Abstract
1. The impulse/quantum (I/Q) ratio was measured as a function of background illumination for rod-dominated, pure central, linear square-wave responses of retinal ganglion cells in the cat.
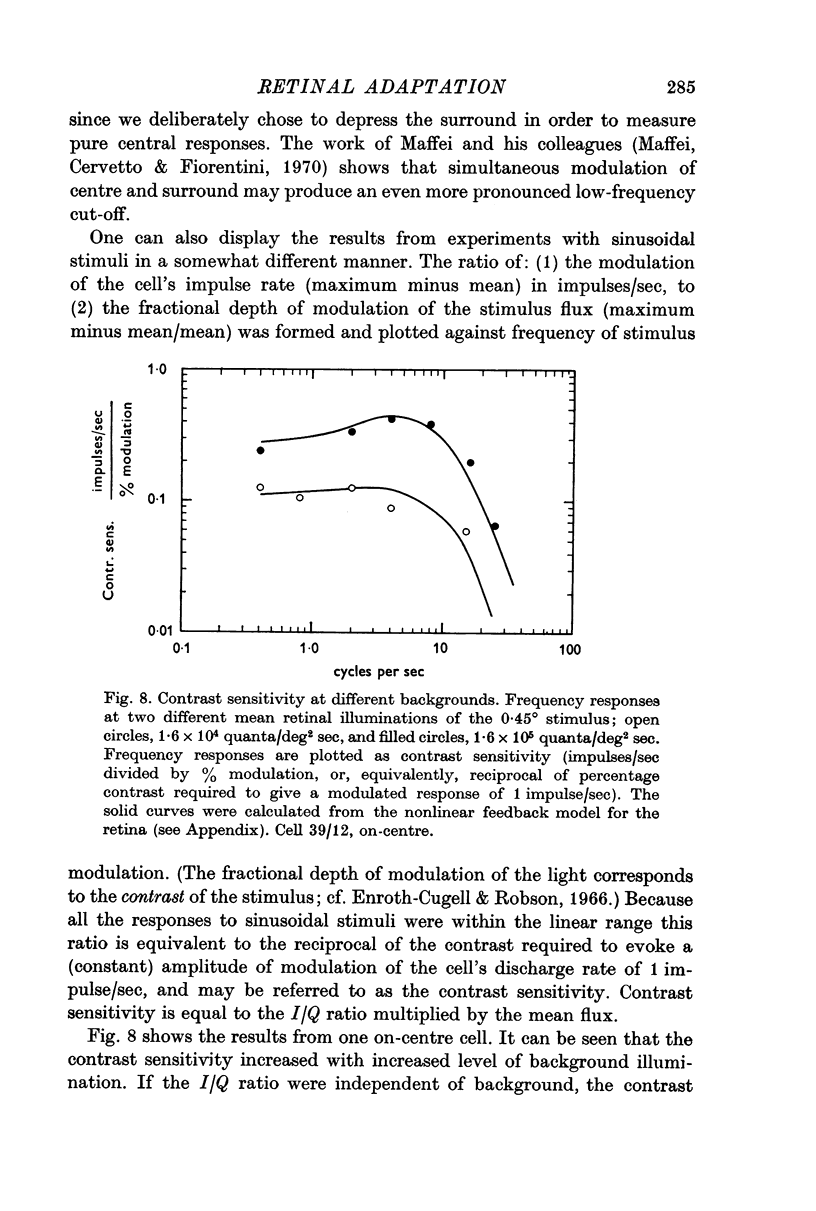
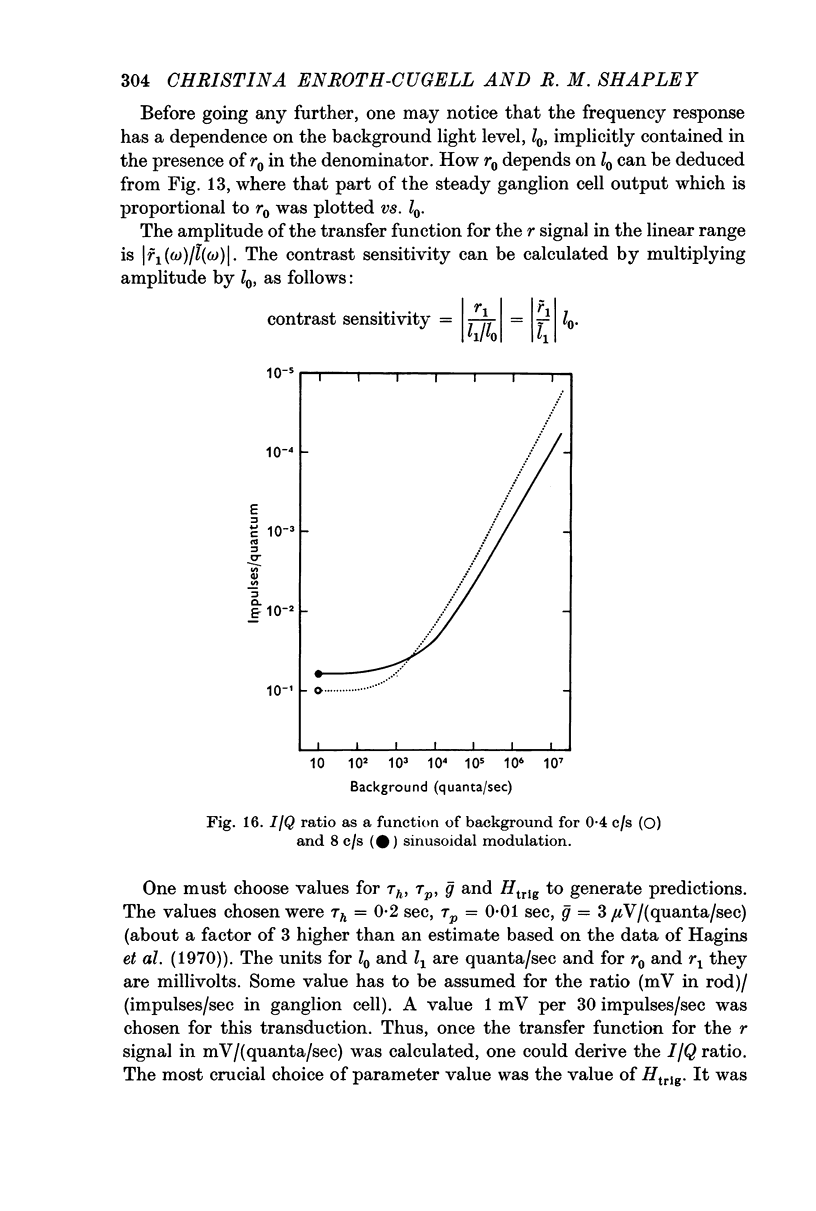
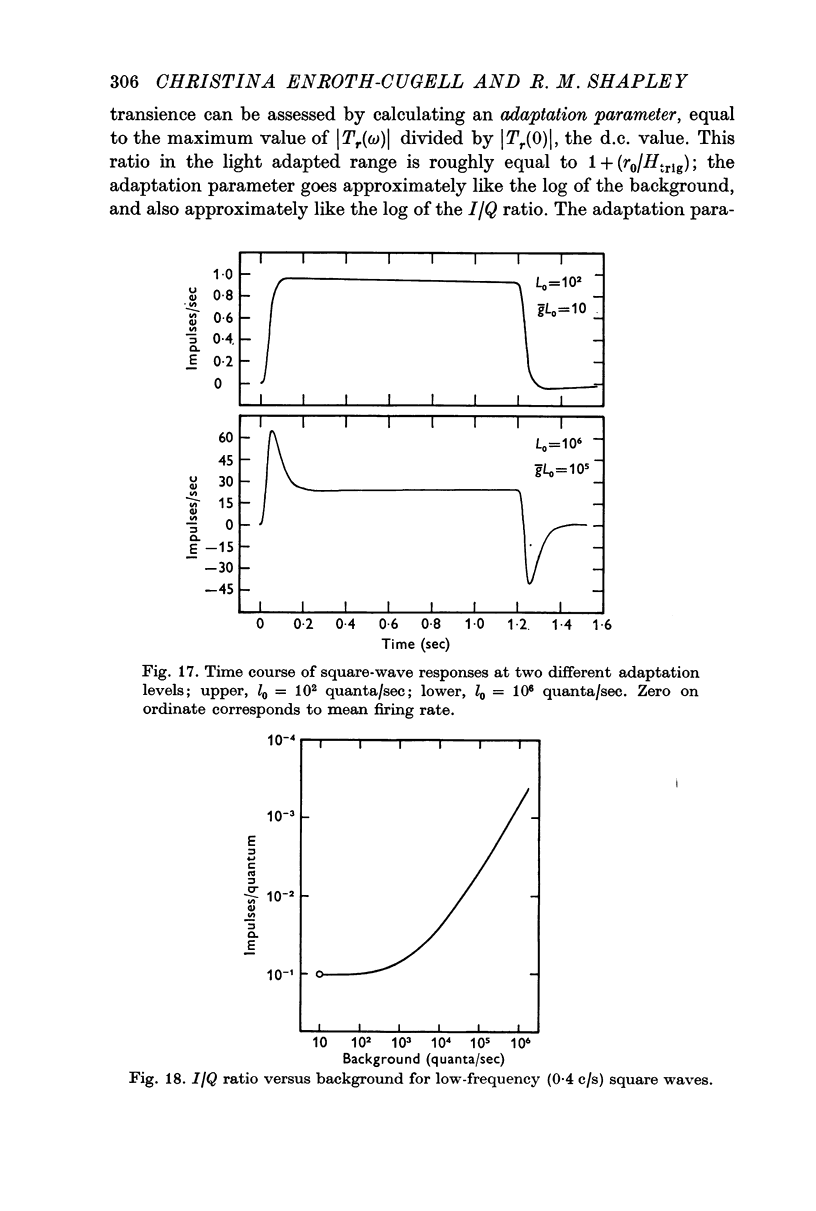
2. The I/Q ratio was constant at low backgrounds (dark adapted state) and inversely proportional to the 0·9 power of the background at high backgrounds (the light adapted state). There was an abrupt transition from the dark-adapted state to the light-adapted state.
