Abstract
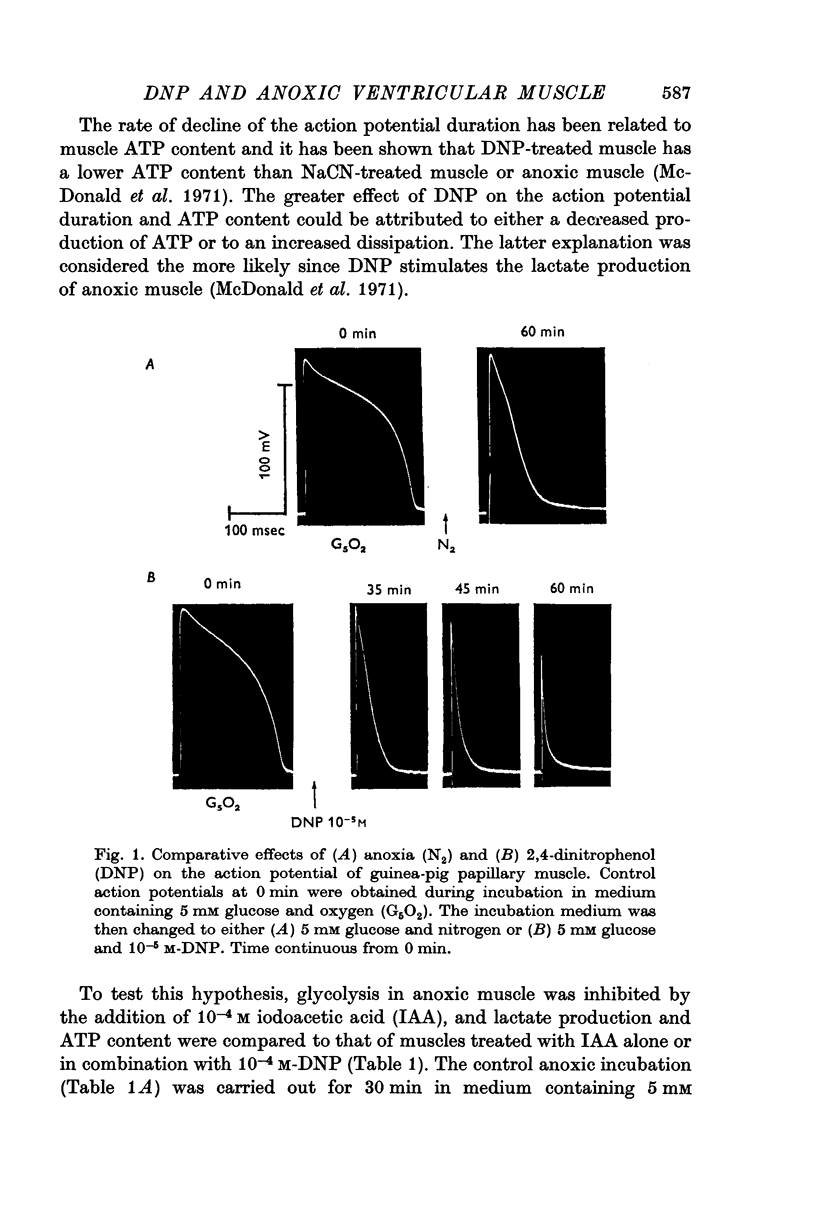
1. During aerobic incubation in 5 mM glucose medium, 10-5 M-DNP reduced the action potential duration and amplitude and the developed tension of guinea-pig ventricular muscle more rapidly and to a greater extent than anoxia.
