Abstract
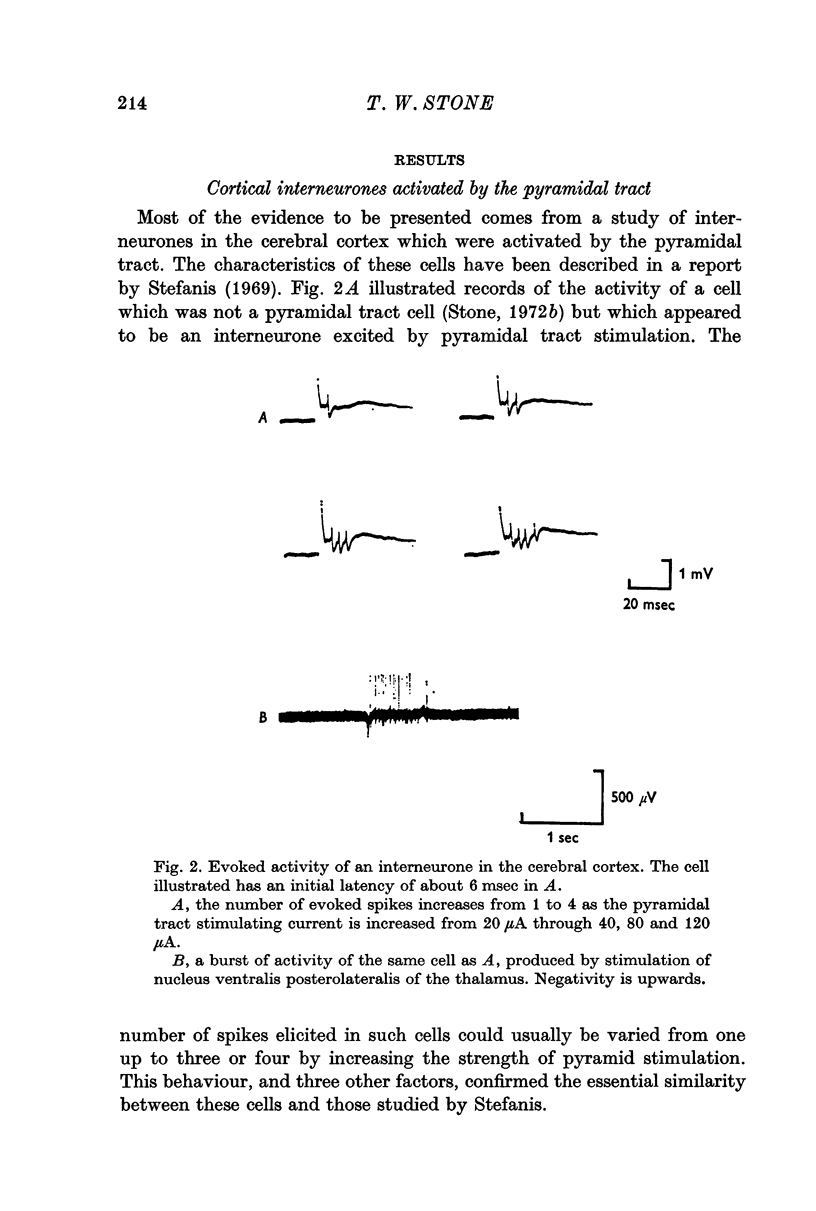
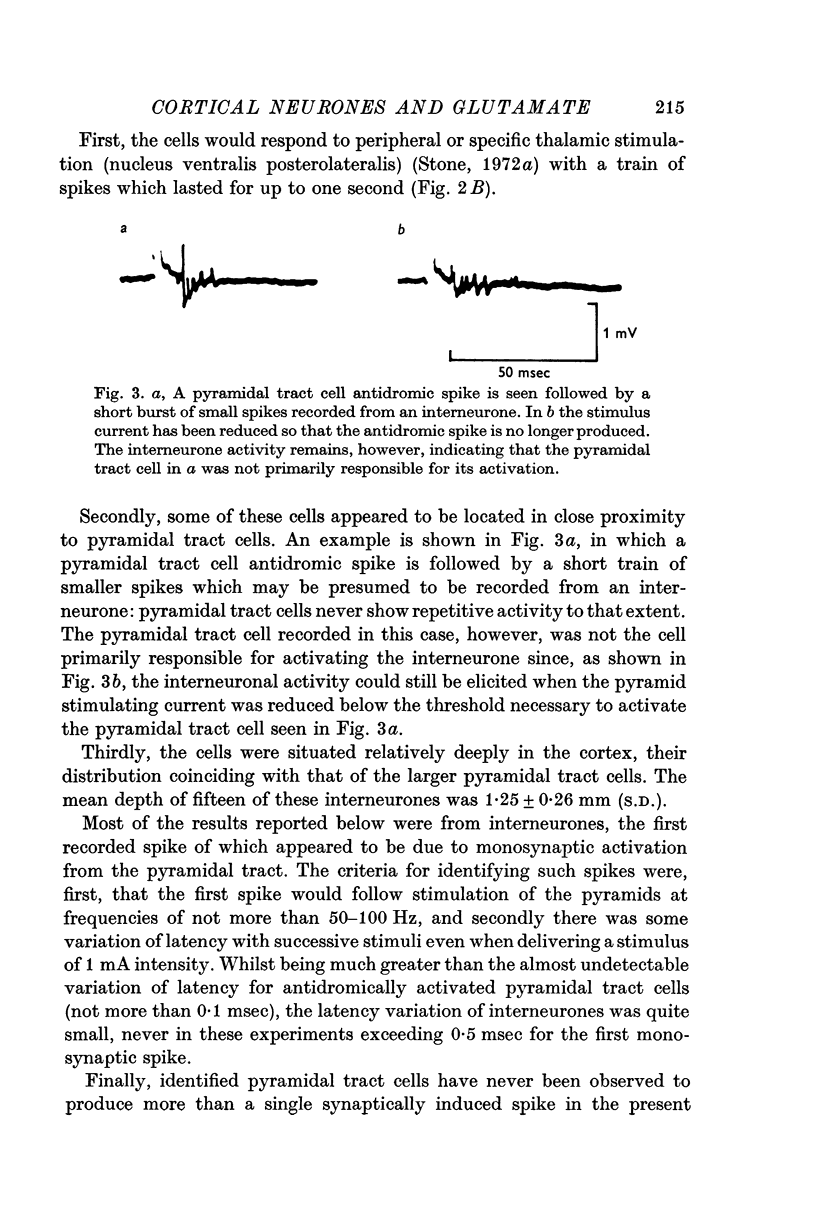
1. Pyramidal tract interneurones, defined as neurones which are activated synaptically as a result of pyramidal tract stimulation, have been identified in the rat cerebral cortex. The number of evoked spikes depended upon stimulus strength, and stimulation in a specific thalamic nucleus produced a burst of activity lasting for up to 1 sec.
