Abstract
1. The experiments were done on single Ranvier nodes of Xenopus laevis (voltage clamp) and Rana esculenta (action potentials). Rate and size of the effect of tetrodotoxin were determined by the reversible reduction of either the sodium inward current (Xenopus) or of V̇A, the maximum rate of rise of the action potential (Rana).
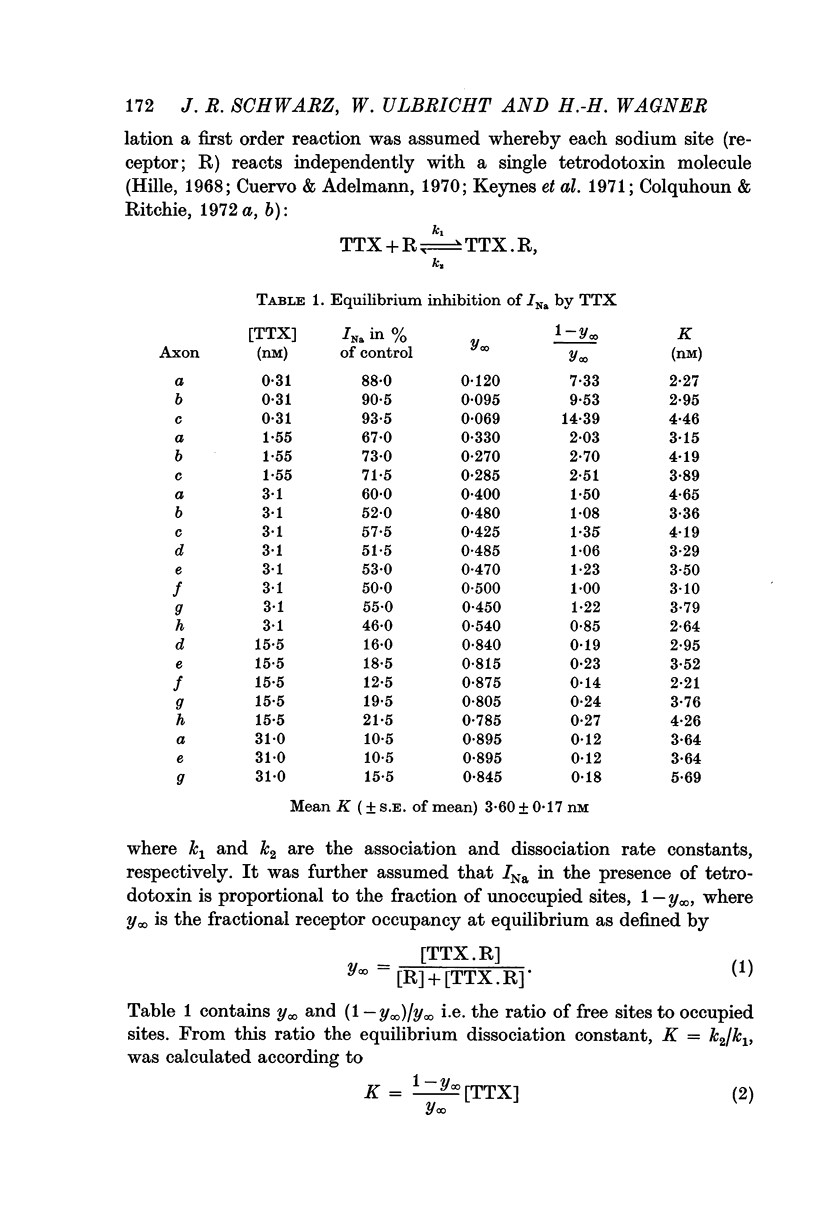
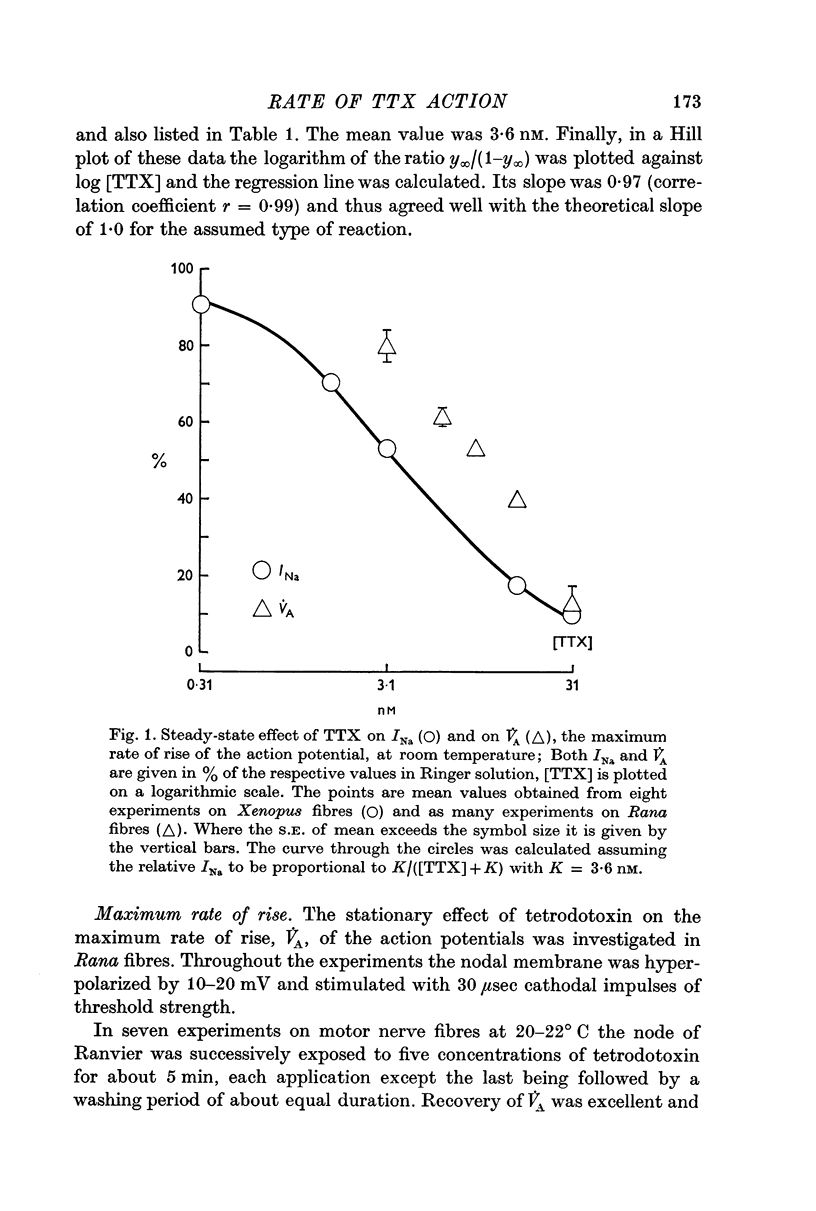
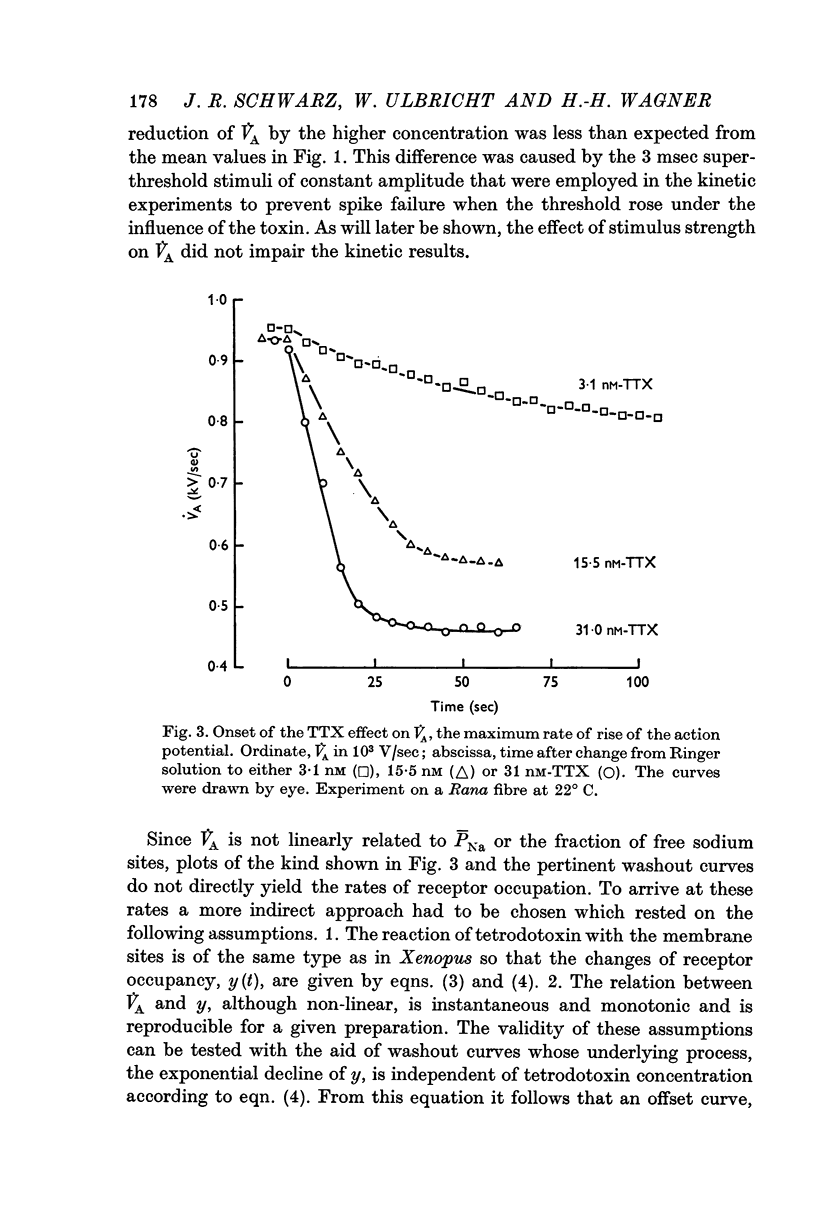
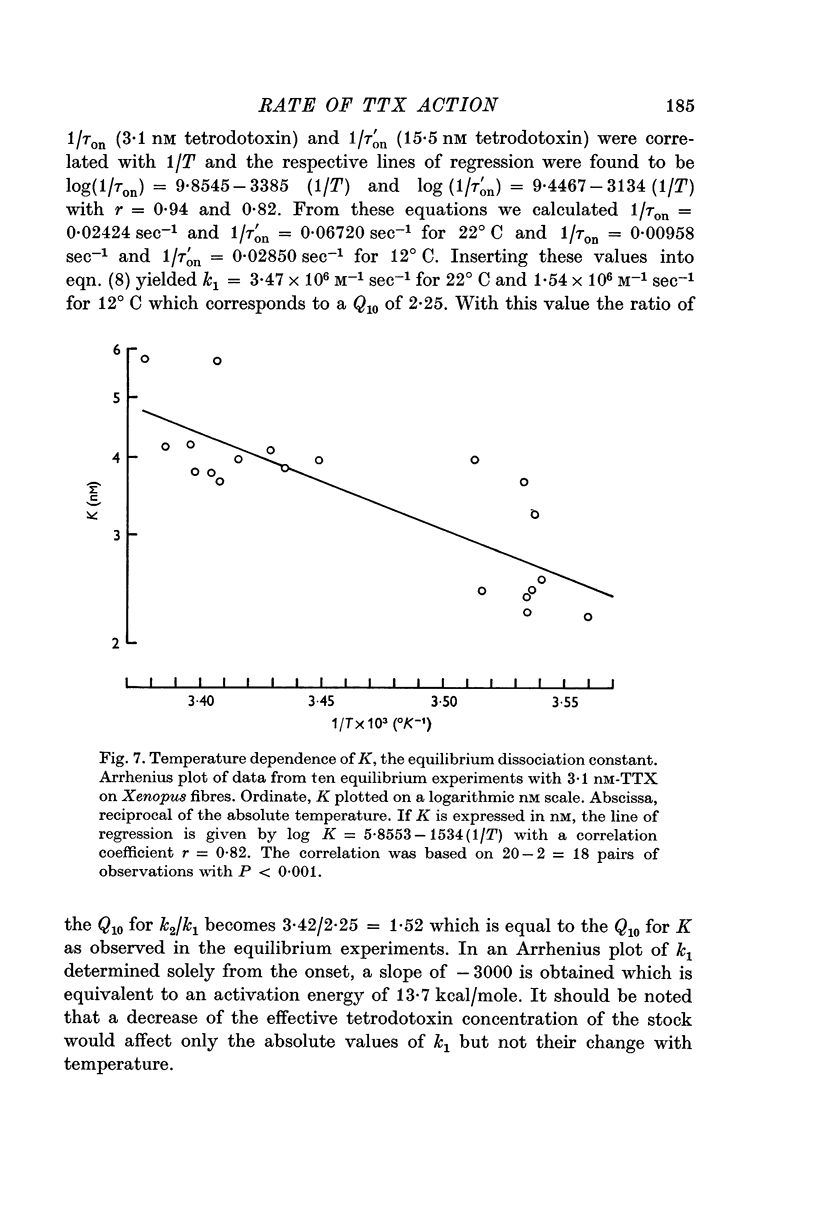
2. The results of tetrodotoxin block at equilibrium could be excellently fitted by assuming a one-to-one reaction between toxin molecules and sodium channels of the Xenopus membrane with an equilibrium dissociation constant K = 3·60 nM at room temperature. V̇A was not linearly related to the fraction of unblocked sodium channels and 10·9 nM tetrodotoxin was necessary on the average to reduce V̇A to 50% in Rana motor fibres; in sensory fibres a lower concentration sufficed.