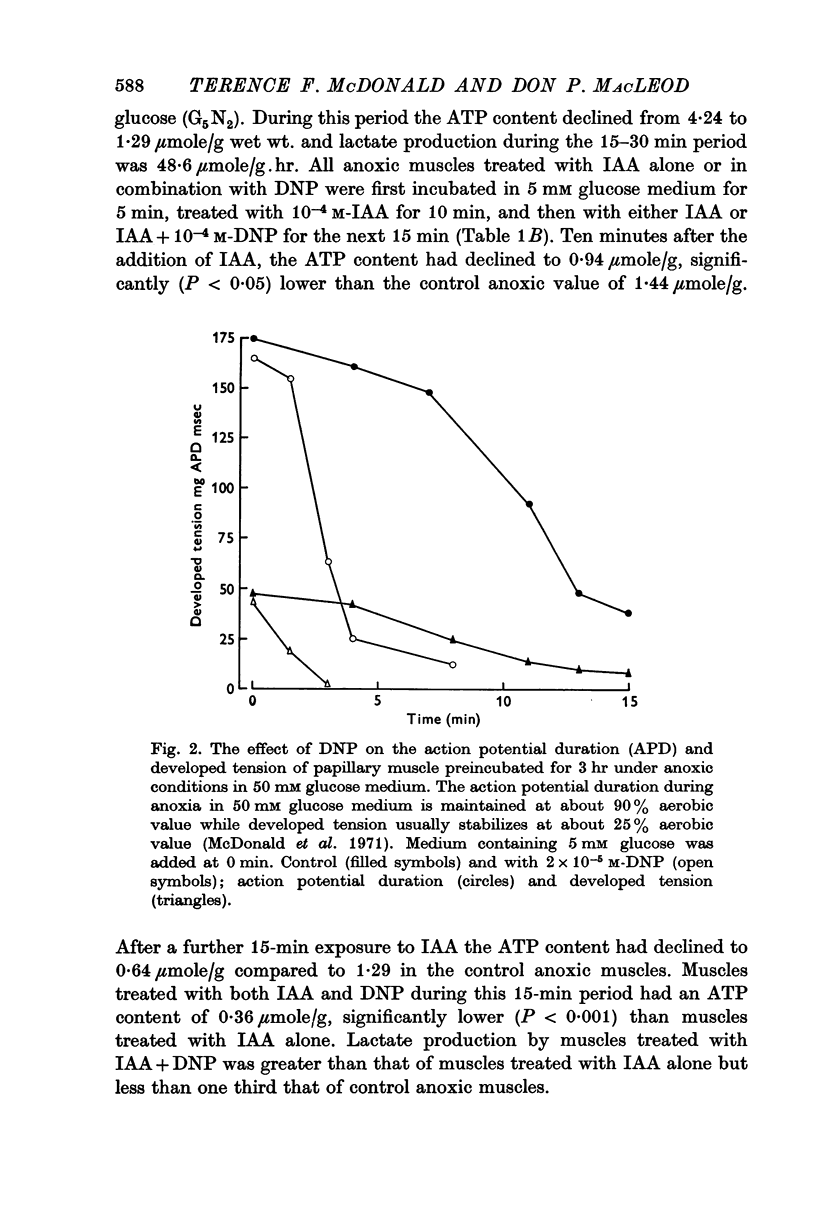
2. The DNP effect on electrical and mechanical activity was even more pronounced following prolonged anoxic incubation. Since the action potential duration and developed tension of anoxic ventricular muscle have previously been shown to be dependent on glycolytic ATP, and since the effects of DNP could not be duplicated with NaCN, it was concluded that DNP was exerting an effect in addition to its uncoupling of oxidative phosphorylation.