3. It was possible to define the adaptation level at a particular background as the ratio (I/Q ratio at that background)/(dark adapted I/Q ratio).
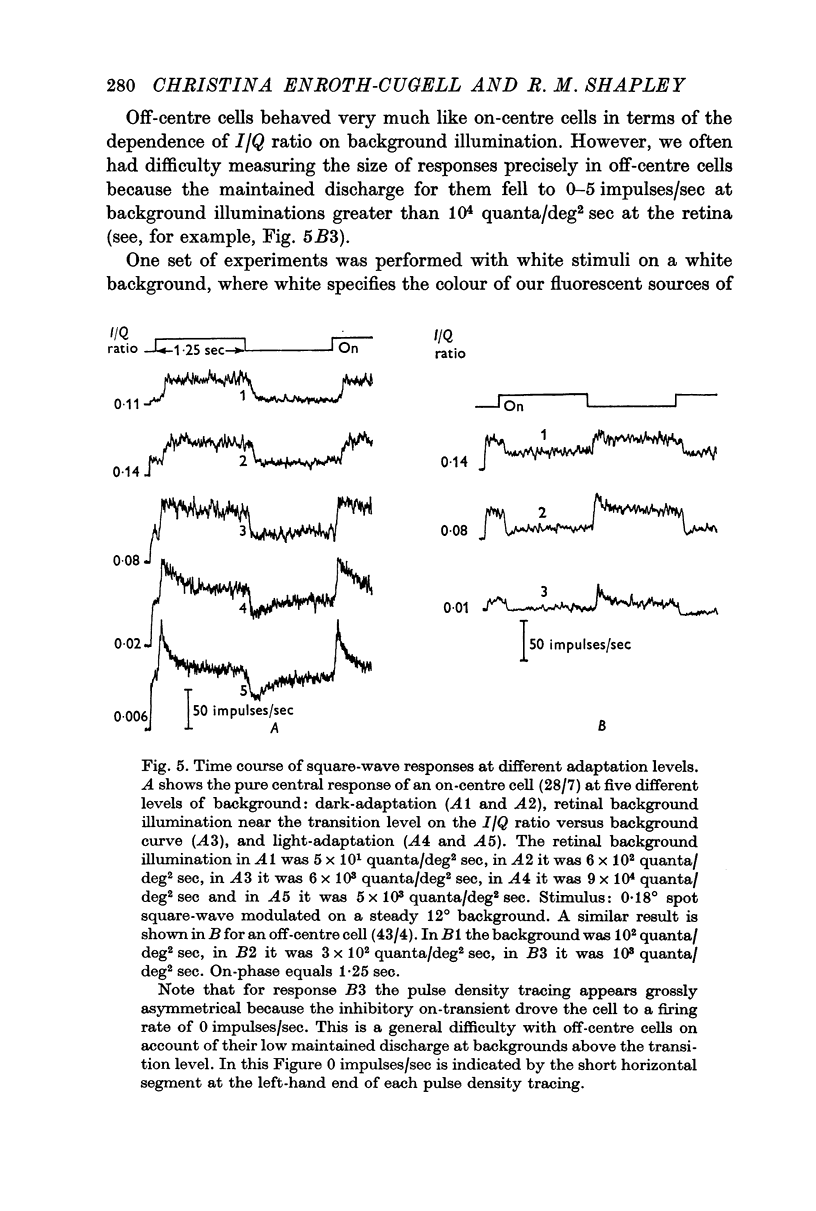
4. The time course of the square-wave response was correlated with the adaptation level. The response was sustained in the dark-adapted state, partially transient at the transition level, and progressively more transient the lower the impulse/quantum ratio of the ganglion cell became. This was true both for on-centre and off-centre cells.
