Abstract
Pertussis toxin of Bordetella pertussis is secreted by a type IV secretion system comprised of the products of the nine ptl (pertussis toxin liberation) genes. These proteins are believed to form a complex spanning both the inner and outer membranes and passing through the peptidoglycan layer. Peptidoglycan acts as a barrier for transport through the periplasm of large folded molecules. Assembled pertussis toxin and the secretion component proteins PtlC through PtlH are too large to diffuse through intact peptidoglycan. Therefore, we hypothesized that the Ptl system contains a peptidoglycanase activity. The PtlE protein was found to exhibit a sequence match to the active site of glycohydrolase enzymes. An N-terminally polyhistidine-tagged PtlE fusion protein, constructed and expressed in Escherichia coli and in B. pertussis, exhibited peptidoglycanase activity on activity gels. A fusion protein with alanine substitutions at the putative active site residues (aspartic acid at position 53 and glutamic acid at position 62) lacked peptidoglycanase activity. B. pertussis strains with the amino acid substitutions were deficient for pertussis toxin secretion. Based on these results, we concluded that PtlE is a peptidoglycanase responsible for the local removal or rearrangement of the peptidoglycan layer during Ptl secretion complex assembly.
Pertussis toxin is the most complex toxin yet discovered. It is an AB5 toxin comprised of the products of five genes, S1 through S5 (17, 22). The A subunit of the toxin is the S1 polypeptide, while the pentameric B subunit is comprised of S2, S3, S4, and S5 assembled in a ratio of 1:1:2:1 (26). Secretion of pertussis toxin past the outer membrane of Bordetella pertussis requires the nine ptl (pertussis toxin liberation) genes (8). The ptl genes are located immediately downstream of the pertussis toxin genes, transcribed from the same promoter (7, 31). Based on homology to other type IV systems, such as the Agrobacterium tumefaciens virB operon, and to the P-plasmid tra conjugation genes (32), it is predicted that the Ptl proteins form a large complex spanning both the inner and outer membranes. While they do not share homology, the type IV complexes are comparable to the flagellar basal body or the type III secretion systems, which also span the cytoplasm and cross the peptidoglycan barrier. During assembly of the flagellum, flagellar subunits are secreted from the bacterium through the basal body (1). Likewise, proteins involved in pathogenesis are secreted into the extracellular milieu through the flagellar export apparatus of Salmonella enterica serovar Typhimurium (33).
Pertussis toxin is assembled in the periplasmic space, and the Ptl system mediates secretion from the periplasm past the outer membrane. However, the Ptl secretion system appears to span the inner membrane as well as the outer membrane since Ptl proteins have been found localized to both the inner and outer membranes (13). It is thought that the energy to power secretion comes from the cytoplasm. The PtlC and PtlH proteins contain ATP binding domains that are required for secretion (6, 14) and are predicted to be localized to the cytoplasm, where they could transduce energy to the membrane-bound components of the secretion system. The secretion complex must span the peptidoglycan layer. Since peptidoglycan forms a rigid barrier around the cell (9), a means of removing or rearranging this portion of the peptidoglycan must be available. Recently, peptidoglycanase proteins involved in local rearrangements of peptidoglycan have been found in other systems involving passage through the membrane. An accessory protein of the flagellar basal body, FlgJ, of S. enterica serovar Typhimurium has been found to have peptidoglycanase activity, which is required for motility (21). Peptidoglycan hydrolase homology has been identified in the VirB1 protein of the A. tumefaciens virB type IV secretion system (20) that has been shown to enhance tumorigenesis (16). Similarly, several bacteriophages have been found to have lytic transglycosylase activities thought to be involved in the invasion step. These enzymes include GP16 of T7 (19) and the P7 structural protein of PRD1 (25). The activity involved in phage entry is different from the activity involved in lysis of an infected bacterium in that it affects the peptidoglycan structure only locally and does not promote cell lysis.
This study was carried out to identify a peptidoglycanase in the Ptl system. PtlE was chosen as the candidate peptidoglycanase because it was found to possess sequence similarity to the active site of glycohydrolase enzymes at its N terminus using the program BLOCKS (11). We showed that a polyhistidine-tagged PtlE has peptidoglycanase activity. Mutants with amino acid substitutions at the putative glycohydrolase active site lacked activity and exhibited significantly reduced secretion of pertussis toxin. On the basis of these results, we suggest that PtlE is a peptidoglycanase specific to the pertussis toxin secretion apparatus.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The strains and plasmids used are shown in Table 1. Escherichia coli strains were grown on L agar. B. pertussis strains were grown on Bordet-Gengou (BG) agar or in Stainer-Scholte (SS) broth overlaid onto BG agar as previously described (8, 30).
TABLE 1.