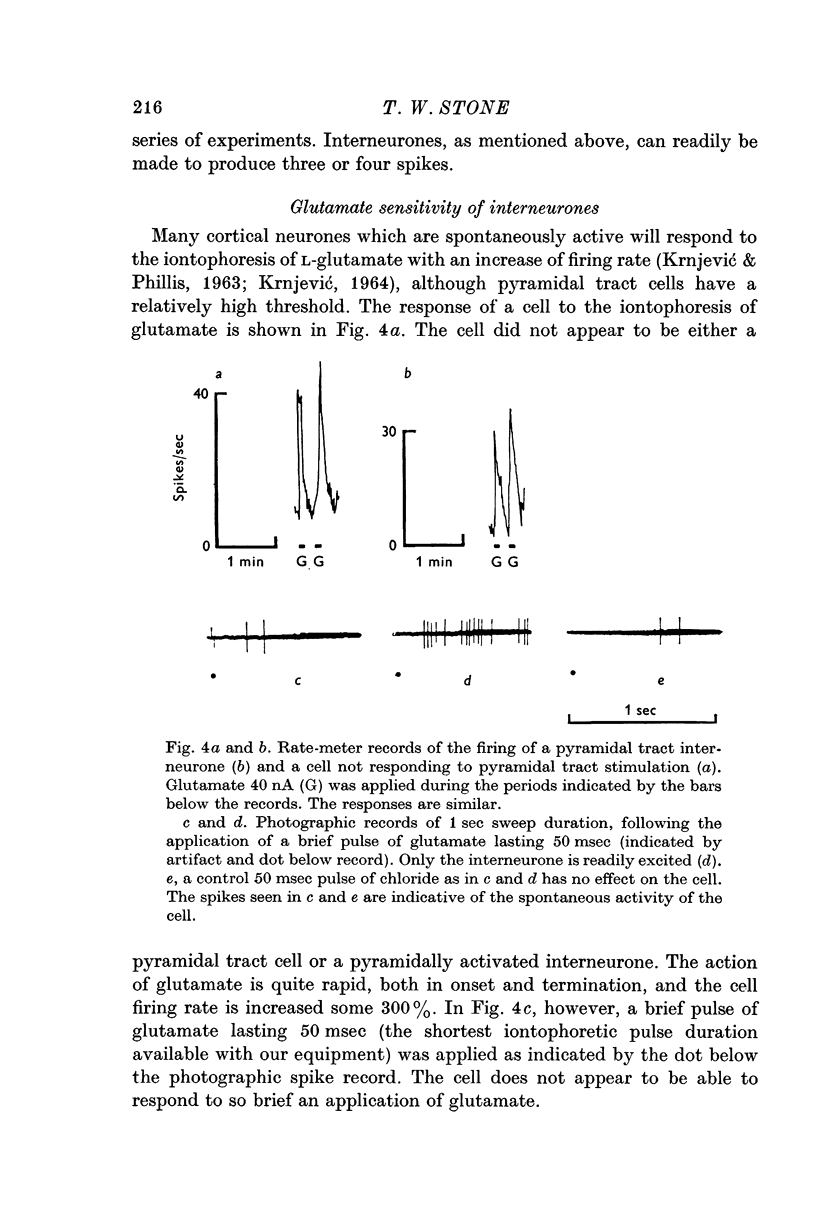
2. These cells are readily excited by a brief (50 msec) pulse of glutamate applied by micro-iontophoresis. Other, unidentified cells are not so responsive.