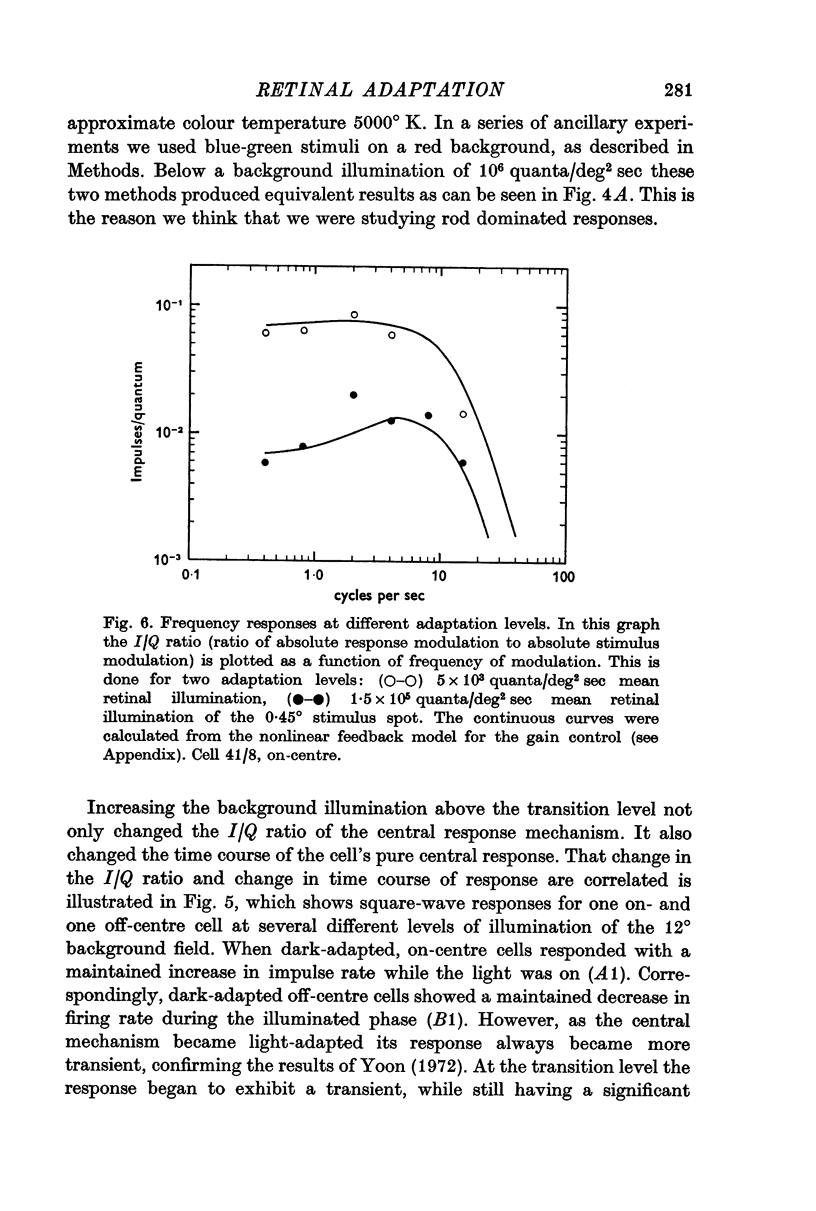
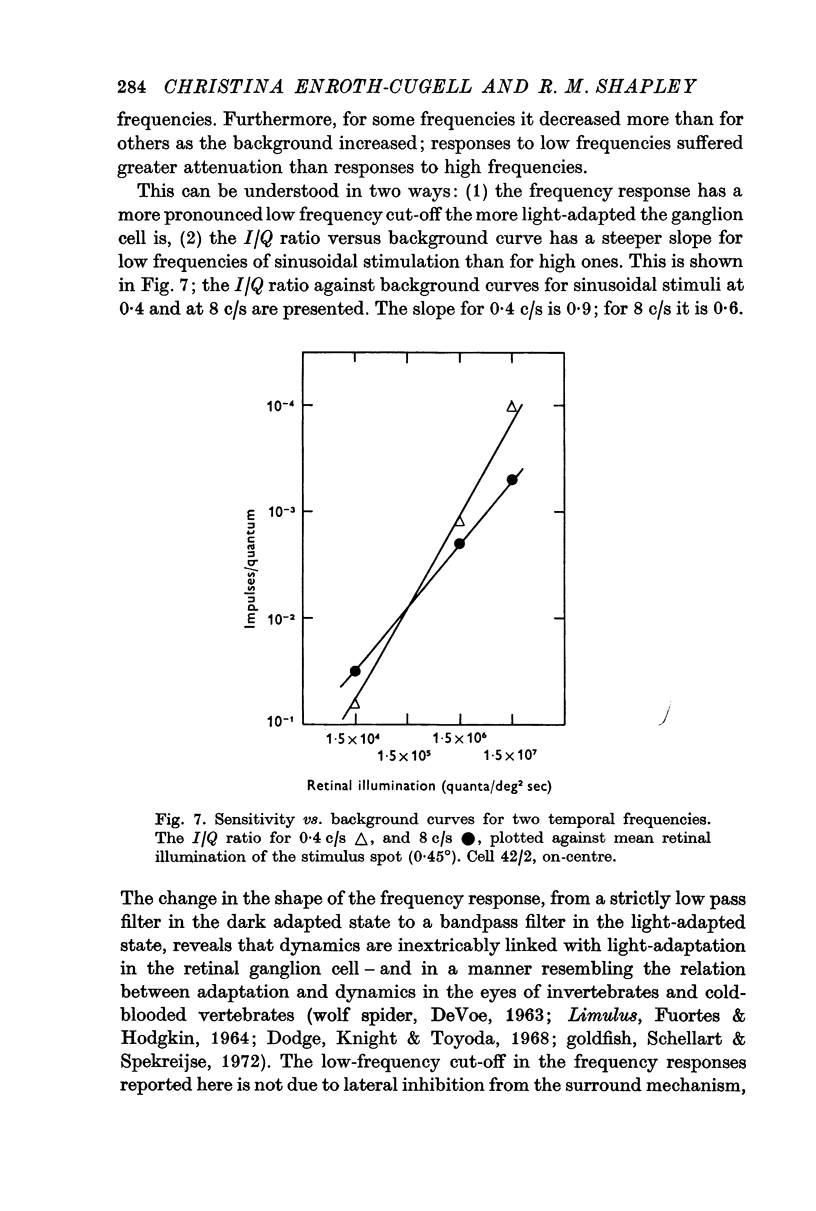
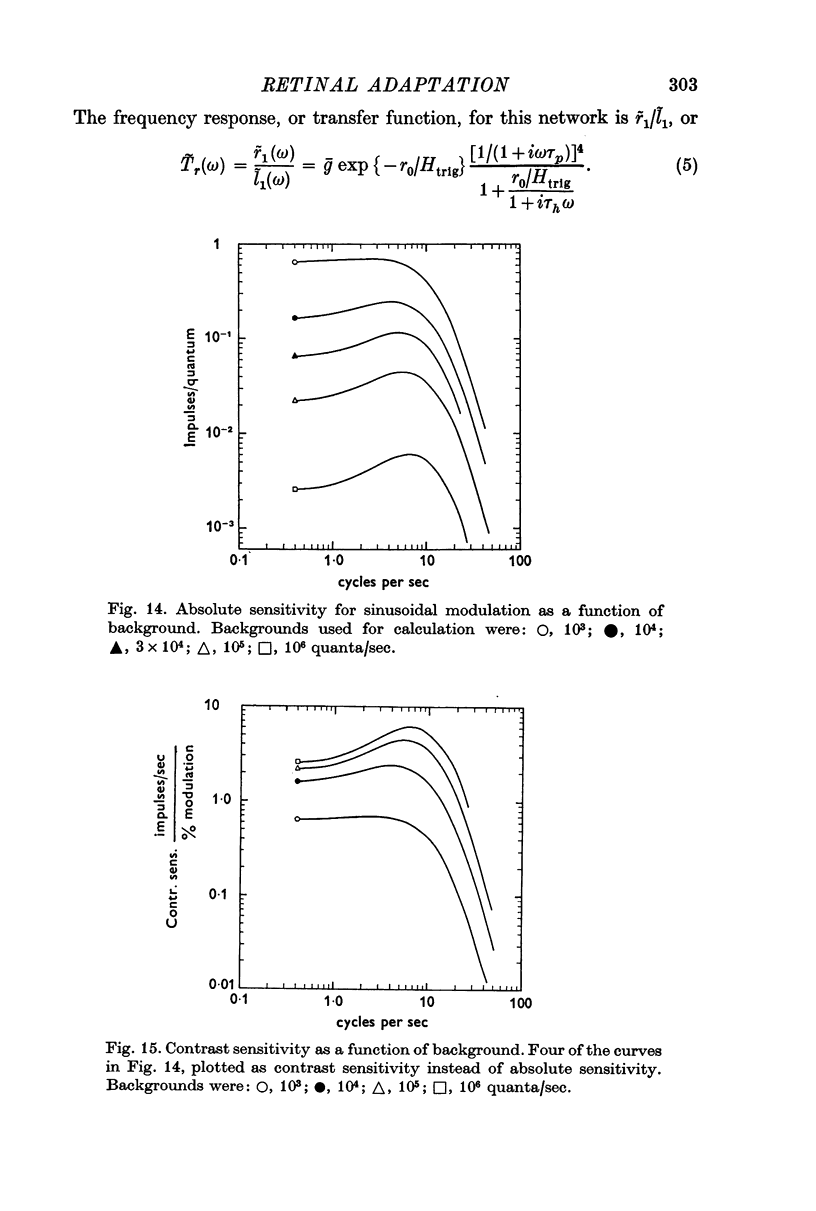
5. The frequency response of the central response mechanism at different adaptation levels was measured. It was a low-pass characteristic in the dark-adapted state and became progressively more of a bandpass characteristic as the cell became more light-adapted.