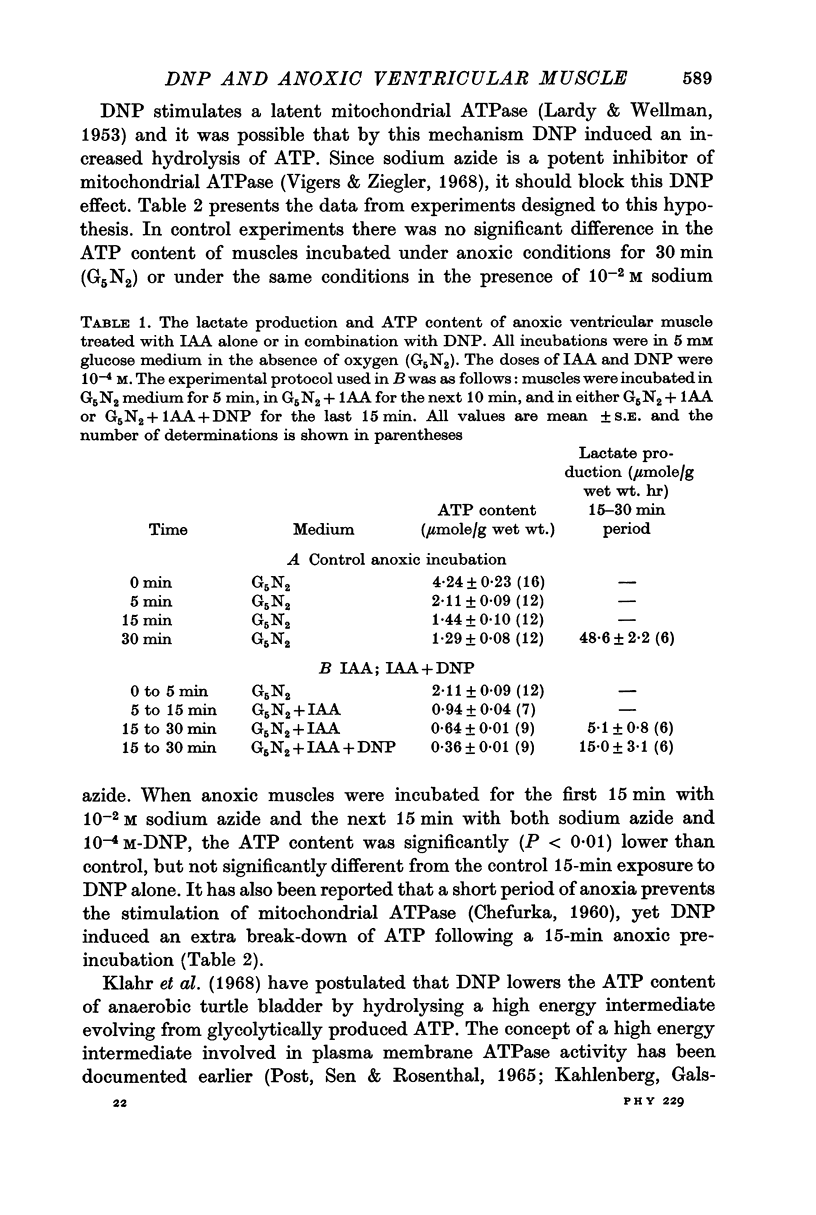
3. Anoxic muscle was incubated with 10-4 M-IAA or with 10-4 M-IAA + 10-4 M-DNP. The ATP content of IAA-treated muscle was significantly lower than control but in the presence of both IAA and DNP there was a further reduction in ATP and an increased lactate production.