Strains and plasmids used
| Strain or plasmid | Relevant features | Source or reference |
|---|---|---|
| E. coli strains | ||
| BL21(DE3)(pLysS) | T7 polymerase | Invitrogen |
| DH5α | High-efficiency transformation, Nalr | Gibco BRL |
| UT5600 | OmpT−mlt-1 | New England Biolabs |
| B. pertussis strains | ||
| BP338 | Tohama I derivative, Nalr | 30 |
| BPRA | Deletion of ptx/ptl promoter and genes for S1 through S5, Nalr Strepr | 2 |
| Plasmids | ||
| Commercial vectors | ||
| pBluescript KS+ | Cloning vector, Ampr | Stratagene |
| pCR2.1 | TA cloning vector, Ampr Kanr | Invitrogen |
| pRSETB | Expression vector, T7 promoter, polyhistidine metal-binding domain, Ampr | Invitrogen |
| pET-21b | Expression vector, T7 lac promoter, Ampr | Novagen |
| pMAL-p2x | Maltose-binding protein fusion vector | New England Biolabs |
| His tagging of PtlE | ||
| pKC34 | ptx/ptl operon in pSP72, Ampr | 8 |
| pAR186 | ptlE in pRSETB | This study |
| pAR253-1 | ptlE D53A in pRSETB | This study |
| pAR278-1 | ptlE E62A in pRSETB | This study |
| pAR278-2 | ptlE D53A E62A in pRSETB | This study |
| pAR312-1 | ptlE (1-84)a in pET-21b | This study |
| pAR312-2 | ptlE D53A (1-84) in pET-21b | This study |
| pAR312-3 | ptlE E62A (1-84) in pET-21b | This study |
| pAR312-4 | ptlE D53A E62A (1-84) in pET-21b | This study |
| Expression in B. pertussis | ||
| pBBR1-MCS2 | Broad-host-range cloning vector, Kanr | 15 |
| pCW211-5 | Plasmid containing B. pertussis cpn10 promoter | 29 |
| pAR228-1 | pBBR1-MCS2 with the B. pertussis cpn10 promoter inserted between the SacI and SacII sites | This study |
| pAR249-1 | His-tagged ptlE in pAR228-1 | This study |
| Cloning of ptlE mutants | ||
| pAR146 | NotI-to-HindIII fragment from pKC34 containing ptlE through ptlH genes in pBluescript | This study |
| pKC109 | oriT Gentr, cycA fragment | 8 |
| pAR287-2 | D53A mutation in pKC34 | This study |
| pAR264-3 | E62A mutation in pKC34 | This study |
| pAR264-4 | D53A and E62A mutations in pKC34 | This study |
Amino acid positions are shown in the parentheses.
Peptidoglycanase activity gels (zymograms).
Peptidoglycanase activity was detected by using the methods of Potvin et al. (23) and Buist et al. (5). Bacterial cells were separated in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gels containing 0.2% (wt/vol) autoclaved, lyophilized Micrococcus lysodeikticus ATCC 4698 cells (Sigma). Proteins were allowed to renature in the gels by shaking the gels in 25 mM Tris-HCl (pH 7.5)-1% (vol/vol) Triton X-100 for 48 h, with several changes of buffer to remove the SDS. Peptidoglycanase activity resulted in the formation of clear bands. Gels were stained for 5 min with 1% methylene blue in 0.1% (wt/vol) KOH to increase contrast and were destained in three to four changes of deionized water. As a further refinement of the procedure, the gel shown in Fig. 8 was renatured in 25 mM Tris-HCl (pH 7.5)-1% (vol/vol) Triton X-100 for 24 h and then in 25 mM Tris-HCl (pH 5.0)-1% (vol/vol) Triton X-100 for an additional 24 h. The optimum pHs for lysozyme and the two major E. coli transglycosylases are in the vicinity of pH 5, so it was thought that decreasing the pH in the final step would increase the activity of the protein.
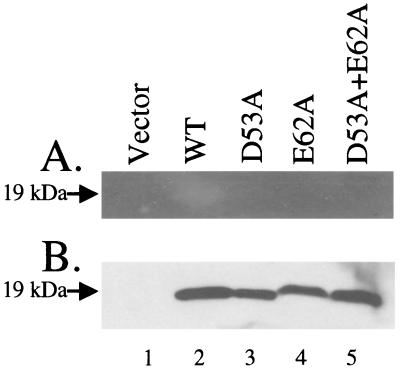
FIG. 8.
Activity of polyhistidine-tagged mutant PtlE proteins expressed in E. coli BL21[DE3]. Lane1, vector control (pET-21b); lane 2, His-tagged PtlE wild type (pAR312-1); lane 3, His-tagged PtlE D53A (pAR312-2); lane 4, His-tagged PtlE E62A (pAR312-3); lane 5, His-tagged PtlE double mutant (pAR312-4). (A) Western blot analysis with anti-polyhistidine antibody; (B) zymogram analysis.
Western blotting.
Western blotting was performed as previously described (8). Samples were mixed at a 1:1 ratio with sample buffer. Proteins were separated on 12% acrylamide SDS-PAGE gels and transferred to polyvinylidene difluoride membranes. The membranes were probed with anti-polyhistidine antibody (Sigma) or anti-PtlF antibody.
Generation of PtlF antibody.
