Abstract
The archaeon Haloferax volcanii was previously shown to contain and transcribe the genes for a 2-oxo acid dehydrogenase (OADH) complex, but their presence remained a mystery because no enzymatic activity with any of the known OADH substrates could be found, and an inactivation of one of the genes did not lead to any phenotype. Here we report the identification of an additional oadh gene cluster in the genome of H. volcanii. In contrast to previously known oadh loci, it contains three genes, oadh2A1, oadh2A2, and oadh2ld, with coding capacity for the E1α and E1β subunits and an unattached lipoyl domain, but it is devoid of the genes for a complete E2 and an E3. The genes were isolated by complementation of a nitrate respiration-deficient mutant of H. volcanii and therefore were shown to be functional in vivo. Phylogenetic analyses revealed that the deduced E1α and E1β subunits of OADH2 group with bacterial acetoin dehydrogenases but not with the OADH1 subunits, and thus, H. volcanii has obtained the two gene groups independently. Comparison of the wild type and the mutant allowed us to exclude a function of OADH2 in the aerobic or anaerobic degradation of acetoin or glucose. Instead, it could be shown that OADH2 is important during nitrate-respirative growth on Casamino Acids. Many physiological and biochemical experiments failed to indicate that OADH2 uses any of the previously known OADH substrates. Growth potentials of the mutant were markedly different in media with a single carbon source versus media with mixed carbon sources.
2-Oxo acid dehydrogenase (OADH) complexes couple the oxidative decarboxylation of 2-oxo acids with the reduction of NAD+ and generate acyl-coenzyme A, CO2, and NADH. Several reviews concentrate on different aspects of OADHs' structure and function (e.g., see references 4 and 28-30). The different steps of the reaction, which require three different subunits called E1, E2, and E3, are summarized in Fig. 1A. The initial decarboxylation is catalyzed by E1, which either is a single polypeptide or consists of two subunits, E1α and E1β. During the next step, the oxidation of the intermediate hydroxyalkylthiamine diphosphate, two electrons are transferred to the oxidized lipoamide bound to subunit E2. E2 is a modular protein which contains at its N terminus one to three lipoyl domains of about 80 amino acids, followed by a binding domain, which is involved in the interaction with E1 and E3. The C-terminal domain of E2 has the catalytic capacity to transfer the acyl group from dihydrolipoamide to coenzyme A. Finally, dihydrolipoamide is reoxidized by the dehydrogenase E3. Known substrates for OADHs are acetoin, 2-oxobutyrate, 2-oxoglutarate, pyruvate, and the three branched-chain 2-oxo acids 2-oxoisovalerate, 2-oxoisocaproate, and 2-oxo-3-methyl-valerate. The last three substrates, which are intermediates in the degradation of branched-chain amino acids, are recognized by a single OADH. The subunits E1 and E2 are substrate specific, while the same E3 can be shared by several different OADHs. E2 is the core component onto which E1 and E3 are assembled. Most if not all OADHs are large complexes with multiple copies of all subunits, e.g., the pyruvate dehydrogenase complexes of different species contain either 24 E2 polypeptides in octahedral symmetry or 60 E2 polypeptides in icosahedral symmetry. The lipoamide is covalently bound to a lysine of the 80-amino-acid-long lipoyl domain of E2. This domain acts as a “swinging arm” and can reach the different catalytic sites on E1, E2, and E3 and thus allows an extreme form of substrate shuttling and of tight coupling of oxidation and rereduction of the covalently bound prosthetic group. Recently the structure of an E1α2E1β2 heterotetramer has been solved, and a model for a whole OADH complex has been proposed by integration of additional substructures determined earlier (2). Most OADHs are involved in catabolic energy-yielding pathways, but branched-chain 2-oxo acid complexes were also found to be important for the production of branched-chain fatty acids for membrane biosynthesis in Bacillus subtilis (26), for cell-cell signaling in Myxococcus xanthus (13), and for the production of an antibiotic in Streptomyces avermitilis (11).
FIG. 1.
Two mechanisms used by different enzyme systems for the oxidative decarboxylation of 2-oxo acids, both leading to the formation of CO2 and acyl-CoA. (A) Reactions performed by OADHs. (B) Reactions performed by 2-oxo acid:ferredoxin oxidoreductases.
