Abstract
1. The relationship between ionic currents and contraction has been investigated in uterine strips of pregnant rat by means of a double sucrose gap apparatus combined with an optical method which permits the measurement of the contraction of the small muscular bundle where potential and current are recorded.
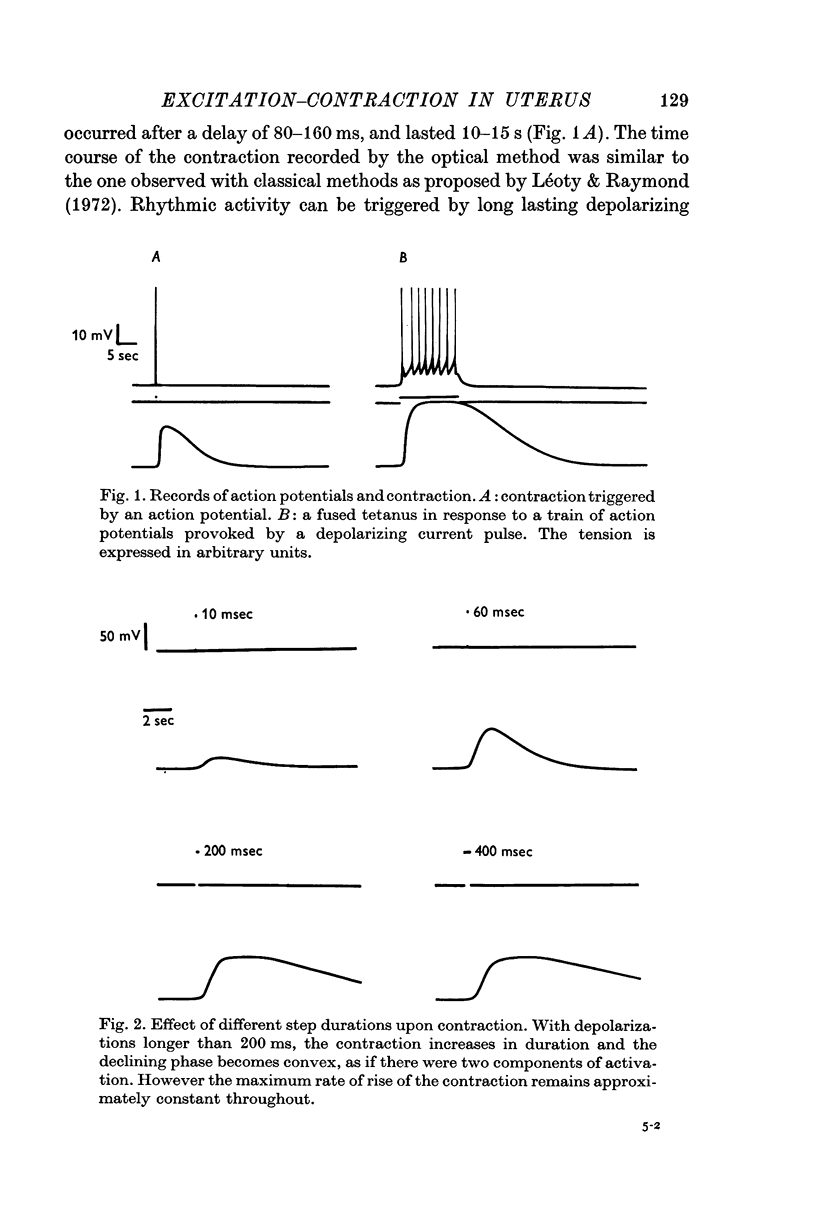
2. Effects of duration, size and frequency of imposed potentials upon contraction have been studied. The uterine muscle shows summation and tetanus phenomena. Tension elicited by depolarizing pulses of different durations and amplitudes can be considered as made of two components.