Hydropathy and secondary-structure data predict that the region of PtlF from amino acid residues 73 to 205 has the highest antigenicity index (12). This region was amplified by PCR by using primers 5-F80 and 3-F80. These primers introduced an XbaI restriction site into the 5′ end and an HindIII restriction site into the 3′ end. The PCR product was cloned into TA cloning vector pCR2.1 by using a TOPO TA cloning kit (Invitrogen, Carlsbad, Calif.) according to the manufacturer's directions. The PCR product was ligated into plasmid pMAL-p2x by using the XbaI and HindIII sites. A maltose-binding protein fusion to this region of PtlF was generated and purified by using the pMAL protein fusion and purification system (New England BioLabs, Inc.). The fusion protein was expressed in E. coli UT5600 to increase expression levels and decrease proteolytic degradation. Rat polyclonal antibodies to the fusion protein were generated at Harlan Bioproducts for Science (Indianapolis, Ind.). Rats were injected with 200 μg of fusion protein with complete Freund's adjuvant at zero time, and this was followed by two boosts consisting of 100 μg with incomplete Freund's adjuvant on days 28 and 56. The final bleed was done on day 70.
Separation of membrane and soluble proteins.
B. pertussis BP338(pAR249-1) was grown in SS broth overlaid onto BG plates as described above. Ten milliliters of a 24-h culture was pelleted by centrifugation, washed two times in 10 ml of 4°C Tris-NaCl (20 mM Tris-HCl [pH 7.4], 200 mM NaCl, 1 mM EDTA), and resuspended in 10 ml of Tris-NaCl with 20 μl of Sigma protease inhibitor cocktail (product no. P 8465). Cells were broken by sonication for 8 min in a Branson 2510 sonicating water bath in 2.5-ml aliquots. Sonicated cultures were centrifuged at 9,000 × g for 10 min to remove unbroken cells. Samples were then centrifuged at 100,000 × g for 1 h to separate the membrane and soluble fractions. Membranes were resuspended in 1 ml of Tris-NaCl by sonication for 1 min. Soluble fractions were precipitated with 20% (wt/vol) trichloroacetic acid and resuspended in 1 ml of Tris-NaCl.
Construction of fusion proteins for overexpression in E. coli.
The PtlE gene was cloned out of pKC34 by using primers 5-E180 and 3-E180, which added the XbaI restriction enzyme site to the 5′ end and the HindIII restriction enzyme site to the 3′ end. The PCR product was TA cloned into pCR2.1 as described above and then ligated into expression vector pRSETB at the NheI and HindIII sites, resulting in an N-terminal polyhistidine fusion, plasmid pAR186 (Table 1).
Overexpression of fusion proteins in E. coli.
The polyhistidine-tagged PtlE fusion proteins were expressed in E. coli BL21[DE3](pLysS). Cultures were grown in L broth containing ampicillin and chloramphenicol at appropriate concentrations. Single, freshly transformed colonies were added to 10 ml of L broth and incubated overnight without shaking at 37°C. The overnight culture (0.2 ml) was used to inoculate 10 ml of fresh L broth, which was incubated with shaking at 37°C until the mid-log phase of growth. Expression of the fusion protein was induced by adding isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 1 mM. Cultures were incubated for an additional 2 h and harvested in 1-ml aliquots by centrifugation. Cell pellets were resuspended to an optical density at 600 nm (OD600) of 4 for analysis.
Construction of fusion proteins for overexpression in B. pertussis.
A vector for the expression of fusion proteins in B. pertussis was constructed in the broad-host-range cloning vector pBBR1-MCS2 (15). Primers 5-HSP and 3-HSP were used to amplify the B. pertussis cpn10 promoter (10) from pCW211-5 (29) and to add the SacI restriction enzyme site to the 5′ end and the SacII restriction enzyme site to the 3′ end. The promoter was ligated into pBBR1-MCS2 at the SacI and SacII sites to create pAR228-1. This placed the promoter immediately upstream of the multiple cloning region. The His-tagged fusion protein gene was ligated into the B. pertussis expression vector pAR228-1 at the XbaI and HindIII sites to generate plasmid pAR249-1. This plasmid was electroporated into B. pertussis BP338 by using the procedure developed by Weingart et al. (29). Colonies were selected with kanamycin, and individual transformants were grown out on BG agar plates at 37°C for 48 h.
Construction of amino acid substitutions in PtlE.
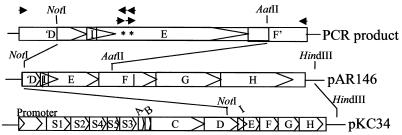
Point mutations D53A and E62A and a double mutation (D53A E62A) were constructed by PCR by using the primers listed in Table 2. The D53A mutation was made by using primers 5-AR156 and 3-AR156. The E62A mutation was made by using primers 5-AR264 and 3-AR264. The nucleotides altered to produce the amino acid substitutions are indicated in Table 2. The double mutation was constructed by using primers 5-AR264 and 3-AR264 and a plasmid containing the PCR fragment with the D53A mutation as the template. Outside primers 5-D80 and 3-F80 were used to clone the mutations into the ptx/ptl operon. The PCR products were TA cloned into pCR2.1. The cloned point mutations were ligated into pAR146 by using NotI and AatII. The NotI-to-HindIII region from the resulting plasmid was then ligated back into the remaining portion of pKC34 to regenerate the complete operon containing the desired nucleotide substitution(s), as shown in Fig. 1. The HindIII fragment of pKC109 containing the gentamicin resistance gene, the origin of transfer, and an internal fragment of the adenylate cyclase gene was ligated into these plasmids to make suicide vectors (for D53A, pAR287-2; for E62A, pAR264-3; and for D53A E62A, pAR264-4) for triparental mating, as shown in Table 1. The suicide plasmids were transformed into E. coli HB101.
TABLE 2.