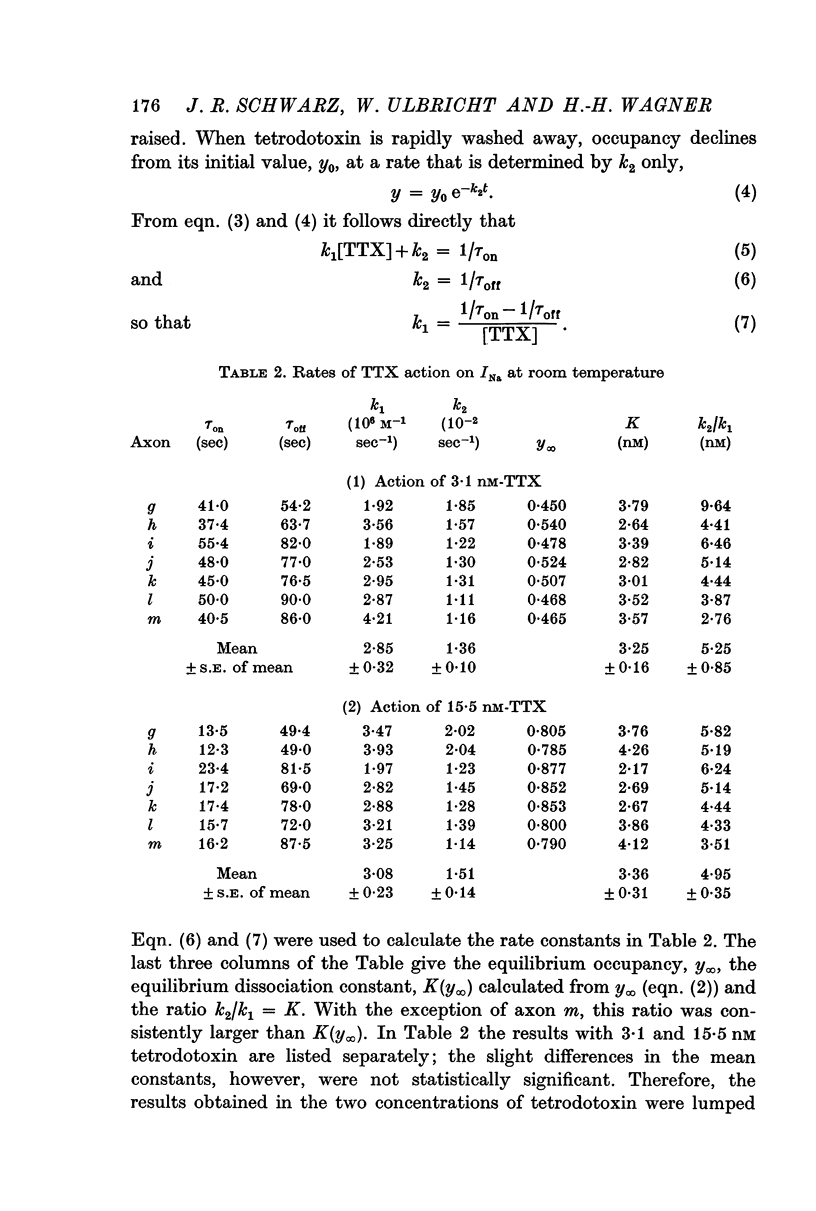
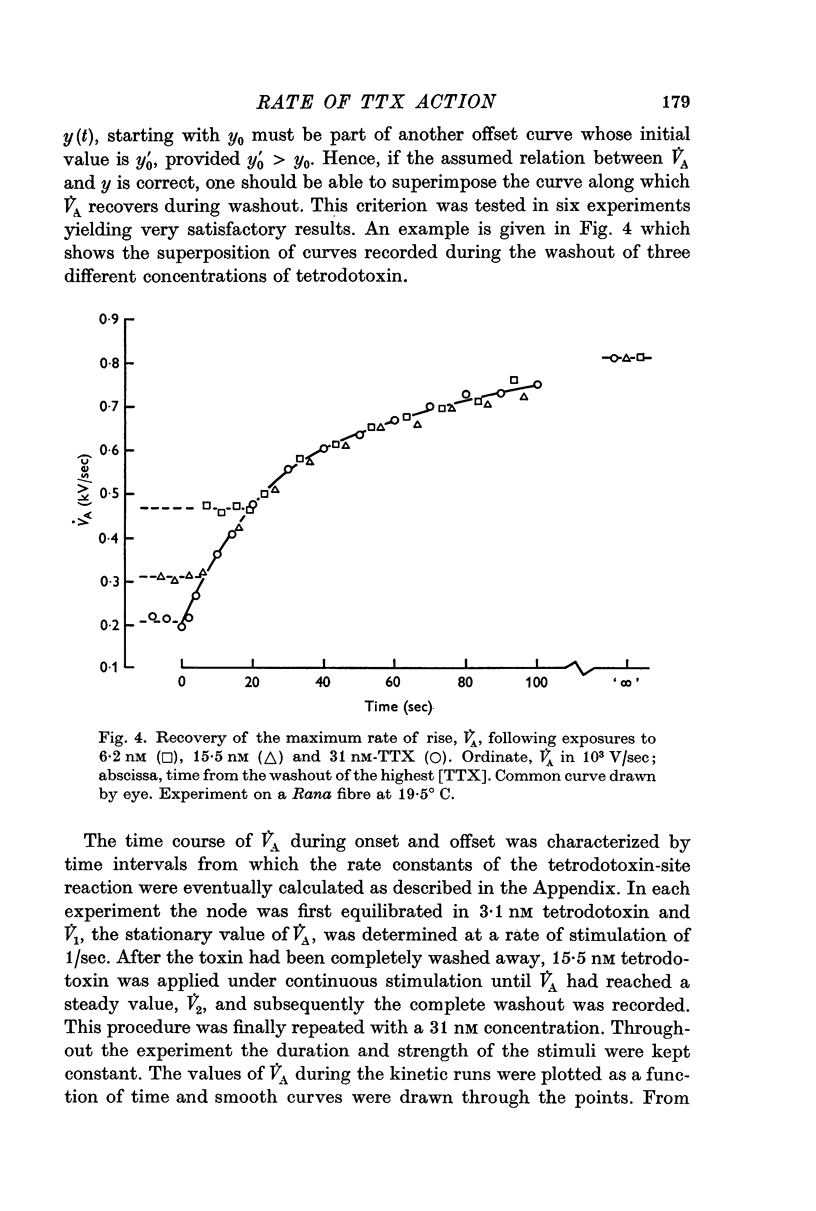
3. Onset and offset of the tetrodotoxin effect on Xenopus nodes could be quantitatively interpreted as being determined by the rates of the tetrodotoxin channel reaction. Experiments with 3·1 and 15·5 nM tetrodotoxin at room temperature yielded an association rate constant, k1, of 2·94 × 106 M-1 sec-1 and a dissociation rate constant, k2, of 1·42 × 10-2 sec-1. In these experiments the equilibrium dissociation