4. Sodium azide (10-2 M), a potent inhibitor of mitochondrial ATPase, did not prevent the reduction of ATP in DNP-treated anoxic muscle.
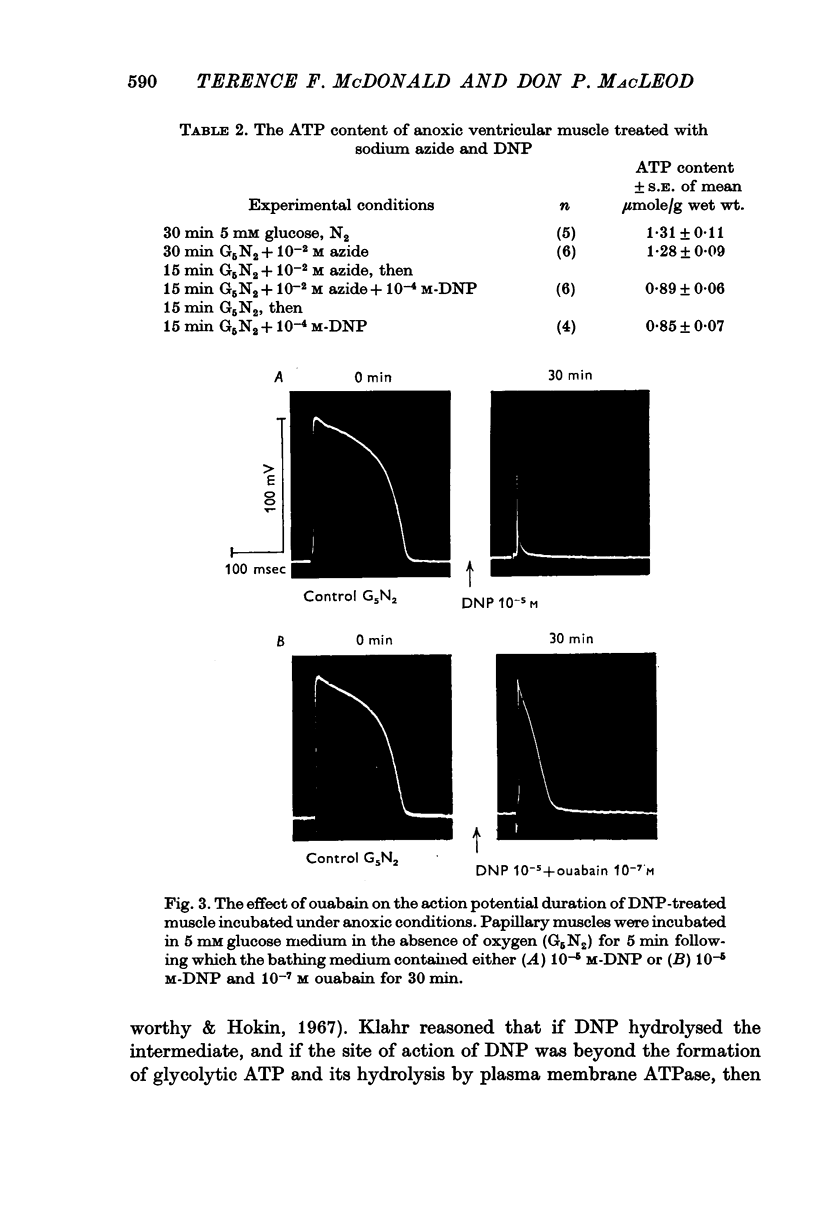
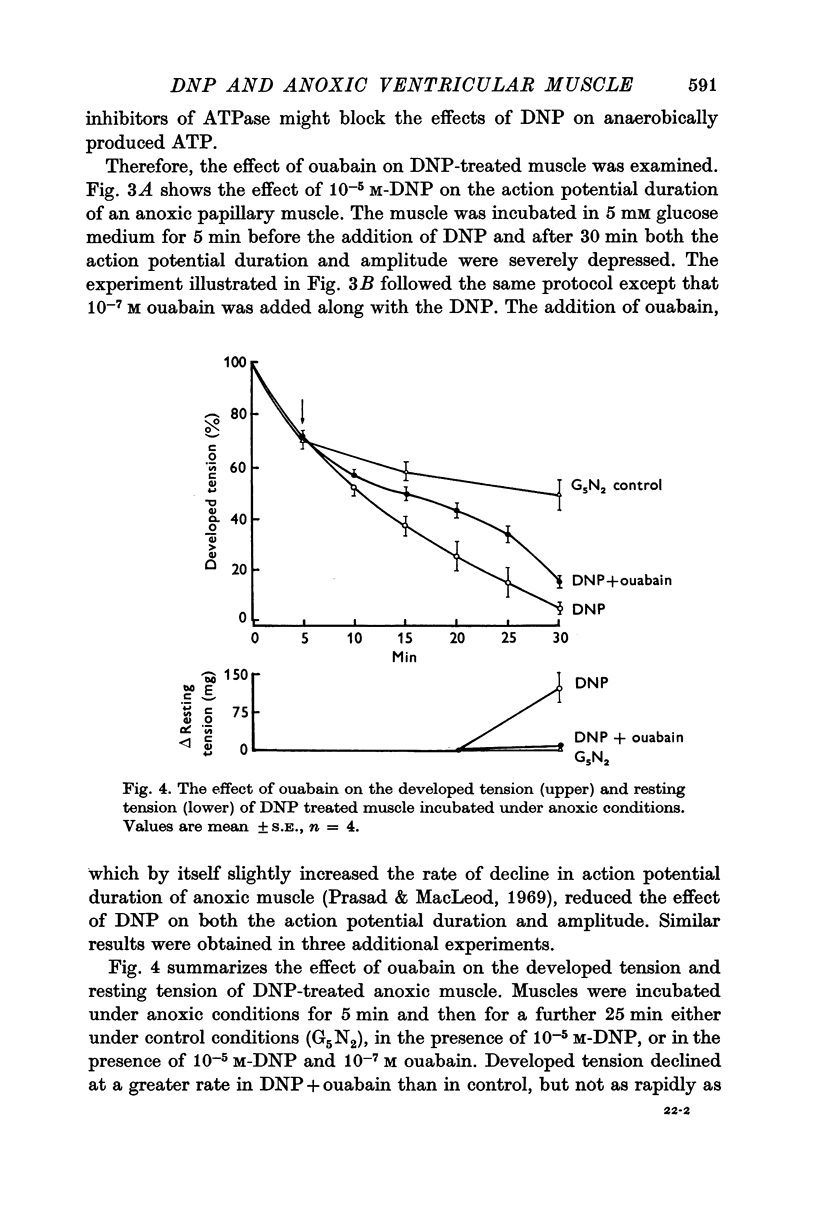
5. Ouabain (10-7 M) partially prevented the rapid decline of action potential duration and developed tension of