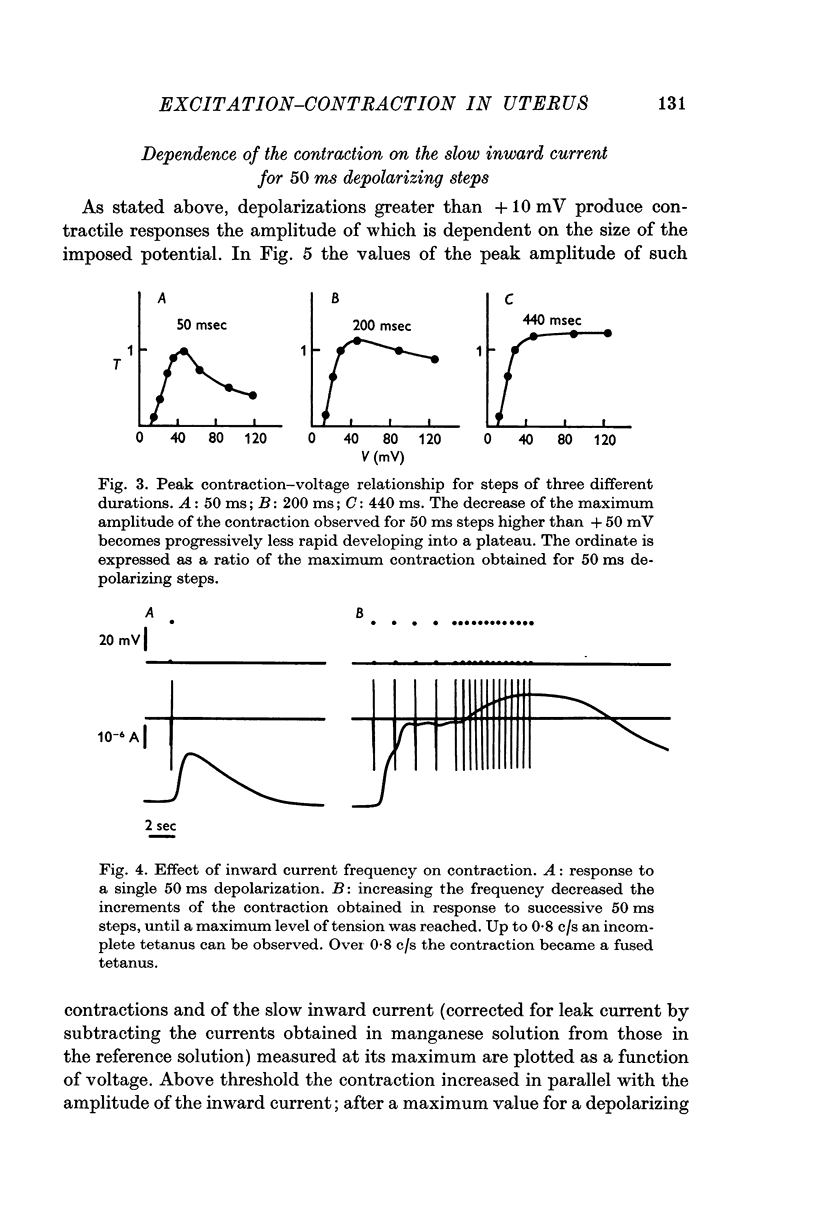
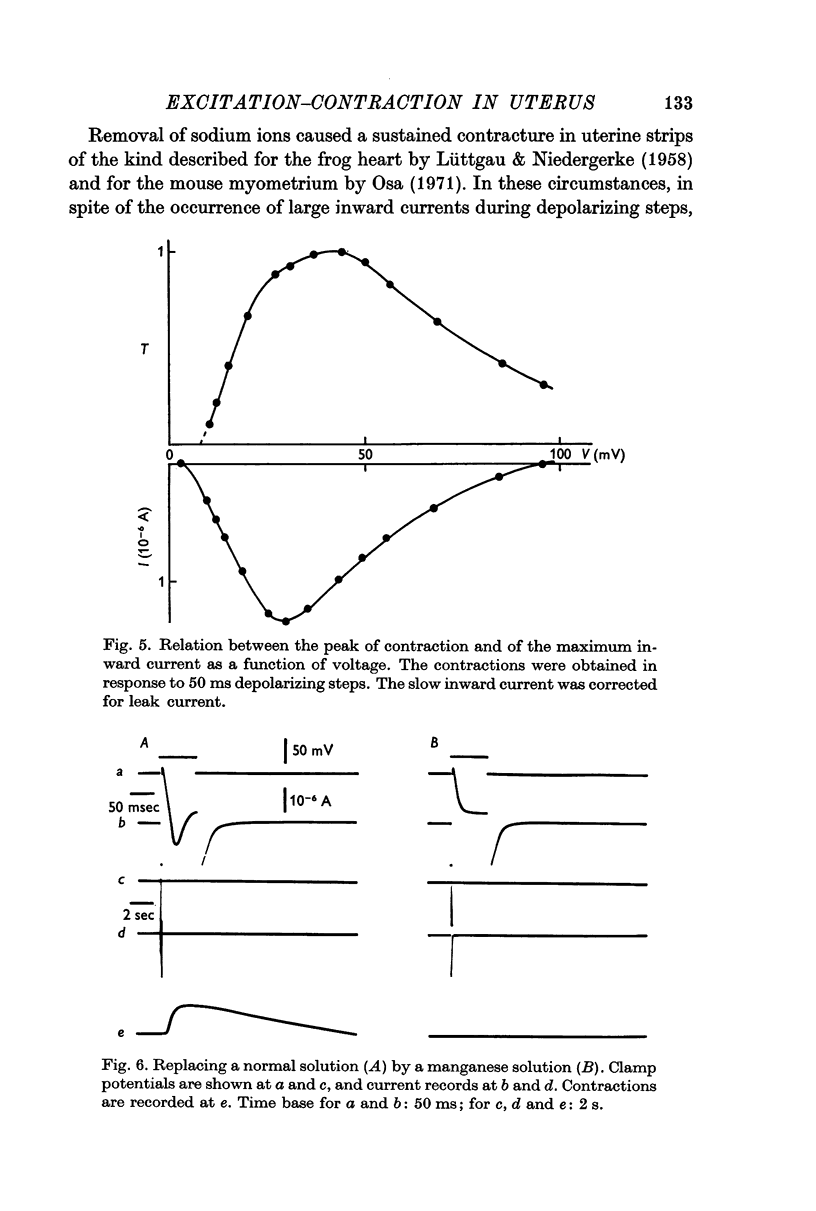
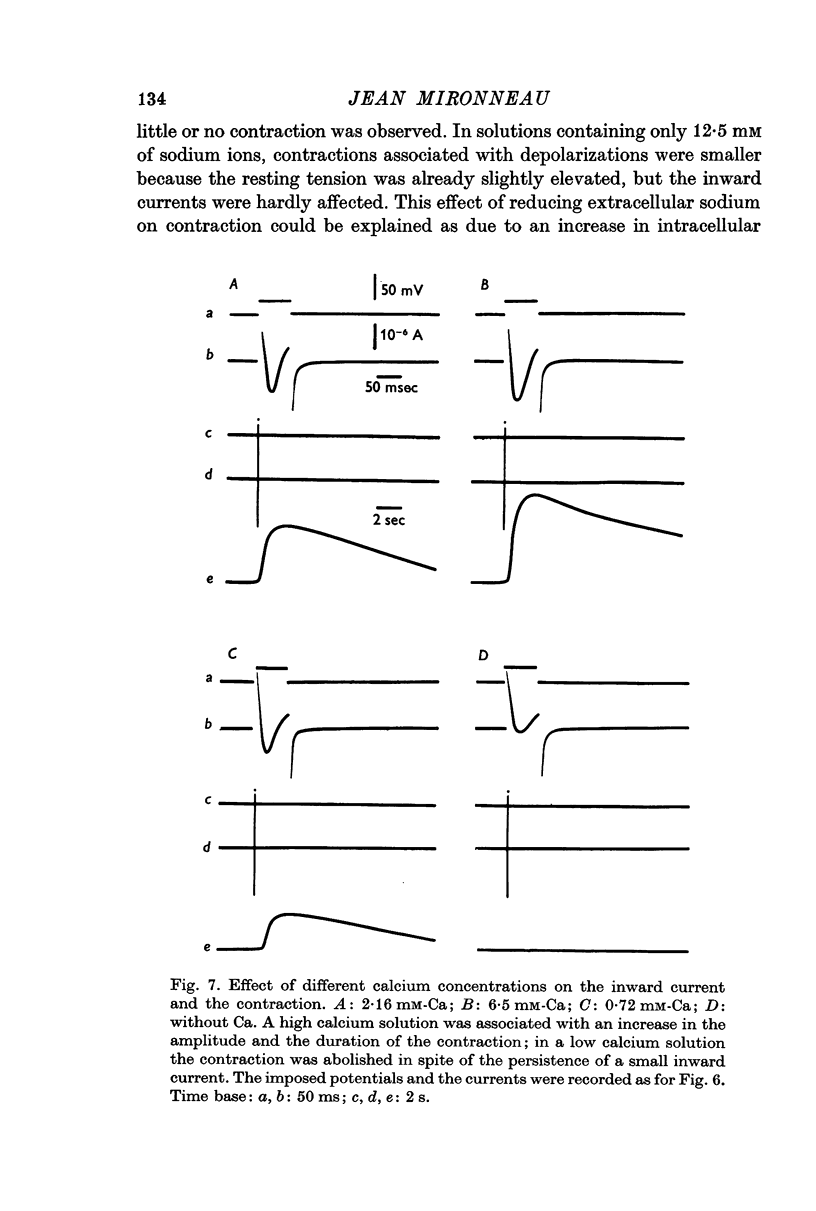
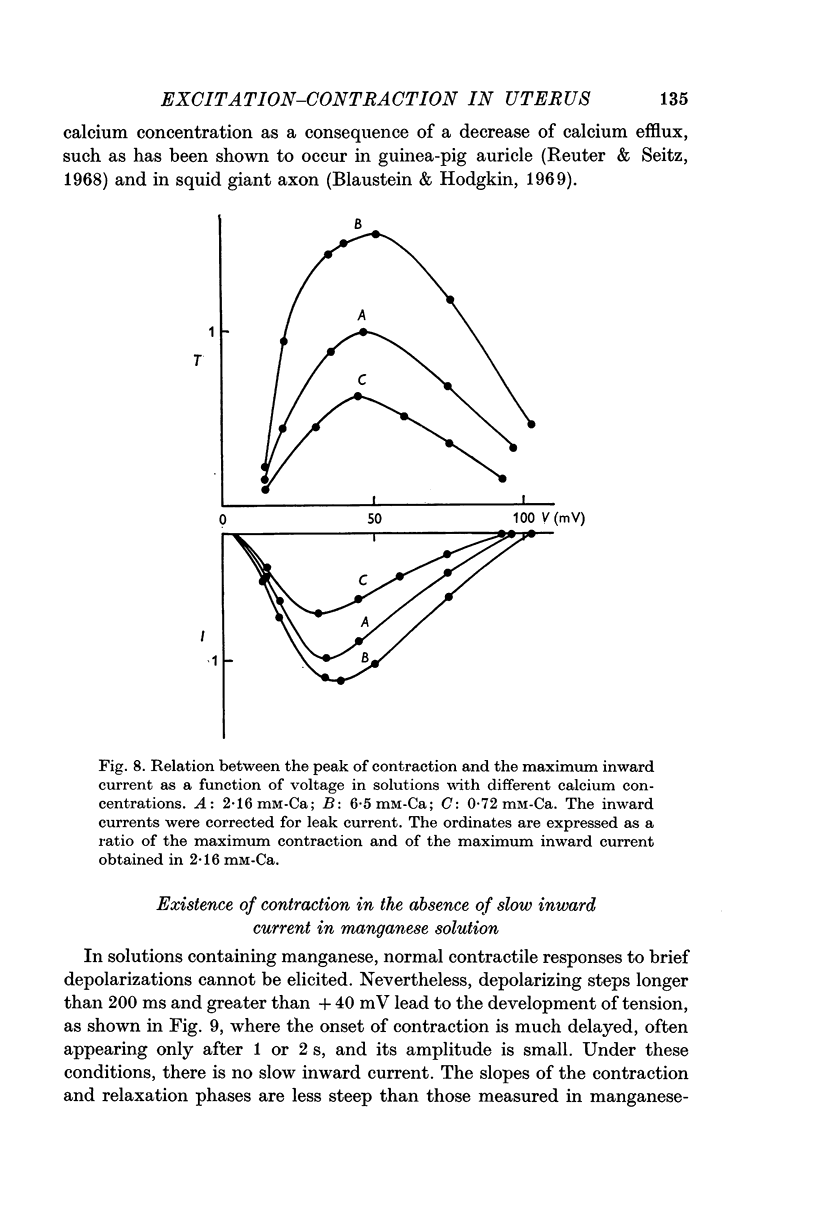
3. The first component of the contraction evoked by short depolarizing steps (about 50 ms) depends on the slow inward current. This contraction is abolished by manganese and lanthanum ions and by compound D 600. The amplitude of the tension can be related to the external calcium concentration and consequently to the calcium influx. The slow inward current is supposed to release a part of the bound calcium without excluding, however, a direct activation of myofibrils.