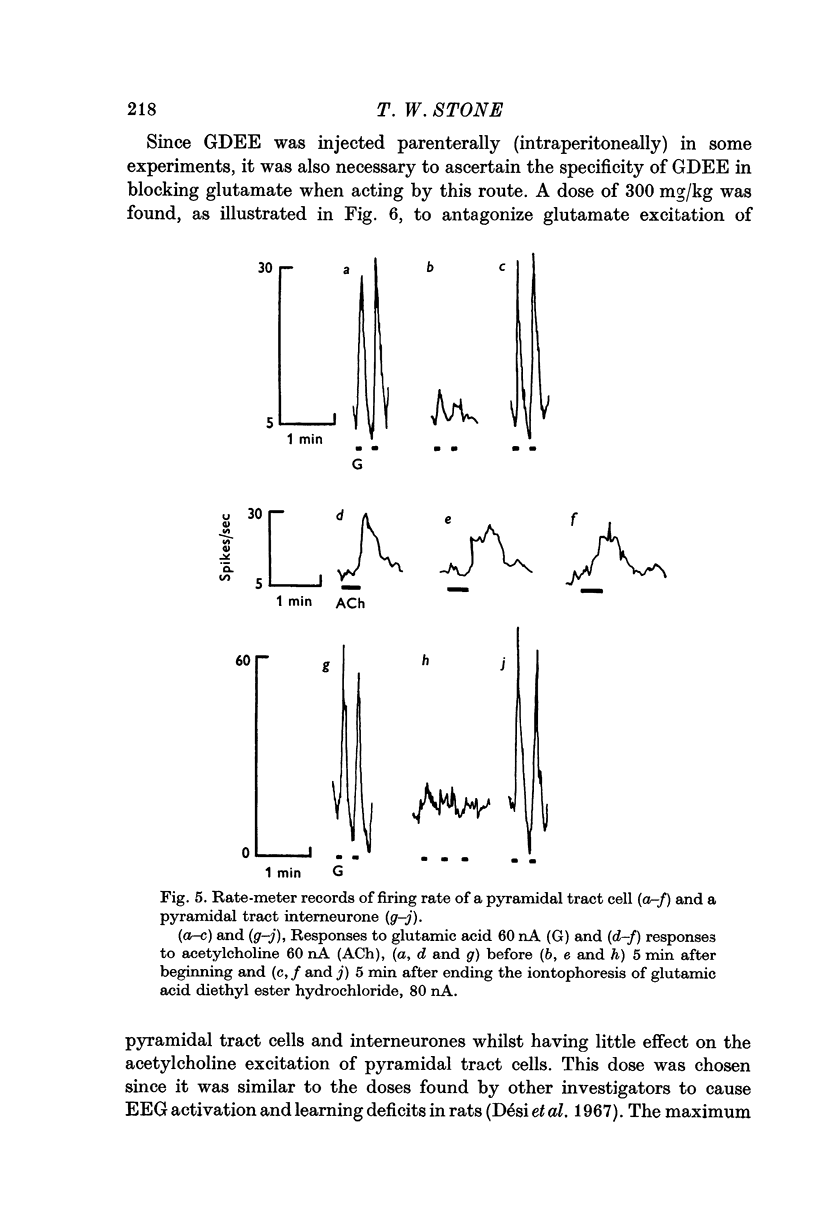
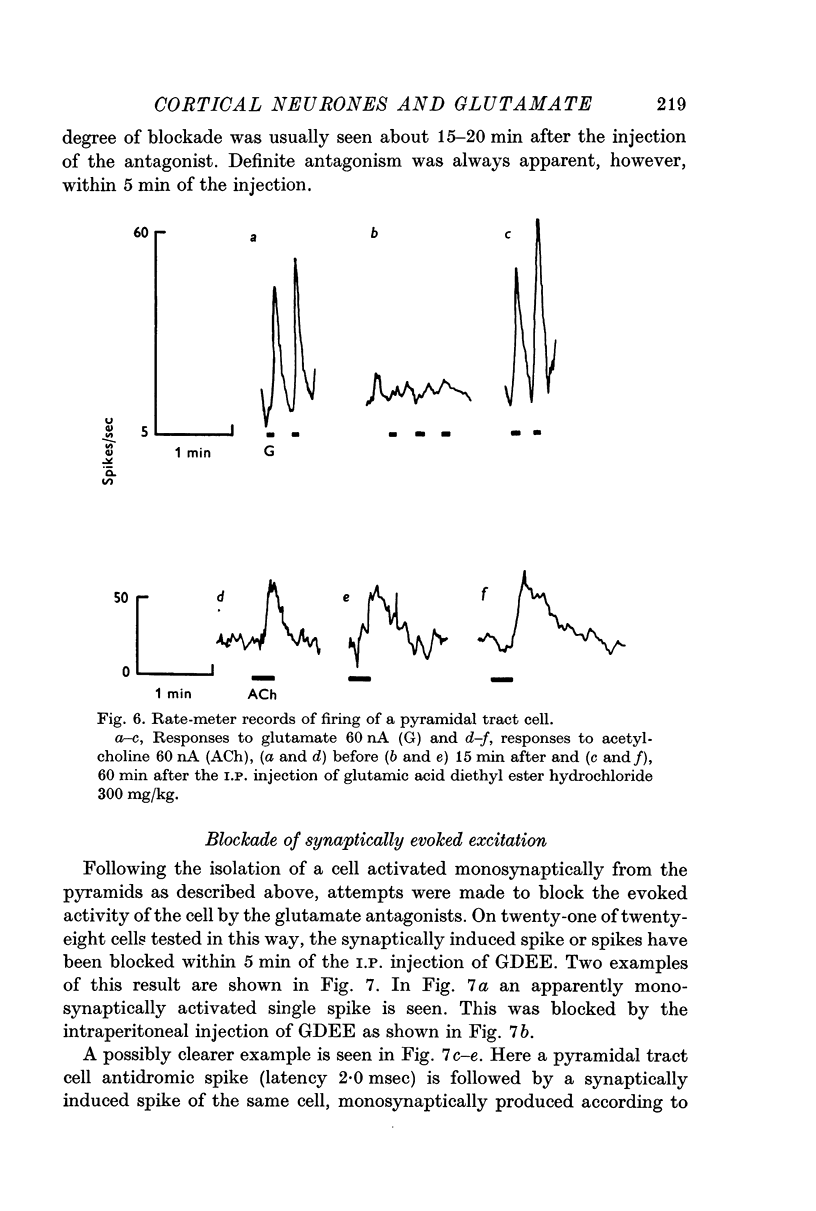
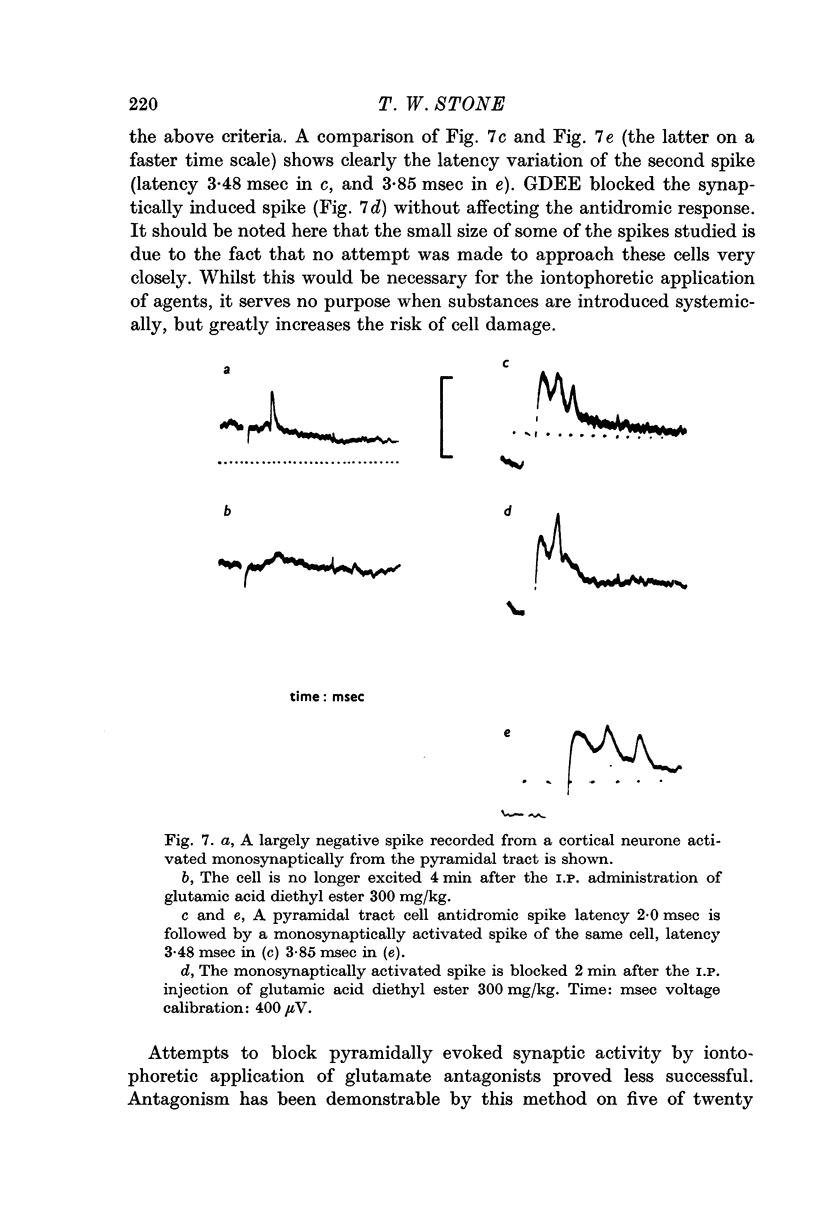
3. Synaptically evoked spikes resulting from pyramidal tract stimulation can be blocked by the iontophoretic and I.P. administration of substances shown to antagonize glutamate excitation of cells.