Primers used
| Primer | Sequencea |
|---|---|
| 5-F80 | GGCTCTAGAGACGGCTGGCAATTCAGCC |
| 3-F80 | CAGAAGCTTACCCGGTCTGAACGTGAGCC |
| 5-E180 | GGCTCTAGAATGGGCCATCCTGGCCATC |
| 3-E280 | CAGAAGCTTCATGGCTGTCCAGCCTCCG |
| 5-HSP | GAGCTCGCGAAGACCCGCC |
| 3-HSP | CCGCGGATGAGGAACTCCTG |
| 5-AR156 | CTTGACGCCTGCCCAGACGC |
| 3-AR156 | GTCTGGGCAGGCGTCAAGGGG |
| 5-AR264 | GCCGCGGCCGCGGTGGAC |
| 3-AR264 | GCGGCCGCGGCATGCCCG |
| 3-AR301 | GAAGCTTCACCATGCACGGCGTTCCGAC |
| 5-D80 | GGCTCTAGAGCGGGCCTGCGGCG |
The underlined nucleotides are the nucleotides altered to produce the amino acid substitutions.
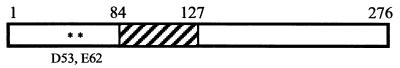
FIG. 1.
Features of the PtlE protein. The putative active site residues and membrane-spanning region are indicated.
Vectors for expression of His-tagged ptlE in E. coli (pAR317-2, pAR317-3, and pAR317-4) containing amino acid substitutions D53A and E62A and the double mutation were generated in the manner described above for plasmid pAR186 by using plasmids pAR287-2, pAR264-3, and pAR264-4 as the templates for PCR. C-terminal truncations of the tagged wild-type and mutant ptlE genes were subcloned from the full-length genes by using the T7 promoter primer and primer 3-AR301. The PCR products were TA cloned and then ligated into expression vector pET-21b at the NdeI and HindIII sites to produce plasmids pAR253-1, pAR278-1, pAR278-2, and pAR312-4. These plasmids expressed the N-terminal portion of PtlE and the PtlE point mutations up to amino acid 84.
Introduction of mutations into the B. pertussis chromosome.
Each plasmid containing a mutant ptlE or the wild-type gene was integrated into the chromosome of B. pertussis via a region of adenylate cyclase homology as described previously (8). Triparental matings were performed as described by Barry et al. (3). The mating mixtures contained 250 μl of E. coli HB101 containing one of the suicide plasmids and 250 μl of an E. coli strain containing helper conjugative plasmid pUW956 (30) suspended in L broth added to 2 ml of B. pertussis in SS broth. The mating mixtures were plated on SS medium plates containing 40 mM MgCl2 and incubated at 37°C. After 6 h, the mixtures were streaked on BG agar plates with nalidixic acid and gentamicin to select for single recombination events where the plasmid had integrated into the B. pertussis chromosome, and they were further screened for recombination into the adenylate cyclase locus by selecting nonhemolytic colonies.
Toxin secretion assay.
Single colonies from independent triparental matings were streaked onto BG agar and incubated for 48 h at 37°C. A suspension of each culture in SS broth with an OD600 of 0.1 was made, and 7 ml of the suspension was overlaid onto a BG agar plate with gentamicin and nalidixic acid and incubated for 24 h at 37°C. Cells were harvested in 1-ml aliquots by centrifugation. Supernatant samples were filter sterilized to remove the remaining bacteria. Cell pellets were washed in 1 ml of phosphate-buffered saline (PBS) (pH 7.4). For Western blot analysis, pellets were resuspended in PBS to an OD600 of 8. For determination of periplasmic toxin, bacterial pellets were resuspended in 100 μl of a 20-mg/ml lysozyme solution and incubated for 30 min at 37°C. The reaction was stopped by addition of 900 μl of PBS-0.05% (vol/vol) Tween 20. Samples were centrifuged to pellet cell debris, and each supernatant (periplasmic fraction) was filter sterilized.
CHO cell assay.
Chinese hamster ovary (CHO) cell assays were performed as described previously (8). Briefly, serial twofold dilutions of samples were made in Ham's F-12 medium containing 1% fetal bovine serum. Samples were added to CHO cell monolayers in 96-well plates and incubated for 48 h at 37°C in a tissue culture incubator. Pertussis toxin causes CHO cells to lose focal adhesion and produces a clusters-of-grapes morphology. Cell morphology in wells containing test samples was compared to cell morphology in wells containing twofold dilutions of purified pertussis toxin (List Biologicals, Campbell, Calif.) and cell morphology in control wells with no toxin. Each sample was assayed in duplicate with at least three independent repeats. The limit of detection for purified pertussis toxin was determined on each plate, and the last positive well of the test samples was assigned that value. The standard error of the mean was graphed, and the Student t test was used to determine statistical significance.
RESULTS
PtlE has sequence homology to glycohydrolases.