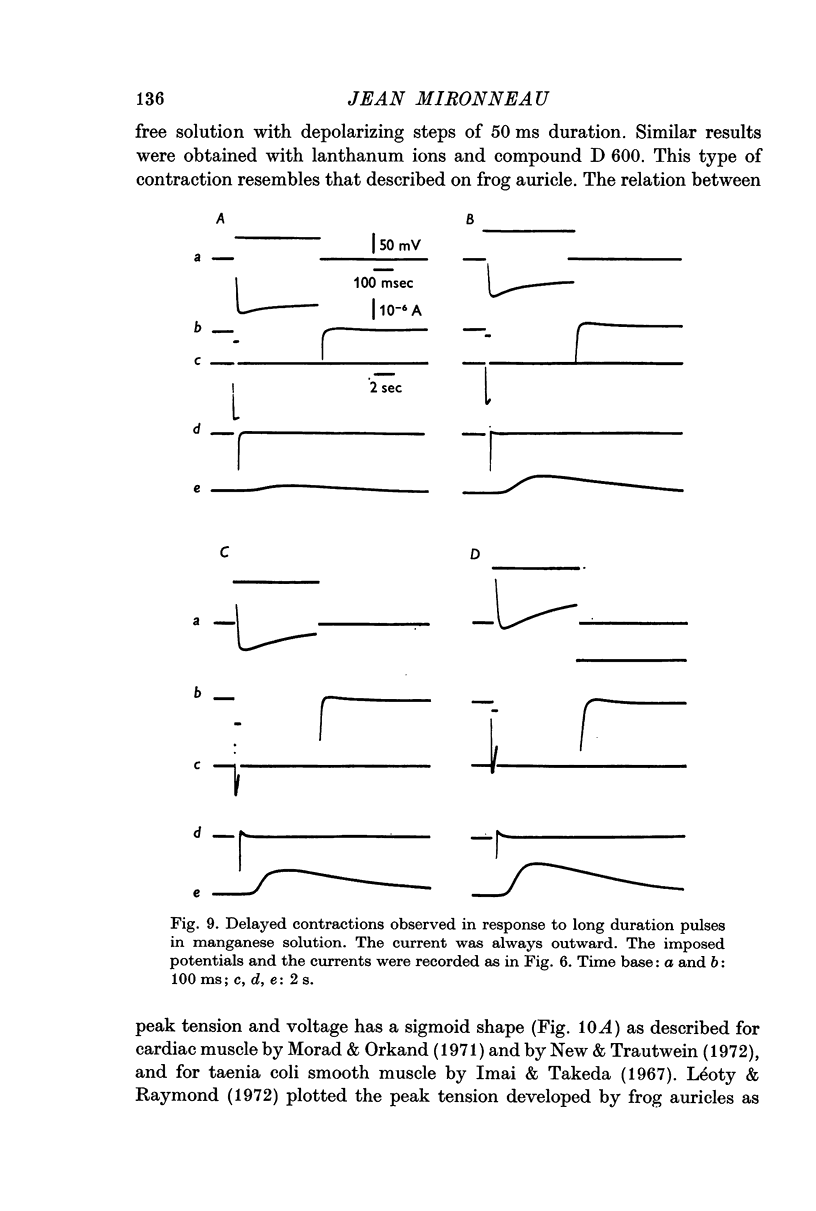
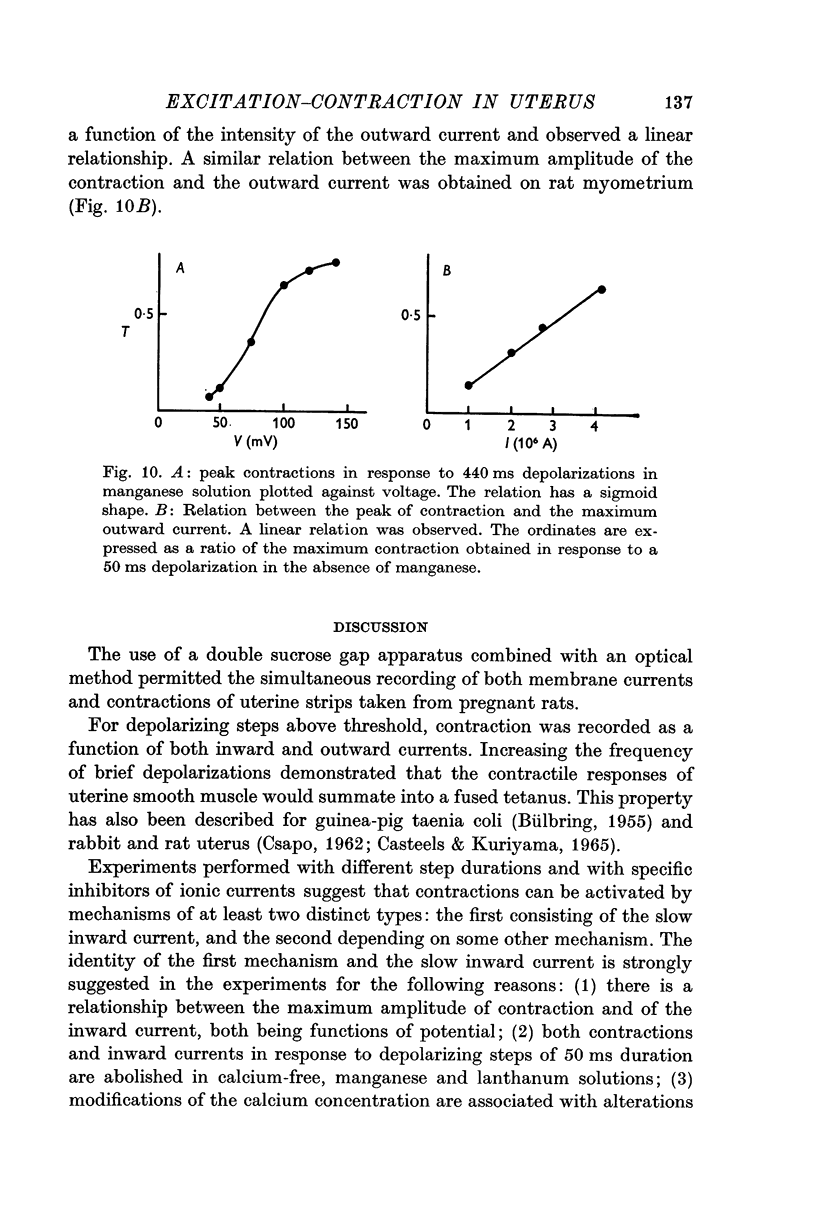
4. The second component of the contraction is observed in manganese containing solution with depolarizations longer than 200 ms and without inward current. Such a component of tension suggests the possibility of release of calcium from intracellular stores which could be located in the sarcoplasmic membrane of the uterine smooth muscle.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y. The effect of sodium and calcium on the action potential of pregnant rat myometrium. J Physiol. 1969 Jan;200(1):1P–2P. [PMC free article] [PubMed] [Google Scholar]
- Anderson N. C., Jr Voltage-clamp studies on uterine smooth muscle. J Gen Physiol. 1969 Aug;54(2):145–165. doi: 10.1085/jgp.54.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. C., Ramon F., Snyder A. Studies on calcium and sodium in uterine smooth muscle excitation under current-clamp and voltage-clamp conditions. J Gen Physiol. 1971 Sep;58(3):322–339. doi: 10.1085/jgp.58.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUER H., GOODFORD P. J., HUETER J. THE CALCIUM CONTENT AND 45-CALCIUM UPTAKE OF THE SMOOTH MUSCLE OF THE GUINEA-PIG TAENIA COLI. J Physiol. 1965 Jan;176:163–179. doi: 10.1113/jphysiol.1965.sp007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOHR D. F. ELECTROLYTES AND SMOOTH MUSCLE CONTRACTION. Pharmacol Rev. 1964 Mar;16:85–127. [PubMed] [Google Scholar]
- BULBRING E. Correlation between membrane potential, spike discharge and tension in smooth muscle. J Physiol. 1955 Apr 28;128(1):200–221. doi: 10.1113/jphysiol.1955.sp005299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. The relation between membrane potential, membrane currents and activation of contraction in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):211–229. doi: 10.1113/jphysiol.1970.sp009057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTEELS R., KURIYAMA H. MEMBRANE POTENTIAL AND IONIC CONTENT IN PREGNANT AND NON-PREGNANT RAT MYOMETRIUM. J Physiol. 