DNP-treated anoxic muscle. In addition, the glycoside partially blocked the DNP-induced break-down of ATP and stimulation of lactate production.
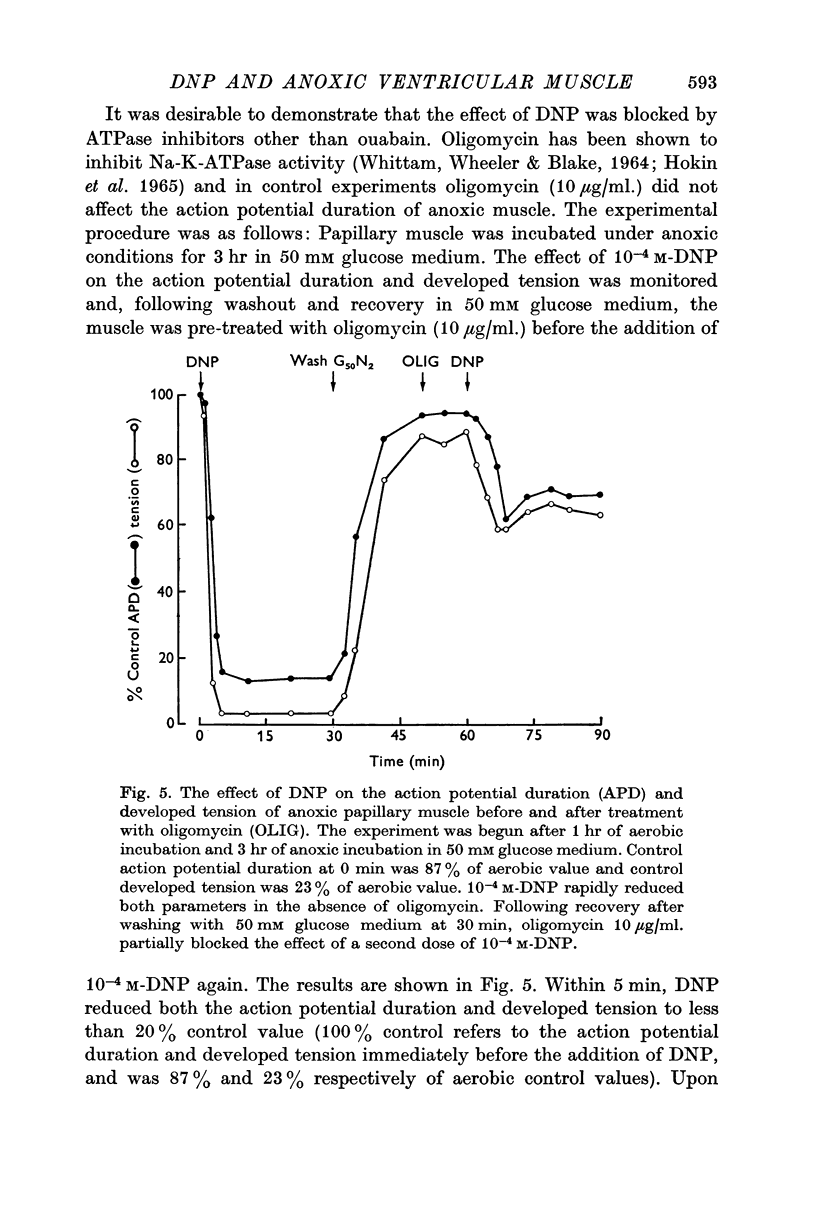
6. Oligomycin (10 μg/ml.) partially prevented the reduction in action potential duration and developed tension of DNP-treated anoxic muscle.