4. The results support suggestions that glutamic acid is a neurotransmitter in the cerebral cortex. The evidence presented further indicates that glutamic acid could be the transmitter released by the pyramidal tract.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERL S., WAELSCH H. Determination of glutamic acid, glutamine, glutathione and gamma-aminobutyric acid and their distribution in brain tissue. J Neurochem. 1958 Dec;3(2):161–169. doi: 10.1111/j.1471-4159.1958.tb12623.x. [DOI] [PubMed] [Google Scholar]
- BROOKS V. B., ASANUMA H. RECURRENT CORTICAL EFFECTS FOLLOWING STIMULATION OF MEDULLARY PYRAMID. Arch Ital Biol. 1965 Jun 10;103:247–278. [PubMed] [Google Scholar]
- Balcar V. J., Johnston G. A. Glutamate uptake by brain slices and its relation to the depolarization of neurones by acidic amino acids. J Neurobiol. 1972;3(4):295–301. doi: 10.1002/neu.480030403. [DOI] [PubMed] [Google Scholar]
- Bradford H. F. Metabolic response of synaptosomes to electrical stimulation: release of amino acids. Brain Res. 1970 Apr 14;19(2):239–247. doi: 10.1016/0006-8993(70)90437-3. [DOI] [PubMed] [Google Scholar]
- Bradley P. B., Dray A. The effects of different anaesthetics on responses of brain stem neurones to iontophoretically applied transmitter substances. Br J Pharmacol. 1972 May;45(1):169P–170P. [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD J. M., CURTIS D. R. THE EXCITATION AND DEPRESSION OF MAMMALIAN CORTICAL NEURONES BY AMINO ACIDS. Br J Pharmacol Chemother. 1964 Oct;23:313–329. doi: 10.1111/j.1476-5381.1964.tb01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross B. A., Dyer R. G. Unit activity in rat diencephalic islands--the effect of anaesthetics. J Physiol. 1971 Jan;212(2):467–481. doi: 10.1113/jphysiol.1971.sp009336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A., Teb ecis A. K., Watkins J. C. Excitation of mammalian central neurones by acidic amino acids. Brain Res. 1972 Jun 22;41(2):283–301. doi: 10.1016/0006-8993(72)90503-3. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Watkins J. C. The pharmacology of amino acids related to gamma-aminobutyric acid. Pharmacol Rev. 1965 Dec;17(4):347–391. [PubMed] [Google Scholar]
- Dési I., Farkas I., Sós J., Balogh A. Neurophysiological effects of glutamic acid ethylester. Acta Physiol Acad Sci Hung. 1967;32(4):323–335. [PubMed] [Google Scholar]
- Feldberg W., Vogt M. Acetylcholine synthesis in different regions of the central nervous system. J Physiol. 1948 Jun 25;107(3):372–381. doi: 10.1113/jphysiol.1948.sp004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldeman S., Huffman R. D., Marshall K. C., McLennan H. The antagonism of the glutamate-induced and synaptic excitations of thalamic neurones. Brain Res. 1972 Apr 28;39(2):419–425. doi: 10.1016/0006-8993(72)90445-3. [DOI] [PubMed] [Google Scholar]
- Haldeman S., McLennan H. The antagonistic action of glutamic acid diethylester towards amino acid-induced and synaptic excitations of central neurones. Brain Res. 1972 Oct 27;45(2):393–400. doi: 10.1016/0006-8993(72)90470-2. [DOI] [PubMed] [Google Scholar]