1965 Mar;177:263–287. doi: 10.1113/jphysiol.1965.sp007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSAPO A. I. Smooth muscle as a contractile unit. Physiol Rev Suppl. 1962 Jul;5:7–33. [PubMed] [Google Scholar]
- Casteels R., Van Breemen C., Mayer C. J. A study of calcium distribution in smooth muscle cells of the guinea-pig taenia coli using La 3+ . Arch Int Pharmacodyn Ther. 1972 Sep;199(1):193–194. [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Gargouïl Y. M., Léoty C., Poindessault J. P., Raymond G. Evolution du courant initial global et de la contraction sur la fibre sinoauriculaire de grenouille. C R Acad Sci Hebd Seances Acad Sci D. 1969 Oct 29;269(17):1686–1689. [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S., Takeda K. Actions of calcium and certain multivalent cations on potassium contracture of guinea-pig's taenia coli. J Physiol. 1967 May;190(1):155–169. doi: 10.1113/jphysiol.1967.sp008199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Kuriyama H. Caffeine and excitation-contraction coupling in the guinea pig taenia coli. J Gen Physiol. 1971 Apr;57(4):448–463. doi: 10.1085/jgp.57.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIYAMA H., TOMITA T. THE RESPONSES OF SINGLE SMOOTH MUSCLE CELLS OF GUINEA-PIG TAENIA COLI TO INTRACELLULARLY APPLIED CURRENTS, AND THEIR EFFECT ON THE SPONTANEOUS ELECTRICAL ACTIVITY. J Physiol. 1965 May;178:270–289. doi: 10.1113/jphysiol.1965.sp007627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. M. Regulation of cardiac muscle contractility. J Gen Physiol. 1967 Jul;50(6 Suppl):185–196. doi: 10.1085/jgp.50.6.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDA J., WEST T. C. Transmembrane potentials and contractility in the pregnant rat uterus. Am J Physiol. 1956 Nov;187(2):333–337. doi: 10.1152/ajplegacy.1956.187.2.333. [DOI] [PubMed] [Google Scholar]