OADHs are present in eukarya and in many bacteria, but most archaeal genomes are devoid of oadh genes. Experimental evidence is available only for a single archaeal species, Haloferax volcanii. More than 10 years ago it was found to harbor lipoic acid and the enzymatic activity of a dihydrolipoamide dehydrogenase (E3) (9, 32). Subsequently the ldh gene has been cloned and sequenced (42) and was found to be situated in an operon together with three other genes with coding capacities for E1α, E1β, and E2 (20). It was shown that a polycistronic mRNA is transcribed from all four genes under anaerobic as well as aerobic conditions. However, the function of this presumptive OADH still remains a mystery. It was not possible to measure an enzymatic activity with any of the known substrates. The gene for the E3 component was inactivated, and it was confirmed that the mutant lacked the dihydrolipoamide dehydrogenase activity, but the mutant did not show any phenotype different from the wild type in a variety of media with different carbon sources chosen to test the presence of all of the known OADHs (21). Taken together, H. volcanii was shown to transcribe all genes for an OADH and to contain the activity of E3, but in spite of considerable efforts no clues for a functional role of an OADH could be detected. The discovery of the oadh genes had been surprising, since it had been shown very early that in haloarchaea the oxidative decarboxylation of pyruvate and 2-oxoglutarate is catalyzed by a pyruvate:ferredoxin oxidoreductase and a 2-oxoglutarate:ferredoxin oxidoreductase, respectively (22, 23). These oxidoreductases are totally different from OADHs. They are composed of only two subunits, do not contain lipoamide, and have a different catalytic mechanism (31), i.e., the oxidation is performed via two consecutive one-electron transfer steps and a substrate radical intermediate is involved (Fig. 1B). Since H. volcanii does not grow on branched-chain amino acids, methionine, or acetoin, it did not seem to possess an OADH for any of the known substrates.
After more than 10 years of an unsuccessful search for the function of the putative OADH in H. volcanii, we were even more surprised to discover genes with coding capacity for subunits of a second presumptive OADH. However, as the genes were identified by complementation of a nitrate respiration-deficient mutant, the second OADH clearly has a functional role in vivo. We report the characterization of genes and deduced gene products, the comparative characterization of mutant and wild-type cells, and the search for the substrate for the newly discovered OADH.
MATERIALS AND METHODS
Organisms, media, and growth conditions.
Haloferax volcanii WR340 was kindly provided by Moshe Mevarech (Tel Aviv University, Tel Aviv, Israel). The nitrate respiration-deficient mutant 170/29 was isolated by 5-bromo-2′deoxyuridine selection (38) as described previously (43). Escherichia coli XL1 Blue MRF′ was obtained from Stratagene (Heidelberg, Germany).
H. volcanii was grown at 42°C either in complex medium (7) or in synthetic medium (25). Complex medium was composed of 2.9 M NaCl, 150 mM MgSO4, 60 mM KCl, 4 mM CaCl2, 50 mM Tris-HCl (pH 7.2), 0.275% (wt/vol) yeast extract, and 0.45% (wt/vol) tryptone. Yeast extract and tryptone are autoclaved separately from the salts. Synthetic medium was composed of 2.5 M NaCl, 85 mM MgCl2, 85 mM MgSO4, 5 mM KCl, 10 mM NH4Cl, 1 mM K2HPO4, and 20 mM Tris-HCl, (pH 7.2). After autoclaving, 0.02% (wt/vol) histidine, 400 μg of molybdate/liter, 5 mM CaCl2, 1/1,000-volume trace element solution, and the electron and carbon source were added. The trace element solution contained 300 mg of MnCl2, 440 mg of ZnSO4, 2.3 g of FeSO4, and 50 mg of CuSO4 per liter. Electron and carbon sources were 1% (wt/vol) Casamino Acids, 20 mM pyruvate, or glucose at the concentrations indicated (up to 10 mM). For anaerobic growth by nitrate respiration, 50 mM sodium nitrate was added. Anaerobic cultures were grown in Erlenmeyer flasks, which were flushed with nitrogen and sealed with rubber septa after inoculation. Growth of H. volcanii was monitored colorimetrically using a Klett-Colorimeter (Klett Manufacturing Co., New York, N.Y.). E. coli XL1 Blue MRF′ was grown in SOB medium (17). Plasmid-containing cells were selected by the inclusion of ampicillin (100 mg/liter).
Molecular biological methods.
General molecular biological methods were performed according to Sambrook et al. (35). E. coli was transformed by electroporation using the Bio-Rad (Munich, Germany) Gene Pulser. H. volcanii was transformed as described previously (7). Restriction and modification of DNA were performed with enzymes purchased from Boehringer (Mannheim, Germany) or MBI Fermentas (St. Leon-Rot, Germany) according to the manufacturer's instruction. Oligonucleotides were from ARK (Darmstadt, Germany). PCR was performed with a DNA thermal cycler (GeneAmp PCR System 2400; PE Applied Biosystems, Weiterstadt, Germany) using standard protocols. DNA sequencing was performed with BigDye Terminator RR Mix and the ABI Prism sequencer 377 according to the protocol outlined by PE Applied Biosystems. Plasmids were isolated based on the alkaline sodium dodecyl sulfate lysis method and with the JET-Star ion exchange columns from GENOMED (Bad Oeynhausen, Germany). DNA fragments were isolated from an agarose-Tris-borate-EDTA gel using the QiaexII kit (Qiagen, Hilden, Germany). Genomic DNA of H. volcanii was isolated as described by Mevarech and Werczberger (25).