constant, K, was 3·31 nM. If determined solely from the onset in the two tetrodotoxin concentrations, k1 = 3·25 × 106 M-1 sec-1 and K = k2/k1 = 4·08 nM was calculated.
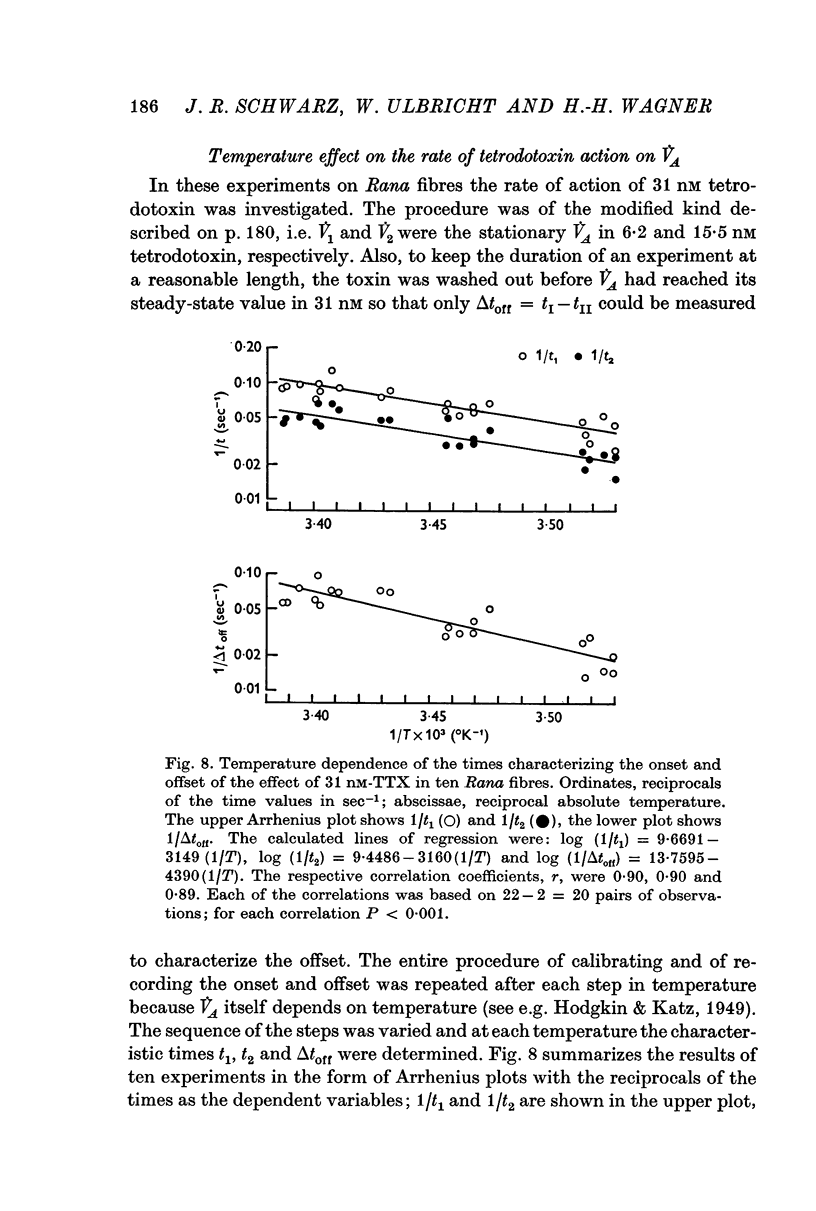
4. In Rana fibres the onset and offset of V̇A reduction by 15·5 and 31 nM tetrodotoxin was evaluated using the equilibrium effects of intermediary tetrodotoxin concentrations for calibration. The average results at room temperature were k1 = 4 × 106 M-1 sec-1, k2 = 1·4 × 10-2 sec-1 and K = 3·4 nM.