The ptlE gene is one of the nine ptl genes located downstream of the pertussis toxin genes and required for the export of folded pertussis toxin past the outer membrane of the bacterium. The PtlE protein is predicted to be 276 amino acids long with a molecular mass of 30 kDa (Fig. 1). It contains a single membrane-spanning domain (amino acids 84 to 127), which we have predicted to be oriented with the N terminus on the periplasmic side of the inner membrane using TMpred (http://www.ch.embnet.org). A preliminary search performed with the program BLOCKS identified a region of homology to glycosyl hydrolase family 9 (11). This region of homology spans amino acids 51 through 76 of PtlE and is predicted to be localized to the periplasmic space. The most conserved region contains two acidic amino acids, located nine residues apart (Fig. 1, residues D53 and E62). These residues, an aspartic acid and a glutamic acid residue, have been shown to be catalytically important in another member of this family (28).
Peptidoglycanase activity of PtlE.
Peptidoglycanase activity was monitored by using activity gels. Bacterial proteins were separated on SDS-PAGE gels containing M. lysodeikticus cells as a source of peptidoglycan. Following electrophoresis, protein was allowed to renature, and peptidoglycanase activity was detected as uncolored bands following staining with methylene blue. In initial studies, no differences were detected between wild-type B. pertussis and BPRA, the pertussis toxin and Ptl deletion mutant. The failure to detect this activity in B. pertussis was not unexpected and was likely due to the need to tightly regulate this potentially dangerous activity.
Construction and expression of fusion proteins.
To improve sensitivity, PtlE was overexpressed as a polyhistidine-tagged fusion. PtlE has two potential initiation codons. Since it is not known which of these codons is the actual initiation site of PtlE, the first possible start site was chosen for the fusion protein to ensure that the entire protein would be included in the resulting fusion protein. The PtlE gene was cloned out of pKC34 by PCR using primers 5-E180 and 3-E180 (Table 2), which added the XbaI restriction enzyme site to the 5′ end and the HindIII restriction enzyme site to the 3′ end, and ligated into expression vector pRSETB at the NheI and HindIII sites, resulting in an N-terminal polyhistidine fusion.
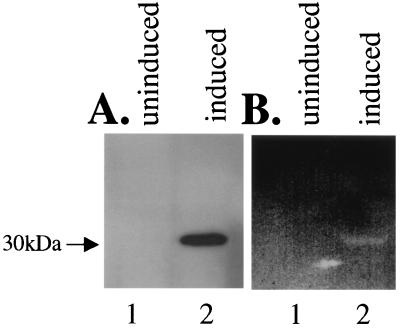
The polyhistidine-tagged PtlE protein was overexpressed in E. coli. This fusion protein migrated at 30 kDa, as determined by Western blotting using antibody to polyhistidine (Fig. 2A). Peptidoglycanase activity was monitored by using a pepditoglycanase activity gel. Peptidoglycanase activity comigrated with the fusion protein (Fig. 2B, lane 2). No peptidoglycanase activity was detected at that molecular weight in the uninduced control (Fig. 2B, lane 1).
FIG. 2.
Analysis of polyhistidine-tagged PtlE expressed in E. coli BL21. Lane 1, uninduced BL21(pAR186); lane 2, BL21(pAR186) induced with IPTG. (A) Western blot analysis with anti-polyhistidine antibody; (B) zymogram analysis showing peptidoglycanase activity.
Expression of the PtlE fusion protein in B. pertussis.
For expression in B. pertussis, vector pAR228-1 was constructed by inserting the B. pertussis cpn10 promoter into the broad-host-range cloning vector pBBR1-MCS2, which is capable of replication in B. pertussis. The region encoding the fusion protein was cut out of the pRSETB vector by using XbaI and HindIII and ligated into the B. pertussis expression vector to create pAR249-1.
The His-tagged PtlE fusion protein was expressed by wild-type B. pertussis strain BP338, as determined by Western blotting (Fig. 3A). The major cross-reacting band detected by antibodies to polyhistidine was observed to migrate at 25 kDa, slightly faster than the same fusion protein expressed in E. coli. Since the fusion protein is N terminally tagged, this suggests that the C terminus of the PtlE protein may be processed in B. pertussis.
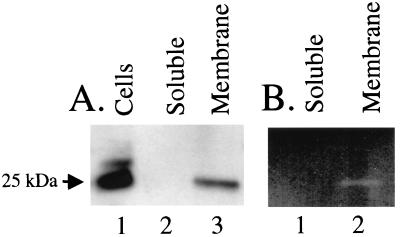
FIG. 3.
Analysis of polyhistidine-tagged PtlE expressed in B. pertussis BP338. (A) Western blot analysis with anti-polyhistidine antibody. Lane 1, cells; lane 2, soluble fraction; lane 3, membrane fraction. (B) Zymogram analysis. Lane 1, soluble fraction; lane 2, membrane fraction.
The native PtlE protein has been shown to localize to the membrane fraction in B. pertussis (13). In order to determine where the fusion protein was localized, B. pertussis BP338(pAR249-1) expressing His-tagged PtlE was separated into soluble and membrane fractions by sonication and high-speed centrifugation. Samples of the fractions were analyzed by using Western blotting and a peptidoglycanase activity gel. The fusion protein was found to localize to the membrane fraction by Western blotting (Fig. 3A). The PtlE fusion protein in the membrane fraction was also found to retain peptidoglycanase activity (Fig. 3B).
Construction of ptlE point mutants within the ptx/ptl operon.
The ptl genes have overlapping reading frames. The stop codon of the ptlI gene overlaps codons 50 and 51 of the ptlE gene, and the stop codon of the ptlE gene overlaps the start codon of the ptlF gene (Fig. 4). Attempting to knock out the ptlE gene for analysis by complementation is therefore impractical, and another strategy had to be used to test the effect of the point mutations on toxin secretion.