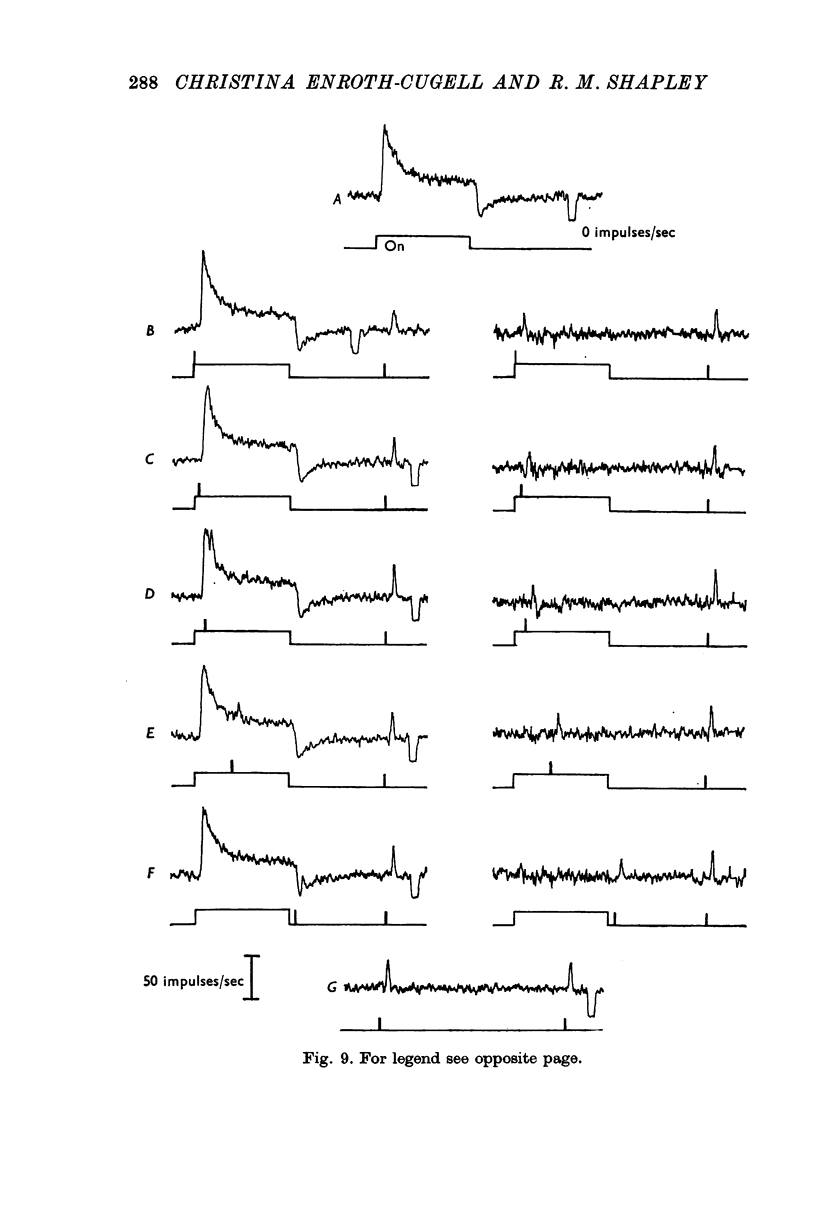
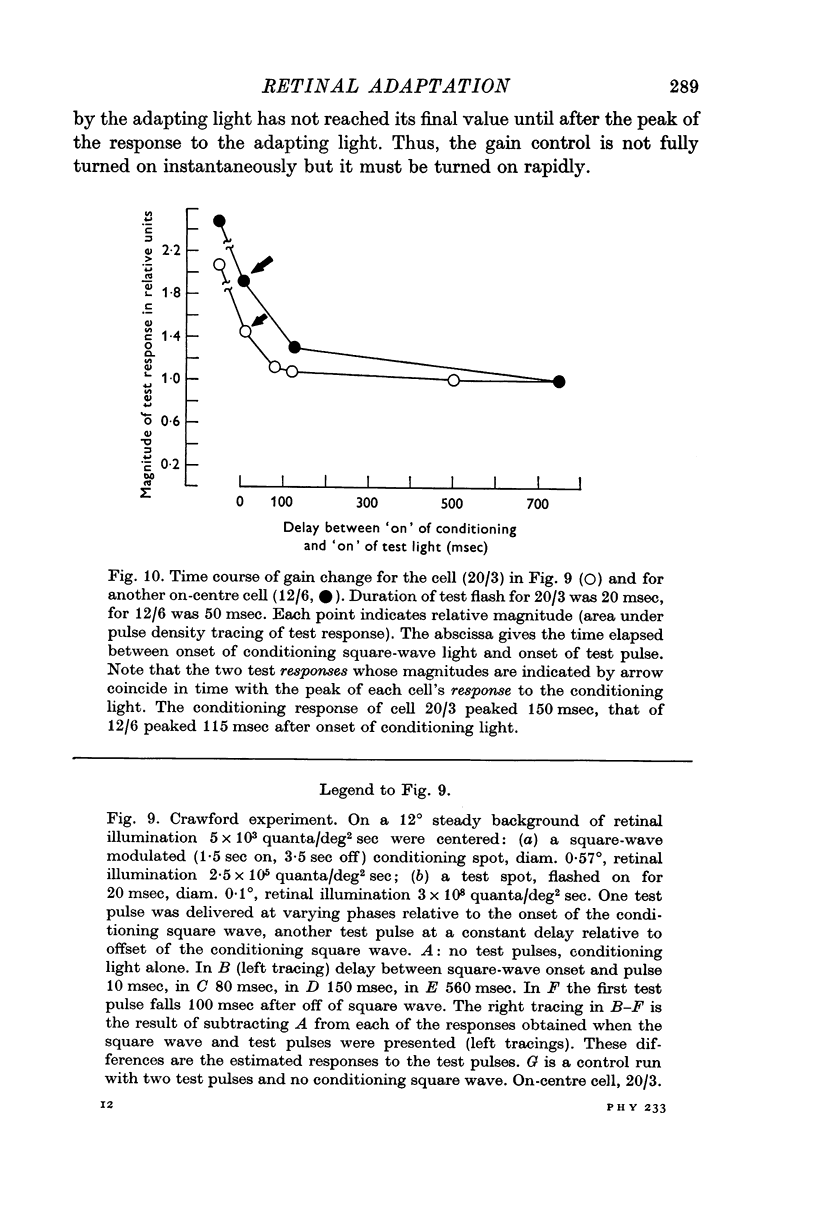
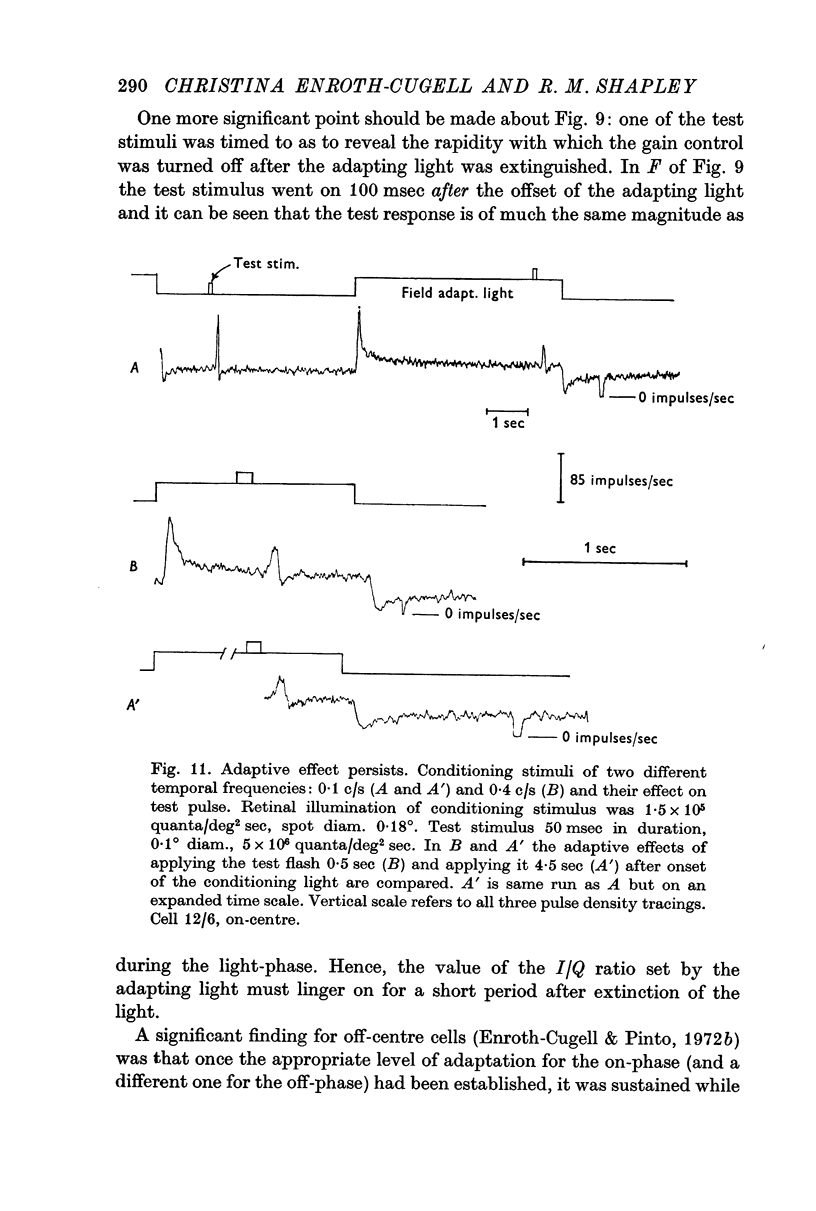
6. The rapidity of onset of adaptation was measured with a time-varying adapting light. The impulse/quantum ratio is reset within 100 msec of the onset of the conditioning light, and is kept at the new value throughout the time the conditioning light is on.