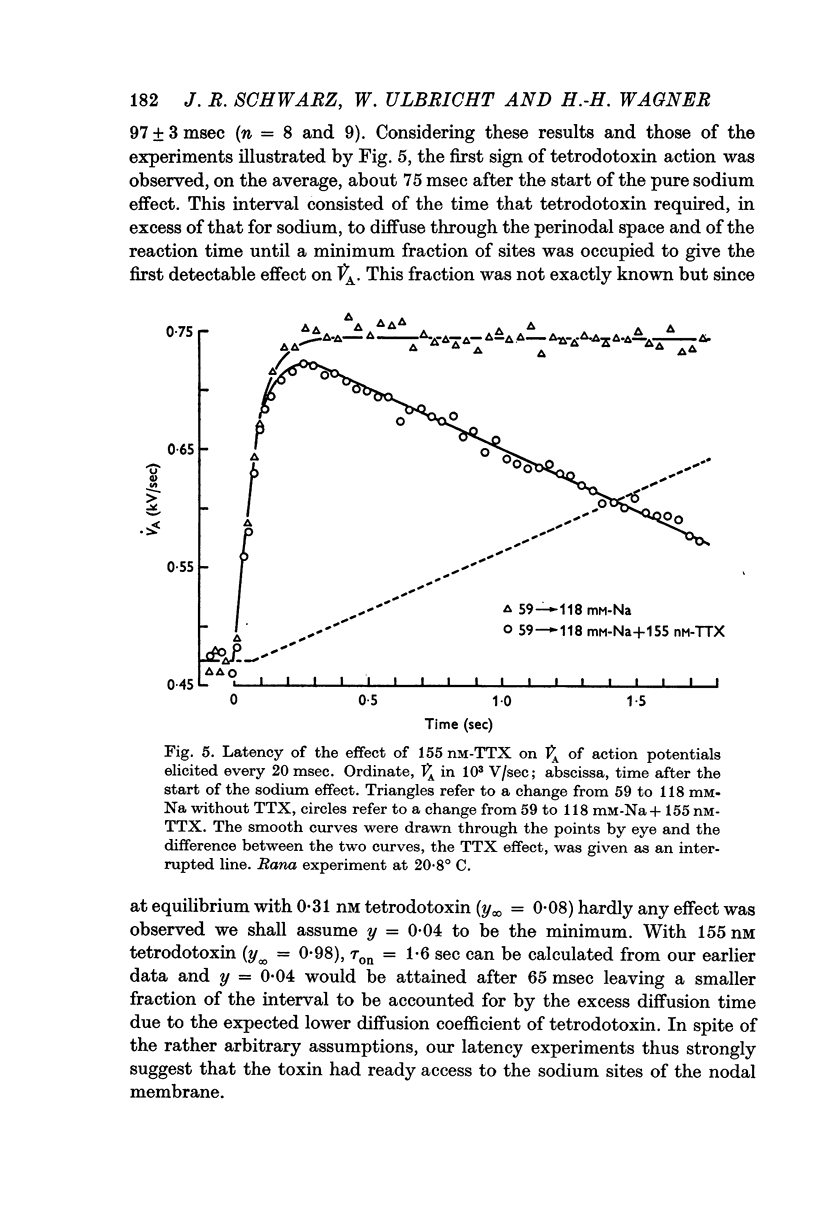
5. The very short latency with which V̇A started to decline when tetrodotoxin was suddenly applied proved that the toxin had ready access to the membrane.