FIG. 4.
Cloning of amino acid substitutions into the ptx/ptl operon. The arrows represent primers used to produce PCR products. The asterisks represent bases that were altered.
Individual amino acid substitutions D53A and E62A were made at the putative active site residues by PCR. A double mutant (D53A E62A) was also constructed. The amino acid substitutions were inserted into the ptlE gene within the ptx/ptl operon by PCR, as diagramed in Fig. 4. Each plasmid containing a mutant ptlE or the wild-type gene was integrated into the chromosome of B. pertussis via a region of adenylate cyclase homology, as described previously (8). For haploid analysis, plasmids were integrated into the chromosome of BPRA, a ptx/ptl mutant strain containing a deletion of the ptx/ptl promoter and the S1, S2, S4, and S5 genes (2). For merodiploid analysis, wild-type strain BP338 was used.
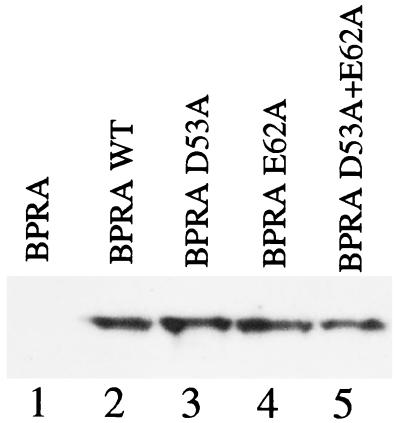
To ensure that the amino acid substitutions had not resulted in polar mutations, immunoblot analysis was performed on the haploid mutant strains by using an antibody to PtlF, the product of the gene immediately downstream of the ptlE gene. All three mutant plasmids and the control wild-type plasmid restored expression of PtlF in BPRA, and similar levels of expression were observed for all strains (Fig. 5).
FIG. 5.
Western blot of PtlF expression for the wild type and PtlE mutants. The ptx/ptl operon containing wild-type PtlE or point mutants was introduced into the chromosome of pertussis toxin deletion strain BPRA at the adneylate cyclase toxin locus. Expression of PtlF was assessed to monitor polarity. Lane 1, BPRA; lane 2, BPRA with wild-type (WT) PtlE (from pKC34); lane 3, BPRA with PtlE D53A (from pAR287-2); lane 4, BPRA with PtlE E62A (from pAR264-3); lane 5, BPRA PtlE D53A E62A (from pAR264-4).
B. pertussis ptlE mutants are defective for toxin secretion.
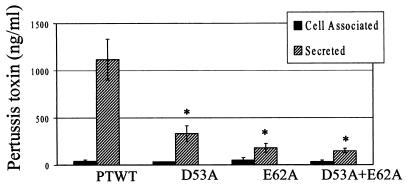
CHO cells were used to determine the concentrations of active toxin in samples of culture supernatant (secreted toxin) and cell lysates (periplasmic toxin). Integration of the wild-type ptx/ptl operon into BPRA restored toxin expression and secretion, as previously demonstrated (8). Secretion was reduced by more than 70% in all three mutants compared to the wild-type control strain (Fig. 6), and the differences were statistically significant. The defect in secretion did not result in increased levels of periplasmic toxin, a result that has been observed for several other ptl mutants (8).
FIG. 6.
Toxin expression and secretion in haploid strains (ptx/ptl mutant background). PTWT, BPRA with wild-type PtlE (from pKC34); D53A, BPRA with mutant PtlE from pAR287-2; E62A, BPRA with mutant PtlE from pAR264-3; D53A+E62A, BPRA with mutant PtlE from pAR264-4. Cell-associated (periplasmic) and secreted toxins were quantified by using the CHO cell activity assay. Secretion of toxin was significantly reduced in strains expressing mutant ptlE genes, including D53A (P < 0.008), E62A, and the double mutant (P < 0.003) (n = 4).
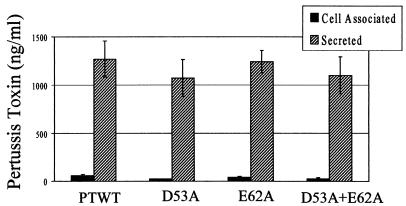
Mutations that disrupt proper folding or localization of a protein that is part of a complex can result in dominant negative effects. A previous mutant of PtlE was found to have a dominant negative phenotype. The mutants from this study were therefore tested for dominant negative effects by integrating the mutant Ptl operon into the adenylate cyclase toxin gene of wild-type strain BP338. The amino acid substitutions did not affect toxin expression or secretion in the wild-type background (Fig. 7), suggesting that these mutants do not have a dominant negative phenotype.
FIG. 7.
Toxin expression and secretion in merodiploid strains (wild-type background). PTWT, BP338 with a second copy of wild-type PtlE (from pKC34); D53A, BP338 with a second copy of mutant PtlE from pAR287-2; E62A, BP338 with a second copy of mutant PtlE from pAR264-3; D53A+E62A, BP338 with a second copy of mutant PtlE from pAR264-4. Cell-associated (periplasmic) and secreted toxins were quantified by using the CHO cell activity assay. Periplasmic and secreted toxin levels were not significantly different for any of the strains tested.