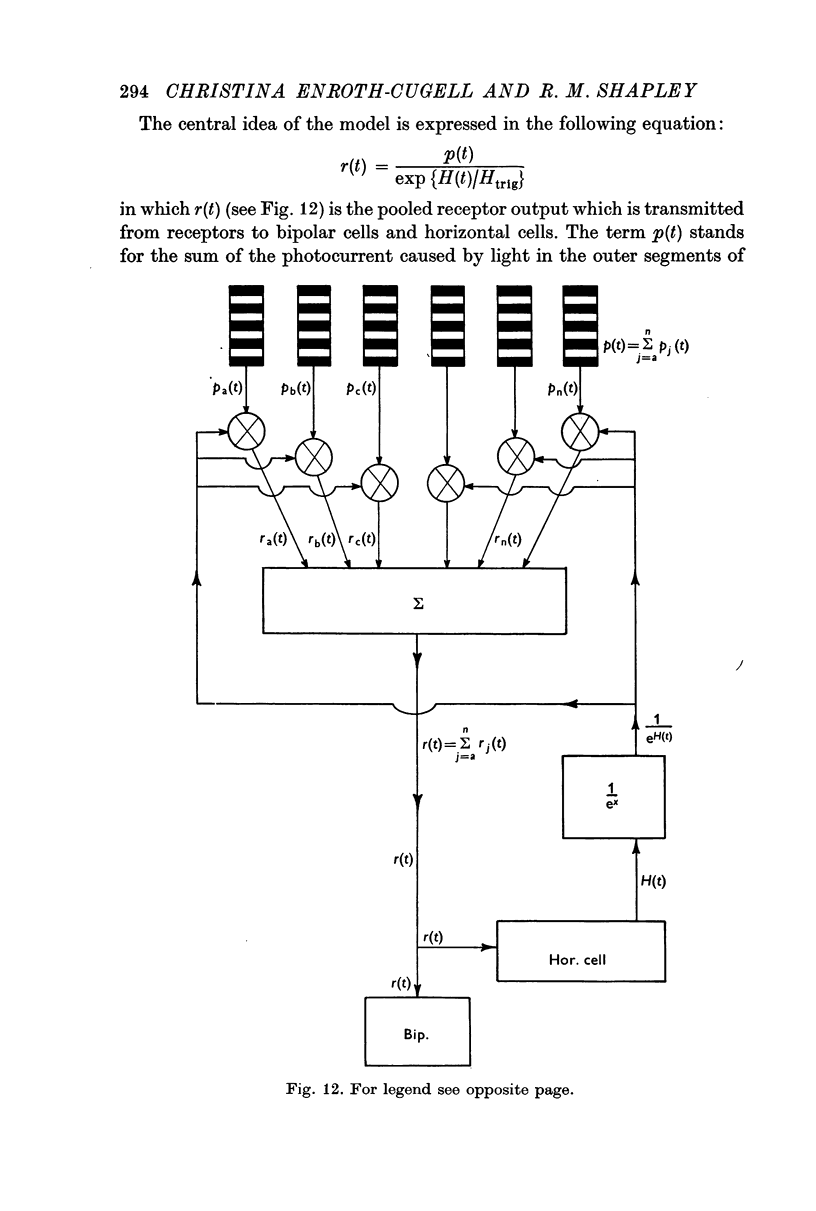
7. These results can be explained by a nonlinear feedback model. In the model, it is postulated that the exponential function of the horizontal cell potential controls transmission from rods to bipolars. This model has an abrupt transition from dark- to light-adapted states, and its response dynamics are correlated with adaptation level.