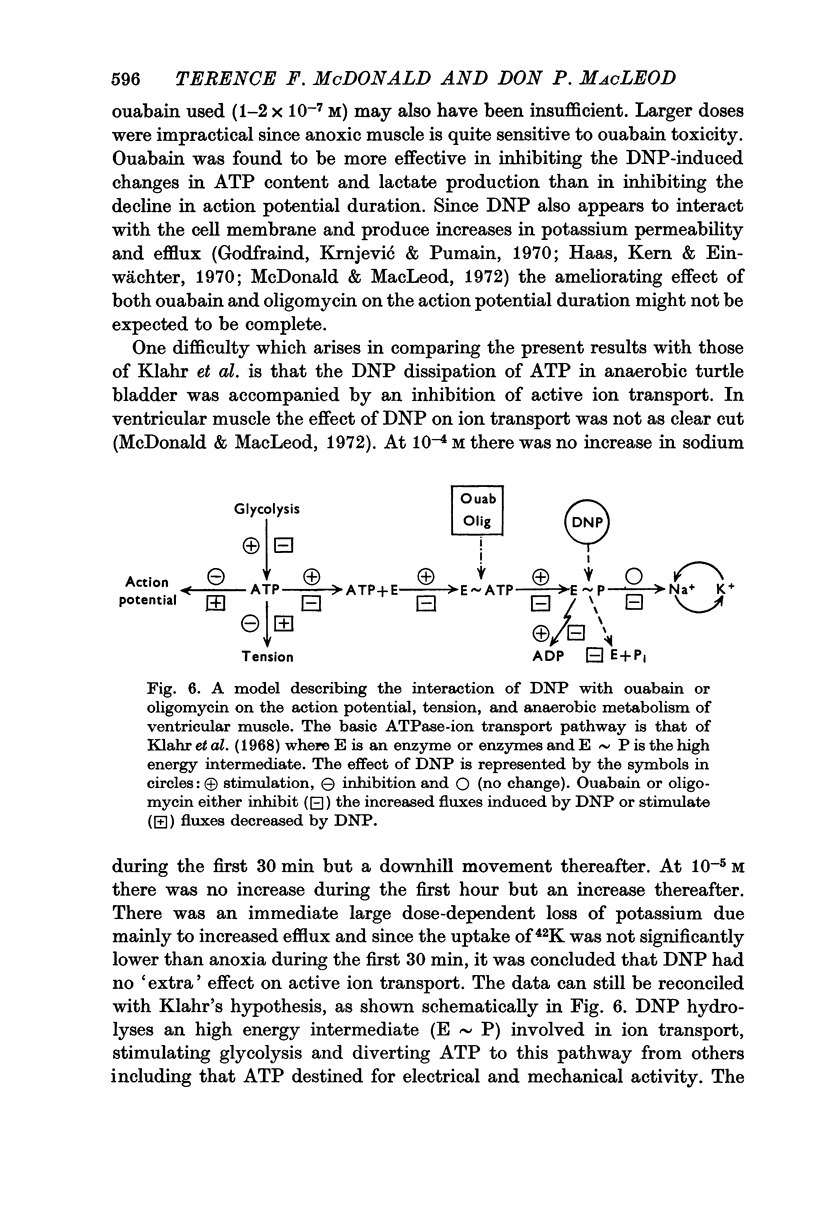
7. It was concluded that DNP induces an `energy leak' by actively promoting the hydrolysis of an high energy glycolytic intermediate at least one step beyond the sites of ATPase inhibition by ouabain and oligomycin.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bricker N. S., Klahr S. Effects of dinitrophenol and oligomycin on the coupling between anaerobic metabolism and anaerobic sodium transport by the isolated turtle bladder. J Gen Physiol. 1966 Jan;49(3):483–499. doi: 10.1085/jgp.49.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEFURKA W. Studies on the inhibition of the mitochondrial ATP-ase by reduction of the respiratory chain. Can J Biochem Physiol. 1960 Oct;38:1195–1214. [PubMed] [Google Scholar]
- ERNSTER L., LEE C. P. BIOLOGICAL OXIDOREDUCTIONS. Annu Rev Biochem. 1964;33:729–790. doi: 10.1146/annurev.bi.33.070164.003501. [DOI] [PubMed] [Google Scholar]
- GLYNN I. M. TRANSPORT ADENOSINETRIPHOSPHATASE' IN ELECTRIC ORGAN. THE RELATION BETWEEN ION TRANSPORT AND OXIDATIVE PHOSPHORYLATION. J Physiol. 1963 Nov;169:452–465. doi: 10.1113/jphysiol.1963.sp007272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREVILLE G. D., REICH E. Effects of 2:4-dinitrophenol and other agents on the nucleoside triphosphatase activities of L-myosin. Biochim Biophys Acta. 1956 May;20(2):440–442. doi: 10.1016/0006-3002(56)90334-1. [DOI] [PubMed] [Google Scholar]
- Godfraind J. M., Krnjević K., Pumain R. Unexpected features of the action of dinitrophenol on cortical neurones. Nature. 1970 Nov 7;228(5271):562–564. doi: 10.1038/228562a0. [DOI] [PubMed] [Google Scholar]
- HARARY I., SLATER E. C. STUDIES IN VITRO ON SINGLE BEATING HEART CELLS. 8. THE EFFECT OF OLIGOMYCIN, DINITROPHENOL, AND OUABAIN ON THE BEATING RATE. Biochim Biophys Acta. 1965 May 18;99:227–233. doi: 10.1016/s0926-6593(65)80119-9. [DOI] [PubMed] [Google Scholar]
- HOFFMAN J. F. Cation transport and structure of the red-cell plasma membrane. Circulation. 1962 Nov;26:1202–1213. doi: 10.1161/01.cir.26.5.1201. [DOI] [PubMed] [Google Scholar]
- HOLLANDER P. B., WEBB J. L. Metabolic aspects of the relationship between the contractility and membrane potentials of the rat atrium. Circ Res. 1956 Sep;4(5):618–626. doi: 10.1161/01.res.4.5.618. [DOI] [PubMed] [Google Scholar]