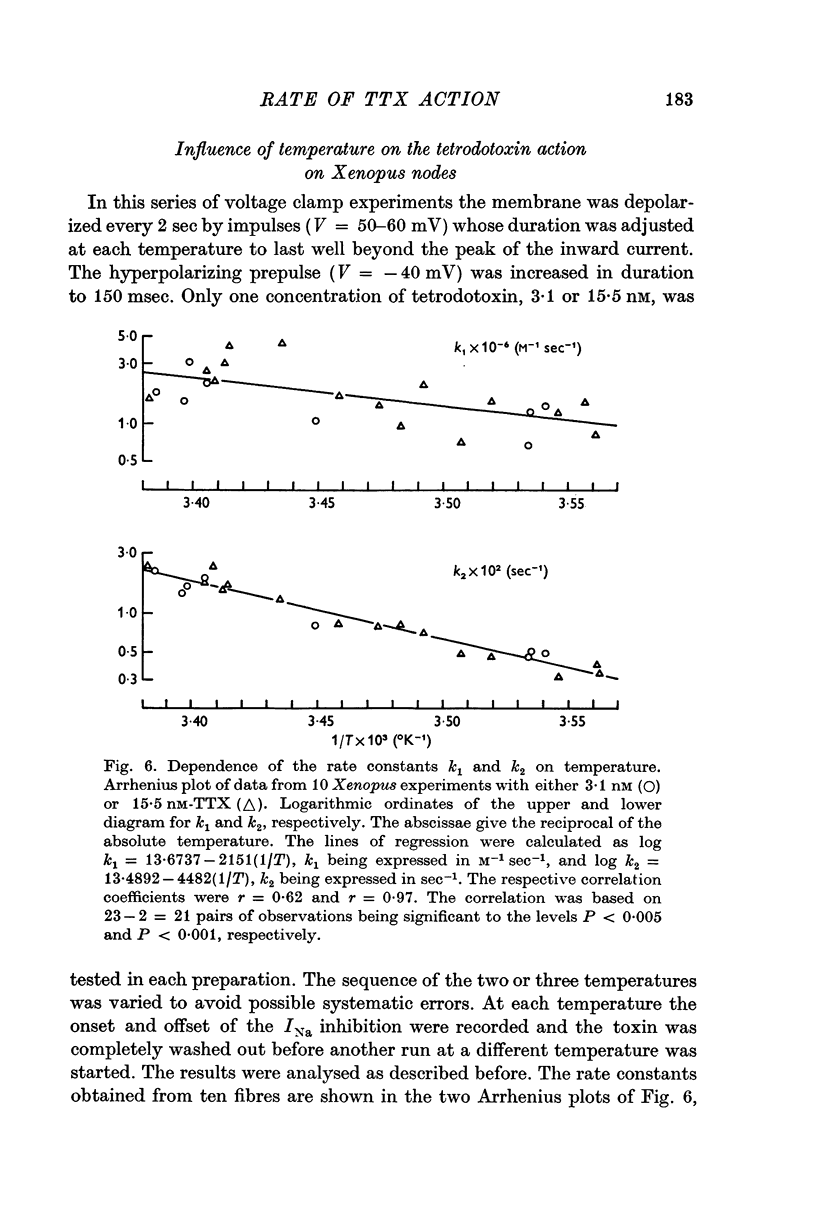
6. The temperature dependence of k1, k2 and K in the Xenopus experiments could be described by Arrhenius plots yielding activation energies, Ea, of 9·8, 20·5 and 7·0 kcal/mole, respectively, corresponding to Q10 values of 1·82, 3·42 and 1·53 (between 12 and 22° C). For k1, determined from onset alone, Ea = 13·7 kcal/mole (Q10 = 2·25) was obtained. Although in Rana the temperature dependence of the rate constants could not be determined directly, the Q10 for k2 must have been of the order of 3.