Isolation of a genomic fragment containing the oadh2 genes.
The mutant 170/29 was complemented with a genomic library of H. volcanii as described previously for three additional mutants (43). For library construction the plasmid pBN1 was used (B. Nowosad and J. Soppa, unpublished data), which is composed of the E. coli vector pSK+ and a haloarchaeal novobiocin resistance gene (19) but is devoid of a haloarchaeal replication origin. Genomic DNA of wild-type H. volcanii was partially digested with MspI, and fragments of 3 to 5 kbp were isolated and cloned into pBN1. The library contained more than 106 independent clones. The H. volcanii mutant 170/29 was transformed with the library, and plasmid-containing clones were selected based on their novobiocin resistance. After that, complemented clones were selected which had regained the ability to grow via nitrate respiration. Complementation of the mutant involves homologous recombination between the defective gene located on the chromosome and a wild-type copy present in the plasmid library. Thereby, the vector pBN1 becomes integrated into the mutant genome between the wild-type and the mutant gene copies. For the isolation of the wild-type copy, aliquots of the genomic DNA of the complemented mutant were digested with different restriction enzymes, diluted to a DNA concentration of 5 ng/μl to ensure monomolecular reactions, and religated. The mixtures of religated genomic fragments were directly used to transform E. coli. The ampicillin resistance marker of pBN1 was used to select clones carrying plasmids with genomic DNA of H. volcanii adjacent to the original integration site. Plasmids of at least 8 kbp, which include at least 3 kbp of genomic haloarchaeal DNA, were chosen to transform the mutant 170/29. Restoration of nitrate-respirative growth was used to identify clones including a wild-type copy of the mutated region. The insert of one plasmid, pCW310, was sequenced on both strands by primer walking.
Isolation of an overlapping genomic fragment.
To extend the sequenced information, a second genomic fragment was cloned. Genomic DNA of the wild type was isolated and digested with the following restriction enzymes: ApaI, SmaI, BglII, Sau3A, PpuMI, BsaI, and ApaI/BglII. The fragments were separated electrophoretically with an agarose-TBE gel and transferred to a charged nylon membrane (Qiagen) by capillary transfer (35). A digoxigenin-labeled probe specific for the 3′ region of the originally cloned genomic fragment was generated by PCR using the two primers 5′CGGTCAAGCGGCTGGCCATC3′ and 5′CGTCTCGCCCGCGTCGACCG3′. Hybridization was performed overnight, and signals were visualized with the chemiluminescence detection kit (Boehringer) according to the instructions of the manufacturer. The ApaI/BglII double digest generated a fragment of 2.6 kbp, which was chosen for cloning. Genomic fragments of this size were isolated and ligated into the vector pSK+ (Stratagene, Amsterdam, The Netherlands), and the plasmid mixture was used to transform E. coli. To identify clones with the desired plasmid, colonies were picked and transferred into 96-well microtiter plates, containing 150 μl of SOB medium supplemented with ampicillin. Microtiter plates were incubated overnight at 37°C on a rotary shaker (700 rpm). Cells were transferred with a multipin replicator onto nylon membranes and soaked with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) plus 5% sodium dodecyl sulfate, and DNA was fixed in a microwave oven (650 W, 2.5 min). Positive clones were detected with the probe described above. One clone was chosen arbitrarily, the 2.6-kbp insert was found to overlap the originally cloned genomic fragment by about 1.1 kbp, and the nonoverlapping part was sequenced on both strands.
Primary sequence analyses.
DNA sequence analysis and open reading frame prediction were performed with MacVector (Eastman Kodak Chemical Company, New Haven, Conn.). The search for and retrieval of similar proteins in the SWISSPROT and TREMBL protein sequence databases (3) were performed with FASTA (27) and SRS (14). The deduced protein sequences were analyzed with coils (coiled-coil formation) (24), HTH (helix-turn-helix motifs) (12), Jpred (secondary structure prediction) (8), Prosite (presence of signature peptides of known protein families) (18), and the programs available at the ExPAsY Molecular Biology Server (www.expasy.ch). Whole-genome analyses were performed using the program PEDANT (pedant.gsf.de) (16) or using the programs of the website www-archbac.u-psud.fr/projects/sulfolobus/Blast_Search.html.