- Hokin L. E., Sastry P. S., Galsworthy P. R., Yoda A. Evidence that a phosphorylated intermediate in a brain transport adenosine triphosphatase is an acyl phosphate. Proc Natl Acad Sci U S A. 1965 Jul;54(1):177–184. doi: 10.1073/pnas.54.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlenberg A., Galsworthy P. R., Hokin L. E. Sodium-potassium adenosine triphosphatase: acyl phosphate "intermediate" shown to be L-glutamyl-gamma-phosphate. Science. 1967 Jul 28;157(3787):434–436. doi: 10.1126/science.157.3787.434. [DOI] [PubMed] [Google Scholar]
- Klahr S., Bourgoignie J., Bricker N. S. Coupling of anaerobic metabolism to anaerobic sodium transport: a high energy intermediate. Nature. 1968 May 25;218(5143):769–770. doi: 10.1038/218769a0. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., WELLMAN H. The catalytic effect of 2,4-dinitrophenol on adenosinetriphosphate hydrolysis by cell particles and soluble enzymes. J Biol Chem. 1953 Mar;201(1):357–370. [PubMed] [Google Scholar]
- Laris P. C., Letchworth P. E. Characteristics of an adenosine triphosphatase in erythrocyte membranes stimulated by 2,4-dinitrophenol. J Cell Physiol. 1967 Apr;69(2):143–149. doi: 10.1002/jcp.1040690204. [DOI] [PubMed] [Google Scholar]
- MACFARLANE W. V. The plateau of the action potential of the frog ventricle. Circ Res. 1960 Jan;8:47–56. doi: 10.1161/01.res.8.1.47. [DOI] [PubMed] [Google Scholar]
- MacLeod D. P., Prasad K. Influence of glucose on the transmembrane action potential of papillary muscle. Effects of concentration, phlorizin and insulin, nonmetabolizable sugars, and stimulators of glycolysis. J Gen Physiol. 1969 Jun;53(6):792–815. doi: 10.1085/jgp.53.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Hunter E. G., MacLeod D. P. Adenosinetriphosphate partition in cardiac muscle with respect to transmembrane electrical activity. Pflugers Arch. 1971;322(2):95–108. doi: 10.1007/BF00592292. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., MacLeod D. P. Anoxia-recovery cycle in ventricular muscle: action potential duration, contractility and ATP content. Pflugers Arch. 1971;325(4):305–322. doi: 10.1007/BF00592172. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., MacLeod D. P. The effect of 2,4-dinitrophenol on electrical and mechanical activity, metabolism and ion movements in guinea-pig ventricular muscle. Br J Pharmacol. 