- LUTTGAU H. C., NIEDERGERKE R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958 Oct 31;143(3):486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léoty C., Raymond G. Mechanical activity and ionic currents in frog atrial trabeculae. Pflugers Arch. 1972;334(2):114–128. doi: 10.1007/BF00586785. [DOI] [PubMed] [Google Scholar]
- Mironneau J., Lenfant J. Activité électrique répétive et courants transmembranaires du friaceau musculaire lisse de l'utérus; action de l'ocytocine. C R Acad Sci Hebd Seances Acad Sci D. 1972 Jun 12;274(24):3269–3272. [PubMed] [Google Scholar]
- Mironneau J., Lenfant J. Analyse des réponses électriques de la fibre musculaire lisse d'utérus de ratte: mise en évidence d'un courant lent calcico-sodique. C R Acad Sci Hebd Seances Acad Sci D. 1971 Jan 18;272(3):436–439. [PubMed] [Google Scholar]
- Mironneau J., Lenfant J., Gargouïl Y. M. L'activité électrique de la fibre musculaire lisse de l'utérus: la repolarisation et les processus d'activation et d'inactivation du courant ionique sortant. C R Acad Sci Hebd Seances Acad Sci D. 1971 Dec 8;273(23):2290–2293. [PubMed] [Google Scholar]
- Morad M., Orkand R. K. Excitation-concentration coupling in frog ventricle: evidence from voltage clamp studies. J Physiol. 1971 Dec;219(1):167–189. doi: 10.1113/jphysiol.1971.sp009656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New W., Trautwein W. The ionic nature of slow inward current and its relation to contraction. Pflugers Arch. 1972;334(1):24–38. doi: 10.1007/BF00585998. [DOI] [PubMed] [Google Scholar]
- Ochi R. The slow inward current and the action of manganese ions in guinea-pig's myocardium. Pflugers Arch. 1970;316(1):81–94. doi: 10.1007/BF00587898. [DOI] [PubMed] [Google Scholar]
- Ochi R., Trautwein W. The dependence of cardiac contraction on depolarization and slow inward current. Pflugers Arch. 1971;323(3):187–203. doi: 10.1007/BF00586383. [DOI] [PubMed] [Google Scholar]