Full text
PDF

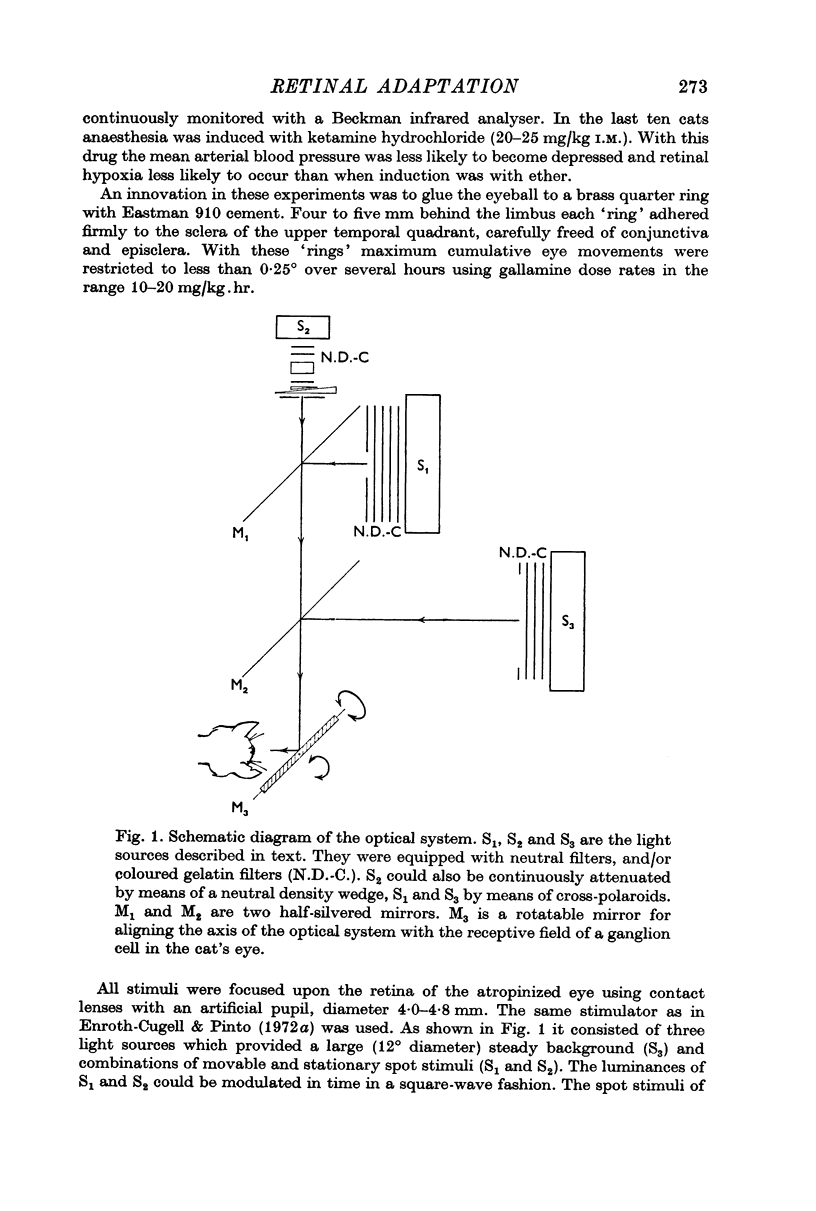
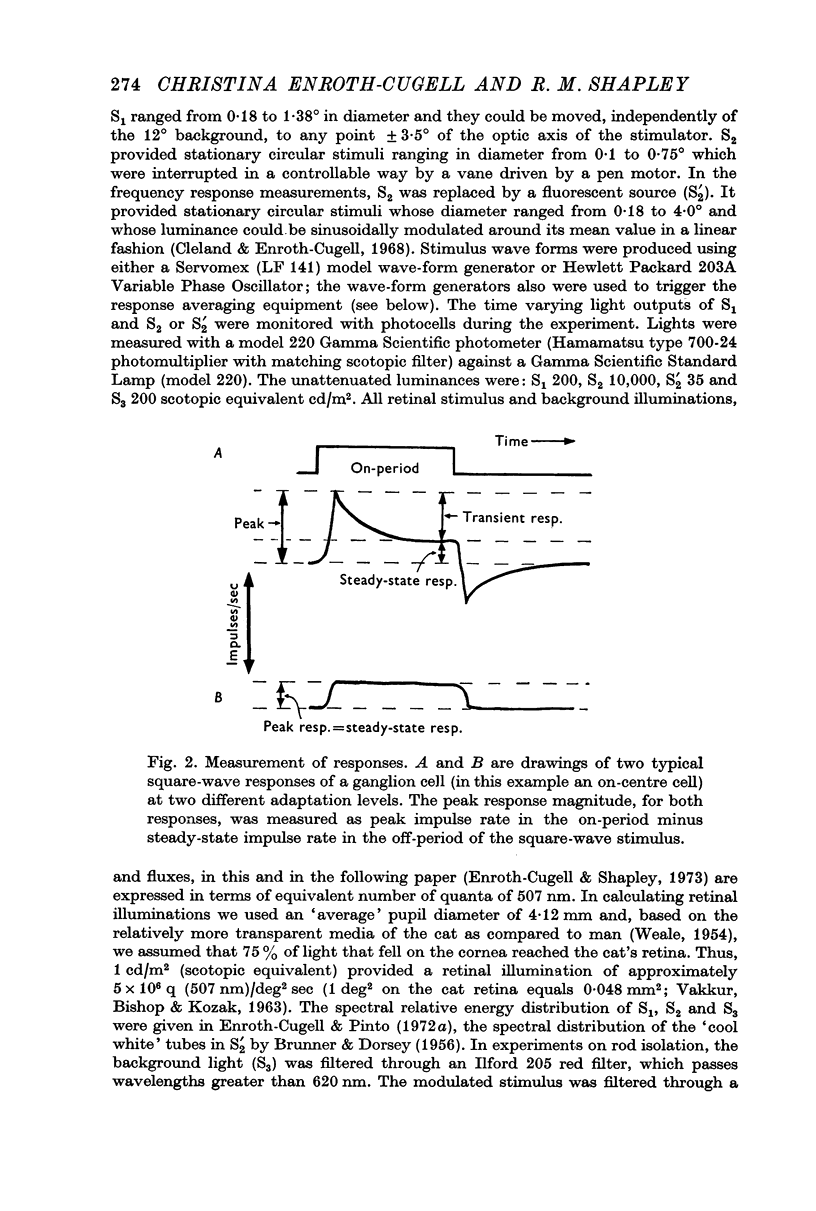

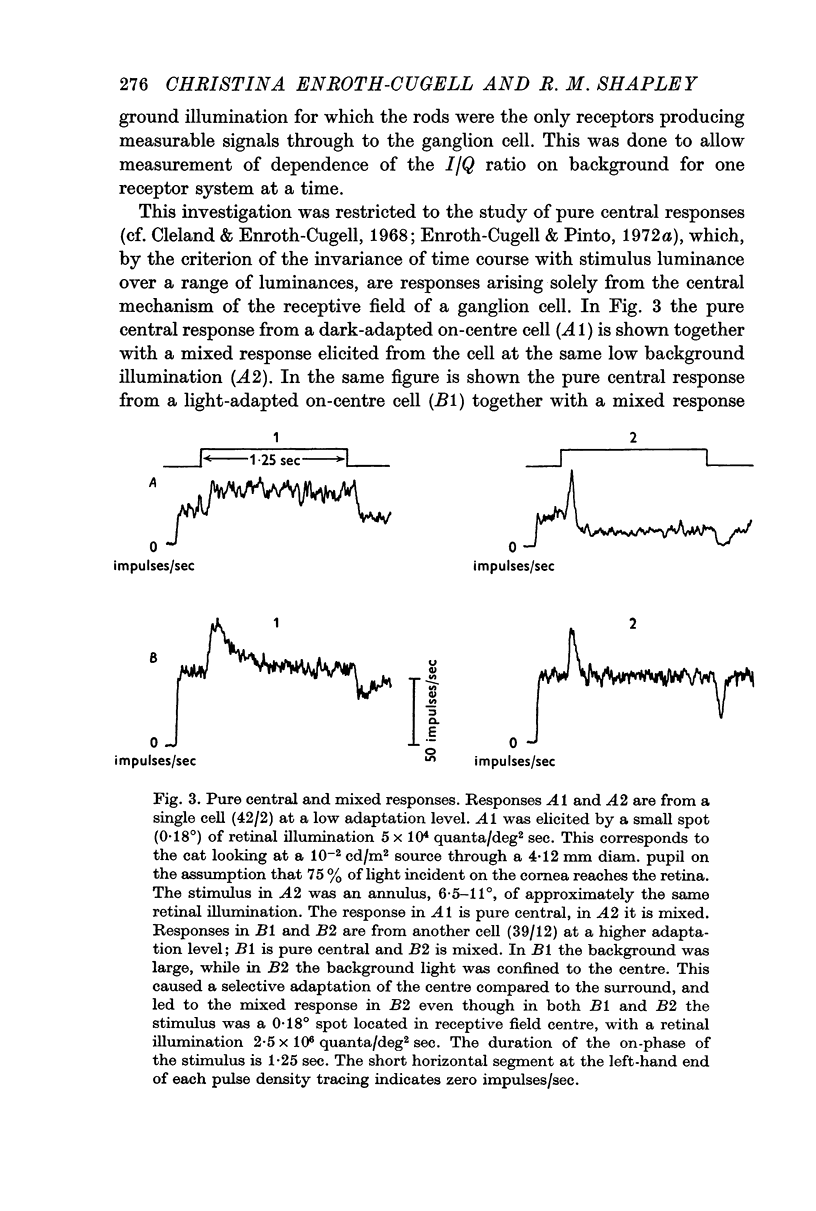

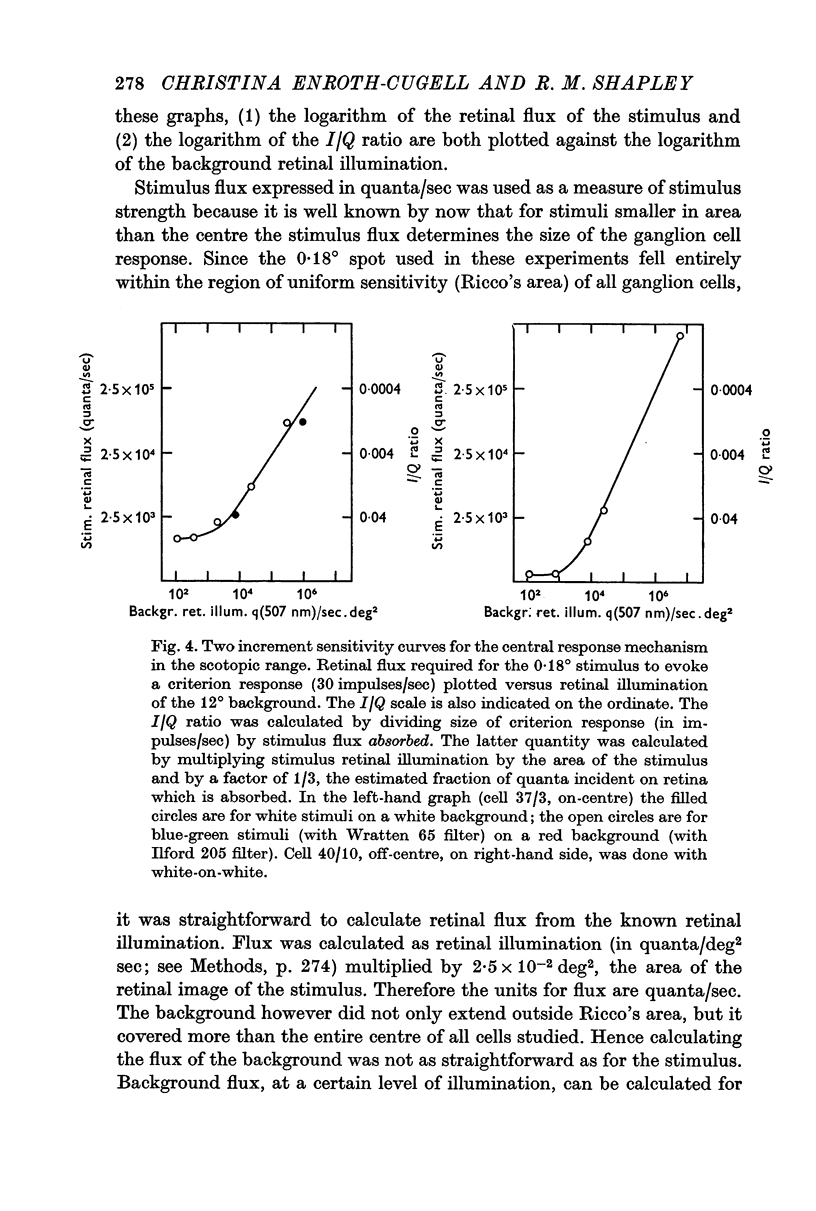













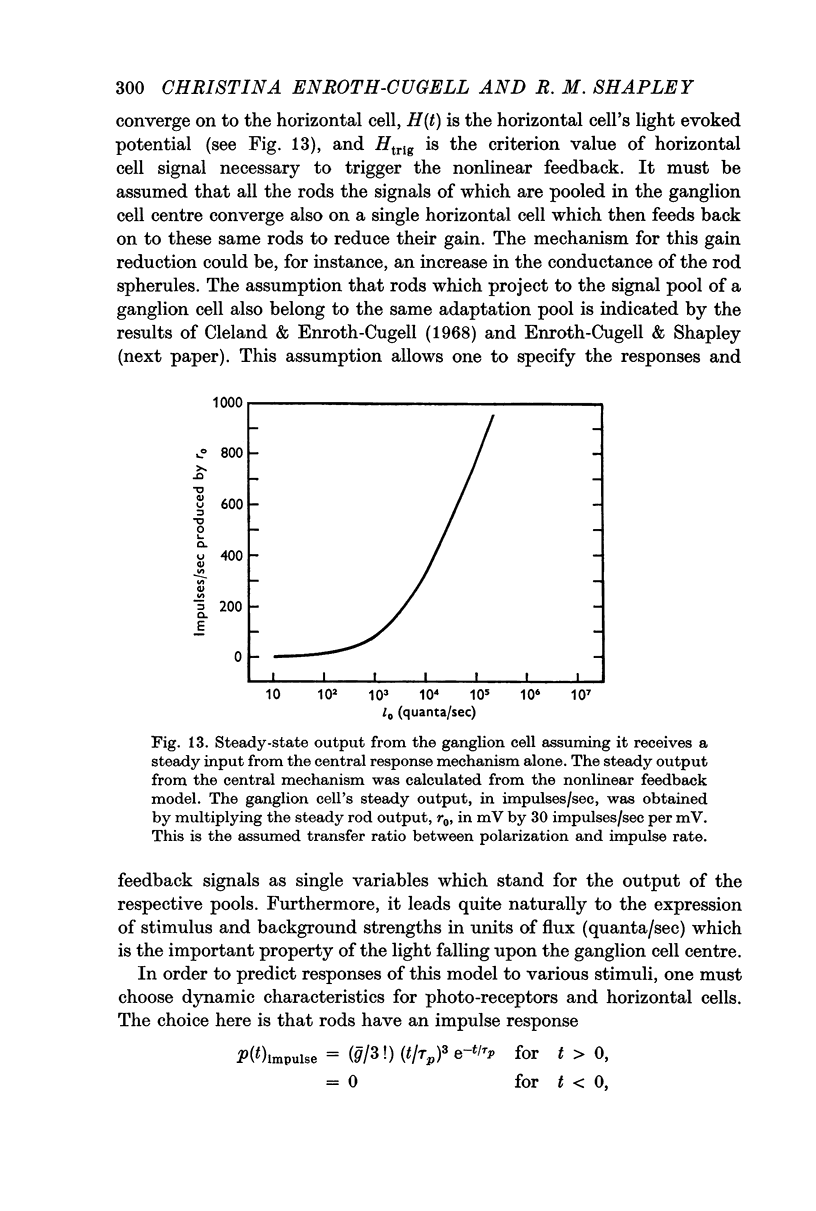






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Increment thresholds at low intensities considered as signal/noise discriminations. J Physiol. 1957 May 23;136(3):469–488. doi: 10.1113/jphysiol.1957.sp005774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Three factors limiting the reliable detection of light by retinal ganglion cells of the cat. J Physiol. 1969 Jan;200(1):1–24. doi: 10.1113/jphysiol.1969.sp008679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R., Yoon M. Responses to single quanta of light in retinal ganglion cells of the cat. Vision Res. 1971;Suppl 3:87–101. doi: 10.1016/0042-6989(71)90033-2. [DOI] [PubMed] [Google Scholar]
- Barlow H. B. Optic nerve impulses and Weber's law. Cold Spring Harb Symp Quant Biol. 1965;30:539–546. doi: 10.1101/sqb.1965.030.01.052. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-Cugell C. Quantitative aspects of gain and latency in the cat retina. J Physiol. 1970 Jan;206(1):73–91. doi: 10.1113/jphysiol.1970.sp008998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVOE R. D. LINEAR RELATIONS BETWEEN STIMULUS AMPLITUDES AND AMPLITUDES OF RETINAL ACTION POTENTIALS FROM THE EYE OF THE WOLF SPIDER. J Gen Physiol. 1963 Sep;47:13–32. doi: 10.1085/jgp.47.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Cat colour vision: one cone process or several? J Physiol. 1969 May;201(3):745–764. doi: 10.1113/jphysiol.1969.sp008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Knight B. W., Toyoda J. Voltage noise in Limulus visual cells. Science. 1968 Apr 5;160(3823):88–90. doi: 10.1126/science.160.3823.88. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Boycott B. B. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966 Nov 15;166(1002):80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Pinto L. H. Properties of the surround response mechanism of cat retinal ganglion cells and centre-surround interaction. J Physiol. 