Wilkins, Baltimore, Md.
- 27.Ishihara, A., J. E. Segall, S. M. Block, and H. C. Berg. 1983. Coordination of flagella on filamentous cells of Escherichia coli. J. Bacteriol. 155:228-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kataoka, M., H. Li, S. Arakawa, and H. Kuramitsu. 1997. Characterization of a methyl-accepting chemotaxis protein gene, dmcA, from the oral spirochete Treponema denticola. Infect. Immun. 65:4011-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy, M. J., and R. J. Yancey, Jr. 1996. Motility and chemotaxis in Serpulina hyodysenteriae. Vet. Microbiol. 49:21-30. [DOI] [PubMed] [Google Scholar]
- 30.Lee, S. H., S. M. Butler, and A. Camilli. 2001. Selection for in vivo regulators of bacterial virulence. Proc. Natl. Acad. Sci. USA 98:6889-6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, C., A. Motaleb, M. Sal, S. F. Goldstein, and N. W. Charon. 2000. Spirochete periplasmic flagella and motility. J. Mol. Microbiol Biotechnol. 2:345-354. [PubMed] [Google Scholar]
- 32.Li, C. Y., J. P. Tsai, Y. W. Han, Z. Yang, L. E. Wolinsky, H. Kuramitsu, and W. Shi. 1998. Chemotaxis and the cheA mutant of Treponema denticola. J. Dent. Res. 77:228. [Google Scholar]
- 33.Li, H., S. Arakawa, Q. D. Deng, and H. Kuramitsu. 1999. Characterization of a novel methyl-accepting chemotaxis gene, dmcB, from the oral spirochete Treponema denticola. Infect. Immun. 67:694-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, H., J. Ruby, N. Charon, and H. Kuramitsu. 1996. Gene inactivation in the oral spirochete Treponema denticola: construction of an flgE mutant. J. Bacteriol. 178:3664-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Limberger, R. J., L. L. Slivienski, J. Izard, and W. A. Samsonoff. 1999. Insertional inactivation of Treponema denticola tap1 results in a nonmotile mutant with elongated flagellar hooks. J. Bacteriol. 181:3743-3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lux, R., J. N. Miller, N. H. Park, and W. Shi. 2001. Motility and chemotaxis in tissue penetration of oral epithelial cell layers by Treponema denticola. Infect. Immun. 69:6276-6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lux, R., A. Moter, and W. Shi. 2000. Chemotaxis in pathogenic spirochetes: directed movement toward targeting tissues? J. Mol. Microbiol Biotechnol. 2:355-364. [PubMed] [Google Scholar]
- 38.Manson, M. D., J. P. Armitage, J. A. Hoch, and R. M. Macnab. 1998. Bacterial locomotion and signal transduction. J. Bacteriol. 180:1009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milner, J. A., and R. Sellwood. 1994. Chemotactic response to mucin by Serpulina hyodysenteriae and other porcine spirochetes: potential role in intestinal colonization. Infect. Immun. 62:4095-4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore, D. 1999. Preparation and analysis of DNA, p. 2.0.1-2.12.7. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 41.Ohta, K., K. K. Makinen, and W. J. Loesche. 1986. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect. Immun. 53:213-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Toole, R., D. L. Milton, and H. Wolf-Watz. 1996. Chemotactic motility is required for invasion of the host by the fish pathogen Vibrio anguillarum. Mol. Microbiol. 19:625-637. [DOI] [PubMed] [Google Scholar]
- 43.Parkinson, J. S. 1977. Behavioral genetics in bacteria. Annu. Rev. Genet. 11:397-414. [DOI] [PubMed] [Google Scholar]
- 44.Parkinson, J. S. 1976. cheA, cheB, and cheC genes of Escherichia coli and their role in chemotaxis. J. Bacteriol. 126:758-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudolph, J., and D. Oesterhelt. 1996. Deletion analysis of the che operon in the archaeon Halobacterium salinarium. J. Mol. Biol. 258:548-554. [DOI] [PubMed] [Google Scholar]
- 46.Segall, J. E., A. Ishihara, and H. C. Berg. 1985. Chemotactic signaling in filamentous cells of Escherichia coli. J. Bacteriol. 161:51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi, W., Z. Yang, Y. Geng, L. E. Wolinsky, and M. A. Lovett. 1998. Chemotaxis in Borrelia burgdorferi. J. Bacteriol. 180:231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umemoto, T., T. Jinno, Y. Taiji, and T. Ogawa. 2001. Chemotaxis of oral treponemes toward sera and albumin of rabbit. Microbiol. Immunol. 45:571-577. [DOI] [PubMed] [Google Scholar]
- 49.Vieira, J., and J. Messing. 1982. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259-268. [DOI] [PubMed] [Google Scholar]
- 50.Ward, M. J., A. W. Bell, P. A. Hamblin, H. L. Packer, and J. P. Armitage. 1995. Identification of a chemotaxis operon with two cheY genes in Rhodobacter sphaeroides. Mol. Microbiol. 17:357-366. [DOI] [PubMed] [Google Scholar]
- 51.Yuri, K., Y. Takamoto, M. Okada, T. Hiramune, N. Kikuchi, and R. Yanagawa. 1993. Chemotaxis of leptospires to hemoglobin in relation to virulence. Infect. Immun. 61:2270-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]