Amino acid substitutions at the glycohydrolase active site residues reduce peptidoglycanase activity.
To demonstrate that the loss of secretion was due to the loss of glycohydrolase activity in PtlE, His-tagged ptlE genes containing the D53A, E62A, and D53A plus E62A amino acid substitutions were expressed in E. coli by using truncated ptlE genes expressing only the N-terminal domain of the protein (through residue 84) to improve the solubility of the protein and allow higher levels of overexpression. The recovery of mutant or wild-type protein was similar in all cases, as determined by Western blotting (Fig. 8A). The proteins were tested for peptidoglycanase activity by using the activity gel assay (Fig. 8B) and gels loaded with the same amount of protein as the Western blot. Peptidoglycanase activity was detected with the wild-type protein but not with the mutant proteins (Fig. 8B).
DISCUSSION
The peptidoglycan of gram-negative bacteria is thought to restrict passage of folded proteins larger than 25 kDa through the periplasm (24). Local cleavage of peptidoglycan has been shown to be essential for the formation of large transmembrane complexes in a number of diverse systems of gram-negative bacteria, including flagellar assembly (21), conjugation (4), and phage entry into the cytoplasm (19, 25).
Soon after the sequence of the Ptl operon was completed (31), we identified PtlE as a possible candidate for performing this function in pertussis toxin secretion when it was found to match a family of glycohydrolases using the program BLOCKS (11). While the overall match was very poor, there was conservation of acidic residues known to be essential for catalytic activity. Interestingly, as more sequence data has become available, the PtlE glycohydrolase match is no longer reported using this program. In addition, a Blast search using amino acids 1 through 84 of PtlE does not yield any significant matches.
The peptidoglycanases, which include lysozymes and transglycosylases, have been grouped into several families based on low levels of homology in the region of the active site groove. However, the level of similarity across families is too low to establish a consensus sequence (27). In spite of the low sequence homology, the peptidoglycanases that have been crystallized have had remarkably similar structures in the region of the catalytic fold (27). All of the families of peptidoglycanases have a catalytic glutamic acid residue in the active site, and some glycanases also have a catalytic aspartic acid residue. These residues are essential for the glycanase activity (18).
Each of the Ptl proteins has a strong match to proteins in the VirB operon of A. tumafaciens. The VirB operon promotes the transfer of DNA from the bacteria into a plant cell utilizing a large protein complex that appears to be similar to the Ptl secretion complex (32). The ptlE gene and its homolog, virB8, have 50% amino acid similarity in the C-terminal region but have no significant similarity in N-terminal region (amino acids 1 to 102 of PtlE and amino acids 1 to 53 of VirB8). Homology to glycohydrolases has been identified in the VirB system (20); however, the match is in the virB1 gene. There is no homolog of VirB1 in the ptl operon. These results suggest that in spite of the high level of overall homology between these proteins, the N-terminal domains of PtlE and VirB8 likely perform different functions.
We have demonstrated that PtlE, a protein essential for secretion of pertussis toxin (8), also possesses peptidoglycanase activity, and this activity is essential for pertussis toxin secretion. Local removal of peptidoglycan is needed for formation of the transmembrane secretion complex. We suggest that this mechanism is universal and that all type III and type IV secretion systems require a peptidoglycanase activity for assembly.
Acknowledgments
This work was supported by grant RO1 AI23695 from the National Institutes of Health.
REFERENCES
- 1.Aizawa, S. I. 1996. Flagellar assembly in Salmonella typhimurium. Mol. Microbiol. 19:1-5. [DOI] [PubMed] [Google Scholar]
- 2.Antoine, R., and C. Locht. 1990. Roles of the disulfide bond and the carboxy-terminal region of the S1 subunit in the assembly and biosynthesis of pertussis toxin. Infect. Immun. 58:1518-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry, E. M., A. A. Weiss, I. E. Ehrmann, M. C. Gray, E. L. Hewlett, and M. S. Goodwin. 1991. Bordetella pertussis adenylate cyclase toxin and hemolytic activities require a second gene, cyaC, for activation. J. Bacteriol. 173:720-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayer, M., R. Iberer, K. Bischof, E. Rassi, E. Stabentheiner, G. Zellnig, and G. Koraimann. 2001. Functional and mutational analysis of p19, a DNA transfer protein with muramidase activity. J. Bacteriol. 183:3176-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buist, G., J. Kok, K. J. Leenhouts, M. Dabrowska, G. Venema, and A. J. Haandrikman. 1995. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J. Bacteriol. 177:1554-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook, D. M., K. M. Farizo, and D. L. Burns. 1999. Identification and characterization of PtlC, an essential component of the pertussis toxin secretion system. Infect. Immun. 67:754-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covacci, A., and R. Rappuoli. 1993. Pertussis toxin export requires accessory genes located downstream from the pertussis toxin operon. Mol. Microbiol. 8:429-434. [DOI] [PubMed] [Google Scholar]