1972 Jan;220(2):403–439. doi: 10.1113/jphysiol.1972.sp009714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Pinto L. H. Pure central responses from off-centre cells and pure surround responses from on-centre cells. J Physiol. 1972 Jan;220(2):441–464. doi: 10.1113/jphysiol.1972.sp009715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Shapley R. M. Flux, not retinal illumination, is what cat retinal ganglion cells really care about. J Physiol. 1973 Sep;233(2):311–326. doi: 10.1113/jphysiol.1973.sp010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y. Receptive field organization of cat optic nerve fibers with special reference to conduction velocity. Vision Res. 1971 Mar;11(3):209–226. doi: 10.1016/0042-6989(71)90186-6. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei L., Cervetto L., Fiorentini A. Transfer characteristics of excitation and inhibition in cat retinal ganglion cells. J Neurophysiol. 1970 Mar;33(2):276–284. doi: 10.1152/jn.1970.33.2.276. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A., Cervetto L. Homeostasis in retinal receptive fields. J Neurophysiol. 1971 Jul;34(4):579–587. doi: 10.1152/jn.1971.34.4.579. [DOI] [PubMed] [Google Scholar]
- Naka K. I. The horizontal cells. Vision Res. 1972 Apr;12(4):573–588. doi: 10.1016/0042-6989(72)90153-8. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. THE SENSITIVITY OF RODS UNDER ILLUMINATION. J Physiol. 1965 May;178:141–160. doi: 10.1113/jphysiol.1965.sp007620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W., Ford R. W. The cat local electroretinogram to incremental stimuli. Vision Res. 1969 Jan;9(1):1–24. doi: 10.1016/0042-6989(69)90028-5. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Creutzfeldt O. D. Scotopic and mesopic light adaptation in the cat's retina. Pflugers Arch. 1969;313(2):168–185. doi: 10.1007/BF00586245. [DOI] [PubMed] [Google Scholar]