7. The results suggest that the rate of the toxin action on the nodal membrane of Xenopus and Rana is limited by the tetrodotoxin-sodium site reaction.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol. 1956 Sep 27;133(3):631–658. doi: 10.1113/jphysiol.1956.sp005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Henderson R., Ritchie J. M. The binding of labelled tetrodotoxin to non-myelinated nerve fibres. J Physiol. 1972 Dec;227(1):95–126. doi: 10.1113/jphysiol.1972.sp010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Ritchie J. M. The interaction at equilibrium between tetrodotoxin and mammalian non-myelinated nerve fibres. J Physiol. 1972 Mar;221(3):533–553. doi: 10.1113/jphysiol.1972.sp009766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Ritchie J. M. The kinetics of the interaction between tetrodotoxin and mammalian nonmyelinated nerve fibers. Mol Pharmacol. 1972 May;8(3):285–292. [PubMed] [Google Scholar]
- Cuervo L. A., Adelman W. J., Jr Equilibrium and kinetic properties of the interaction between tetrodotoxin and the excitable membrane of the squid giant axon. J Gen Physiol. 1970 Mar;55(3):309–335. doi: 10.1085/jgp.55.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Membrane currents in isolated frog nerve fibre under voltage clamp conditions. J Physiol. 1958 Aug 29;143(1):76–90. doi: 10.1113/jphysiol.1958.sp006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HUXLEY A. F. THE ACTION POTENTIAL IN THE MYELINATED NERVE FIBER OF XENOPUS LAEVIS AS COMPUTED ON THE BASIS OF VOLTAGE CLAMP DATA. J Physiol. 1964 Jun;171:302–315. doi: 10.1113/jphysiol.1964.sp007378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of temperature on the electrical activity of the giant axon of the squid. J Physiol. 1949 Aug;109(1-2):240–249. doi: 10.1113/jphysiol.1949.sp004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. V. The mode of action of nicotine and curari, determined by the form of the contraction curve and the method of temperature coefficients. J Physiol. 1909 Dec 23;39(5):361–373. doi: 10.1113/jphysiol.1909.sp001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Common mode of action of three agents that decrease the transient change in sodium permeability in nerves. Nature. 1966 Jun 18;210(5042):1220–1222. doi: 10.1038/2101220a0. [DOI] [PubMed] [Google Scholar]
- Hille B. Pharmacological modifications of the sodium channels of frog nerve. J Gen Physiol. 1968 Feb;51(2):199–219. doi: 10.1085/jgp.51.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M., Rojas E. The binding of tetrodotoxin to nerve membranes. J Physiol. 1971 Feb;213(1):235–254. doi: 10.1113/jphysiol.1971.sp009379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppenhöfer E., Vogel W. Wirkung von Tetrodotoxin und Tetraäthylammoniumchlorid an der Innenseite der Schnürringsmembran von Xenopus laevis. Pflugers Arch. 1969;313(4):361–380. doi: 10.1007/BF00593959. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Moore J. W., Frazier D. T. Dependence of tetrodotoxin blockage of nerve membrane conductance on external pH. J Pharmacol Exp Ther. 1969 Oct;169(2):224–228. [PubMed] [Google Scholar]
- PATON W. D., WAUD D. R. A QUANTITATIVE INVESTIGATION OF THE RELATIONSHIP BETWEEN RATE OF ACCESS OF A DRUG TO RECEPTOR AND THE RATE OF ONSET OR OFFSET OF ACTION. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964 May 11;248:124–143. doi: 10.1007/BF00246668. [DOI] [PubMed] [Google Scholar]
- Rang H. P. The kinetics of action of acetylcholine antagonists in smooth muscle. Proc R Soc Lond B Biol Sci. 1966 Apr 19;164(996):488–510. doi: 10.1098/rspb.1966.0045. [DOI] [PubMed] [Google Scholar]
- STAMPFLI R. Die Strom-Spannungs-Charakteristik der erregbaren Membran eines einzelnen Schnürrings und ihre Abhängigkeit von der Ionenkonzentration. Helv Physiol Pharmacol Acta. 1958;16(2):127–145. [PubMed] [Google Scholar]
- Takata M., Moore J. W., Kao C. Y., Fuhrman F. A. Blockage of sodium conductance increase in lobster giant axon by tarichatoxin (tetrodotoxin). J Gen Physiol. 1966 May;49(5):977–988. doi: 10.1085/jgp.49.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thron C. D., Waud D. R. The rate of action of atropine. J Pharmacol Exp Ther. 1968 Mar;160(1):91–105. [PubMed] [Google Scholar]
- ULBRICHT W. DER ZEITLICHE VERLAUF DER KALIUM-DEPOLARISATION DER SCHNUERRINGSMEMBRAN BEI VERSCHIEDENEN CALCIUM-KONZENTRATIONEN UND ANODISCHER POLARISATION. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963 Jul 2;277:270–284. [PubMed] [Google Scholar]
- Vierhaus J., Ulbricht W. Effect of a sudden change in sodium concentration on repetitively evoked action potentials of single nodes of Ranvier. Pflugers Arch. 1971;326(1):76–87. doi: 10.1007/BF00586795. [DOI] [PubMed] [Google Scholar]
- Vierhaus J., Ulbricht W. Rate of action of tetraethylammonium ions on the duration of action potentials in single Ranvier nodes. Pflugers Arch. 1971;326(1):88–100. doi: 10.1007/BF00586796. [DOI] [PubMed] [Google Scholar]
- Wagner H. H., Schwarz J. R., Ulbricht W. Rate of tetrodotoxin (TTX) action on single nodes of Ranvier. Pflugers Arch. 1972;332(Suppl):R68–R68. [PubMed] [Google Scholar]
- Waud D. R. Pharmacological receptors. Pharmacol Rev. 1968 Jun;20(2):49–88. [PubMed] [Google Scholar]