- 8.Craig-Mylius, K. A., and A. A. Weiss. 1999. Mutants in the ptlA-H genes of Bordetella pertussis are deficient for pertussis toxin secretion. FEMS Microbiol. Lett. 179:479-484. [DOI] [PubMed] [Google Scholar]
- 9.Dijkstra, A. J., and W. Keck. 1996. Peptidoglycan as a barrier to transenvelope transport. J. Bacteriol. 178:5555-5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez, R. C., and A. A. Weiss. 1995. Cloning and sequencing of the Bordetella pertussis cpn10/cpn60 (groESL) homolog. Gene 158:151-152. [DOI] [PubMed] [Google Scholar]
- 11.Henikoff, S., and J. G. Henikoff. 1994. Protein family classification based on searching a database of blocks. Genomics 19:97-107. [DOI] [PubMed] [Google Scholar]
- 12.Jameson, B. A., and H. Wolf. 1988. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput. Appl. Biosci. 4:181-186. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, F. D., and D. L. Burns. 1994. Detection and subcellular localization of three Ptl proteins involved in the secretion of pertussis toxin from Bordetella pertussis. J. Bacteriol. 176:5350-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotob, S. I., and D. L. Burns. 1997. Essential role of the consensus nucleotide-binding site of PtlH in secretion of pertussis toxin from Bordetella pertussis. J. Bacteriol. 179:7577-7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop, 2nd, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 16.Llosa, M., J. Zupan, C. Baron, and, P. Zambryski. 2000. The N- and C-terminal portions of the Agrobacterium VirB1 protein independently enhance tumorigenesis. J. Bacteriol. 182:3437-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locht, C., and J. M. Keith. 1986. Pertussis toxin gene: nucleotide sequence and genetic organization. Science 232:1258-1264. [DOI] [PubMed] [Google Scholar]
- 18.Malcolm, B. A., S. Rosenberg, M. J. Corey, J. S. Allen, A. de Baetselier, and J. F. Kirsch. 1989. Site-directed mutagenesis of the catalytic residues Asp-52 and Glu-35 of chicken egg white lysozyme. Proc. Natl. Acad. Sci. USA 86:133-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moak, M., and I. J. Molineux. 2000. Role of the Gp16 lytic transglycosylase motif in bacteriophage T7 virions at the initiation of infection. Mol. Microbiol. 37:345-355. [DOI] [PubMed] [Google Scholar]
- 20.Mushegian, A. R., K. J. Fullner, E. V. Koonin, and E. W. Nester. 1996. A family of lysozyme-like virulence factors in bacterial pathogens of plants and animals. Proc. Natl. Acad. Sci. USA 93:7321-7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nambu, T., T. Minamino, R. M. Macnab, and K. Kutsukake. 1999. Peptidoglycan-hydrolyzing activity of the FlgJ protein, essential for flagellar rod formation in Salmonella typhimurium. J. Bacteriol. 181:1555-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicosia, A., M. Perugini, C. Franzini, M. C. Casagli, M. G. Borri, G. Antoni, M. Almoni, P. Neri, G. Ratti, and R. Rappuoli. 1986. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc. Natl. Acad. Sci. USA 83:4631-4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potvin, C., D. Leclerc, G. Tremblay, A. Asselin, and G. Bellemare. 1988. Cloning, sequencing and expression of a Bacillus bacteriolytic enzyme in Escherichia coli. Mol. Gen. Genet. 214:241-248. [DOI] [PubMed] [Google Scholar]
- 24.Russel, M. 1998. Macromolecular assembly and secretion across the bacterial cell envelope: type II protein secretion systems. J. Mol. Biol. 279:485-499. [DOI] [PubMed] [Google Scholar]
- 25.Rydman, P. S., and D. H. Bamford. 2000. Bacteriophage PRD1 DNA entry uses a viral membrane-associated transglycosylase activity. Mol. Microbiol. 37:356-363. [DOI] [PubMed] [Google Scholar]
- 26.Tamura, M., K. Nogimori, S. Murai, M. Yajima, K. Ito, T. Katada, M. Ui, and S. Ishii. 1982. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry 21:5516-5522. [DOI] [PubMed] [Google Scholar]
- 27.Thunnissen, A. M., N. W. Isaacs, and B. W. Dijkstra. 1995. The catalytic domain of a bacterial lytic transglycosylase defines a novel class of lysozymes. Proteins 22:245-258. [DOI] [PubMed] [Google Scholar]
- 28.Tomme, P., J. van Beeumen, and M. Claeyssens. 1992. Modification of catalytically important carboxy residues in endoglucanase D from Clostridium thermocellum. Biochem. J. 285:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weingart, C. L., G. Broitman-Maduro, G. Dean, S. Newman, M. Peppler, and A. A. Weiss. 1999. Fluorescent labels influence phagocytosis of Bordetella pertussis by human neutrophils. Infect. Immun. 67:4264-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss, A. A., E. L. Hewlett, G. A. Myers, and S. Falkow. 1983. Tn 5-induced mutations affecting virulence factors of Bordetella pertussis. Infect. Immun. 42:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss, A. A., F. D. Johnson, and D. L. Burns. 1993. Molecular characterization of an operon required for pertussis toxin secretion. Proc. Natl. Acad. Sci. USA 90:2970-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winans, S. C., D. L. Burns, and P. J. Christie. 1996. Adaptation of a conjugal transfer system for the export of pathogenic macromolecules. Trends Microbiol. 4:64-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young, G. M., D. H. Schmiel, and V. L. Miller. 1999. A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc. Natl. Acad. Sci. USA 96:6456-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]