- Schellart N. A., Spekreijse H. Dynamic characteristics of retinal ganglion cell responses in goldfish. J Gen Physiol. 1972 Jan;59(1):1–21. doi: 10.1085/jgp.59.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R., Enroth-Cugell C., Bonds A. B., Kirby A. Gain control in the retina and retinal dynamics. Nature. 1972 Apr 14;236(5346):352–353. doi: 10.1038/236352a0. [DOI] [PubMed] [Google Scholar]
- Smith R. A., Jr Luminance-dependent changes in mesopic visual contrast sensitivity. J Physiol. 1973 Apr;230(1):115–135. doi: 10.1113/jphysiol.1973.sp010178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spekreijse H., van Norren D., van den Berg T. J. Flicker responses in monkey lateral geniculate nucleus and human perception of flicker. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2802–2805. doi: 10.1073/pnas.68.11.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. H. Rod and cone contributions to S-potentials from the cat retina. Vision Res. 1969 Nov;9(11):1319–1329. doi: 10.1016/0042-6989(69)90069-8. [DOI] [PubMed] [Google Scholar]
- Stone J., Fabian M. Summing properties of the cat's retinal ganglion cell. Vision Res. 1968 Aug;8(8):1023–1040. doi: 10.1016/0042-6989(68)90075-8. [DOI] [PubMed] [Google Scholar]
- VAKKUR G. J., BISHOP P. O., KOZAK W. VISUAL OPTICS IN THE CAT, INCLUDING POSTERIOR NODAL DISTANCE AND RETINAL LANDMARKS. Vision Res. 1963 Nov;61:289–314. doi: 10.1016/0042-6989(63)90004-x. [DOI] [PubMed] [Google Scholar]
- WEALE R. A. Light absorption in the crystalline lens of the cat. Nature. 1954 May 29;173(4413):1049–1050. doi: 10.1038/1731049a0. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Yoon M. Influence of adaptation level on response pattern and sensitivity of ganglion cells in the cat's retina. J Physiol. 1972 Feb;221(1):93–104. doi: 10.1113/jphysiol.1972.sp009741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nes F. L., Koenderink J. J., Nas H., Bouman M. A. Spatiotemporal modulation transfer in the human eye. J Opt Soc Am. 1967 Sep;57(9):1082–1088. doi: 10.1364/josa.57.001082. [DOI] [PubMed] [Google Scholar]