- Herz A., Zieglgänsberger W., Färber G. Microelectrophoretic studies concerning the spread of glutamic acid and GABA in brain tissue. Exp Brain Res. 1969;9(3):221–235. doi: 10.1007/BF00234456. [DOI] [PubMed] [Google Scholar]
- Holmes O., Houchin J. Units in the cerebral cortex of the anaesthetized rat and the correlations between their discharges. J Physiol. 1966 Dec;187(3):651–671. doi: 10.1113/jphysiol.1966.sp008116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper H. H., Koyama I. Rate of release of amino acids from the cerebral cortex in the cat as affected by brainstem and thalamic stimulation. Can J Physiol Pharmacol. 1969 Oct;47(10):889–905. doi: 10.1139/y69-146. [DOI] [PubMed] [Google Scholar]
- Jasper H., Koyama I. Amino acids released from the cortical surface in cats following stimulation of the mesial thalamus and midbrain reticular formation. Electroencephalogr Clin Neurophysiol. 1968 Mar;24(3):292–292. [PubMed] [Google Scholar]
- Johnson J. L. Glutamic acid as a synaptic transmitter in the nervous system. A review. Brain Res. 1972 Feb 11;37(1):1–19. doi: 10.1016/0006-8993(72)90343-5. [DOI] [PubMed] [Google Scholar]
- KOELLE G. B. The histochemical localization of cholinesterases in the central nervous system of the rat. J Comp Neurol. 1954 Feb;100(1):211–235. doi: 10.1002/cne.901000108. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., PHILLIS J. W. Iontophoretic studies of neurones in the mammalian cerebral cortex. J Physiol. 1963 Feb;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama K., Roberts E., Kakefuda T. Association of the gamma-aminobutyric acid system with a synaptic vesicle fraction from mouse brain. Brain Res. 1968 Apr;8(1):132–152. doi: 10.1016/0006-8993(68)90176-5. [DOI] [PubMed] [Google Scholar]
- Logan W. J., Snyder S. H. Unique high affinity uptake systems for glycine, glutamic and aspartic acids in central nervous tissue of the rat. Nature. 1971 Dec 3;234(5327):297–299. doi: 10.1038/234297b0. [DOI] [PubMed] [Google Scholar]
- McLennan H., Huffman R. D., Marshall K. C. Patterns of excitation of thalamic neurones by amino-acids and by acetylcholine. Nature. 1968 Jul 27;219(5152):387–388. doi: 10.1038/219387a0. [DOI] [PubMed] [Google Scholar]
- PHILLIPS C. G. Actions of antidromic pyramidal volleys on single Betz cells in the cat. Q J Exp Physiol Cogn Med Sci. 1959 Jan;44(1):1–25. doi: 10.1113/expphysiol.1959.sp001364. [DOI] [PubMed] [Google Scholar]
- STEFANIS C., JASPER H. RECURRENT COLLATERAL INHIBITION IN PYRAMIDAL TRACT NEURONS. J Neurophysiol. 1964 Sep;27:855–877. doi: 10.1152/jn.1964.27.5.855. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Cholinergic mechanisms in the rat somatosensory cerebral cortex. J Physiol. 1972 Sep;225(2):485–499. doi: 10.1113/jphysiol.1972.sp009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T. W. Cortical responses to pyramidal tract stimulation in the rat. Exp Neurol. 1972 Jun;35(3):492–502. doi: 10.1016/0014-4886(72)90119-7. [DOI] [PubMed] [Google Scholar]