1972 Apr;44(4):711–722. doi: 10.1111/j.1476-5381.1972.tb07309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POST R. L., SEN A. K., ROSENTHAL A. S. A PHOSPHORYLATED INTERMEDIATE IN ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT ACROSS KIDNEY MEMBRANES. J Biol Chem. 1965 Mar;240:1437–1445. [PubMed] [Google Scholar]
- Prasad K., MacLeod D. P. Influence of glucose on the transmembrane action potential of guinea-pig papillary muscle. Metabolic inhibitors, ouabain, and calcium chloride, and their interaction with glucose, sympathomimetic amines, and aminophylline. Circ Res. 1969 Jun;24(6):939–950. doi: 10.1161/01.res.24.6.939. [DOI] [PubMed] [Google Scholar]
- Riemersma J. C. Effects of sodium azide and 2,4-dinitrophenol on phosphorylation reactions and ion fluxes in Saccharomyces cerevisiae. Biochim Biophys Acta. 1968 Jan 15;153(1):80–87. doi: 10.1016/0005-2728(68)90148-5. [DOI] [PubMed] [Google Scholar]
- Robinson J. D. Effects of oligomycin on the (Na + + K + )-dependent adenosine triphosphatase. Mol Pharmacol. 1971 May;7(3):238–246. [PubMed] [Google Scholar]
- VAN GRONINGENH, SLATER E. C. THE EFFECT OF OLIGOMYCIN ON THE (NA+ + K+)-ACTIVATED MAGNESIUM ATPASE OF BRAIN MICROSOMES AND ERYTHROCYTE MEMBRANE. Biochim Biophys Acta. 1963 Jul 9;73:527–530. doi: 10.1016/0006-3002(63)90460-8. [DOI] [PubMed] [Google Scholar]
- Vigers G. A., Ziegler F. D. Azide inhibition of mitochondrial ATPase. Biochem Biophys Res Commun. 1968 Jan 11;30(1):83–88. doi: 10.1016/0006-291x(68)90716-x. [DOI] [PubMed] [Google Scholar]
- WHITTAM R., WHEELER K. P., BLAKE A. OLIGOMYCIN AND ACTIVE TRANSPORT REACTIONS IN CELL MEMBRANES. Nature. 1964 Aug 15;203:720–724. doi: 10.1038/203720a0. [DOI] [PubMed] [Google Scholar]
- WOODBURY J. W., BRADY A. J. Intracellular recording from moving tissues with a flexibly mounted ultramicroelectrode. Science. 1956 Jan 20;123(3186):100–101. doi: 10.1126/science.123.3186.100-a. [DOI] [PubMed] [Google Scholar]
- Wins P., Schoffeniels E. Studies on red-cell ghost ATPase systems: properties of a (Mg2+ + Ca2+)-dependent ATPase. Biochim Biophys Acta. 1966 Jul 13;120(3):341–350. doi: 10.1016/0926-6585(66)90301-3. [DOI] [PubMed] [Google Scholar]