- Osa T. Effect of removing the external sodium on the electrical and mechanical activities of the pregnant mouse myometrium. Jpn J Physiol. 1971 Dec;21(6):607–625. doi: 10.2170/jjphysiol.21.607. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier O., Vassort G., Garnier D., Gargouil Y. M., Coraboeuf E. Existence and role of a slow inward current during the frog atrial action potential. Pflugers Arch. 1969;308(2):91–110. doi: 10.1007/BF00587018. [DOI] [PubMed] [Google Scholar]
- SCHOENBERG C. F. An electron microscope study of smooth muscles in pregnant uterus of the rabbit. J Biophys Biochem Cytol. 1958 Sep 25;4(5):609–614. [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Y., Kuriyama H. The relationship between the electrical and mechanical activity of the guinea-pig stomach. Jpn J Physiol. 1970 Dec 15;20(6):640–656. doi: 10.2170/jjphysiol.20.640. [DOI] [PubMed] [Google Scholar]
- Sandow A. Skeletal muscle. Annu Rev Physiol. 1970;32:87–138. doi: 10.1146/annurev.ph.32.030170.000511. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Vinall P., Somlyo A. P. Excitation-contraction coupling and electrical events in two types of vascular smooth muscle. Microvasc Res. 1969 Oct;1(4):354–373. doi: 10.1016/0026-2862(69)90014-4. [DOI] [PubMed] [Google Scholar]
- Van Breemen C., Farinas B. R., Gerba P., McNaughton E. D. Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium influx. Circ Res. 1972 Jan;30(1):44–54. doi: 10.1161/01.res.30.1.44. [DOI] [PubMed] [Google Scholar]
- Vassort G., Rougier O. Membrane potential and slow inward current dependence of frog cardiac mechanical activity. Pflugers Arch. 1972;331(3):191–203. doi: 10.1007/BF00589126. [DOI] [PubMed] [Google Scholar]
- WEST T. C., HADDEN G., FARAH A. Effect of anoxia on response of the isolated intestine to various drugs and enzyme inhibitors. Am J Physiol. 1951 Feb;164(2):565–572. doi: 10.1152/ajplegacy.1951.164.2.565. [DOI] [PubMed] [Google Scholar]