For phylogenetic analyses, multiple sequence alignments were generated using ClustalW (41). A text editor was used to remove N-terminal and C-terminal extensions, to shorten indels present in less than 10% of the species, and to format the alignments for phylogenetic programs. The programs ProtPars, ProtDist, Fitch, and Consense of the Phylip program package (15) were used at the server of the Institute Pasteur (bioweb.pasteur.fr/seqanal/phylogeny/phylip-uk.html) to calculate phylogenetic trees with parsimony and distance matrix methods with and without 100 bootstrap replications. Phylogenetic trees based on a maximum-likelihood algorithm were calculated with the program Molphy (1) at the following site: bioweb.pasteur.fr/seqanal/interfaces/molphy-simple.html. The trees were visualized with the Macintosh program Treeview (version 1.6.2 [http.//taxonomy.zoology.gla.ac.uk/rod/rod.html]).
Enzymatic assays.
H. volcanii cultures were grown to a cell density of approximately 5 × 108 cells/ml either by aerobic respiration or by nitrate respiration with 1% Casamino Acids or with 10 mM glucose as the carbon and energy source. Cells of a 1-liter culture were collected by centrifugation (8,000 × g, 15 min, 10°C) and resuspended in 5 ml of buffer A (3 M KCl, 20 mM Tris-HCl [pH 7.2]). Cells were disrupted by sonication, debris was removed by centrifugation (14,000 × g, 10 min, room temperature), and various concentrations of the supernatant were tested for enzymatic activity with the 2-oxo acids pyruvate, 2-oxoglutarate, 2-oxobutyrate, 2-oxo-3-methyl-valerate, 2-oxoisocaproate, and 2-oxo-isovalerate and with acetoin (4 mM). The reactions were performed in buffer A at 37°C and contained, in addition to the oxo acid, 0.5 mM thiamine diphosphate, 0.1 mM coenzyme A, 2 mM NAD+ or 2 mM NADP+, 0.2 mM 1,4-dithioerythritol, 10 mM MgCl2, and 5 mM valine. Absorbtion was monitored at 340 nm. Many variations were tested, e.g., each of the constituents was omitted, the concentration of all constituents was varied, and different constituents were used to start the reaction.
Nucleotide sequence accession number.
The combined DNA sequence of the two overlapping genomic fragments is available from EBI under accession number AJ431383.
RESULTS AND DISCUSSION
Identification of genes for an OADH.
In an attempt to identify components which are essential for nitrate-respirative growth of H. volcanii, a collection of more than 40 nitrate respiration-deficient mutants of three phenotypic classes had been isolated (38). Fifteen of these mutants represent the null phenotype and are totally unable to grow anaerobically in complex medium in the presence of nitrate. Mutants of the other two classes were characterized by a reduced growth rate or by a long lag phase before the onset of growth. A library of the wild-type genome, which covers the genome with a probability by far exceeding 99%, was established for E. coli (see Materials and Methods) (43). Complementation of the first three mutants of the null phenotype led to the discovery of three different ABC transporters (43). In the present study the library was used to complement a fourth null mutant with the designation 170/29. The library had been constructed in a suicide vector; therefore, complementation requires that a library plasmid containing the wild-type copy of the mutated gene integrate into the chromosomal DNA of the mutant via homologous recombination. For isolation of the wild-type copy the following procedure was used (see Materials and Methods for details): (i) genomic DNA of the complemented mutant was isolated; (ii) it was digested with a variety of restriction endonucleases; (iii) the resultant fragment mixtures were religated in dilute solution and used to transform E. coli; and (iv) plasmids with a wild-type copy of the essential gene were identified by recomplementation of the mutant 170/29. One plasmid, pCW310, was found to reproducibly complement the mutant, restoring the wild-type phenotype. Since the genomic library was constructed using a suicide vector, which can neither autonomously replicate in H. volcanii nor drive integration into the chromosome, complementation is successful only when homologous recombination occurs between the mutant allele in the chromosome and the cloned wild-type allele in the cognate plasmid of the library. Thus, complementation provides genetic proof that the cloned genomic region contains the wild-type copy of a gene which is essential for nitrate respirative growth of H. volcanii. The insert of the plasmid pCW310 was sequenced and found to contain about 3 kbp of genomic DNA of H. volcanii with coding capacity for three open reading frames, all of which exhibited the haloarchaeal codon usage (39). The three deduced proteins were found to have high levels of similarity to the E1α and E1β components and the N-terminal part of the E2 component of OADHs. Since the genes for the E1 and E2 subunits of nearly all previously known OADHs are clustered, it was expected that the remaining part of E2 and possibly E3 would be encoded downstream of the sequenced region. Therefore, an overlapping BglII-ApaI fragment from the genome of H. volcanii was cloned, which allowed expansion of the sequence information by about 1.5 kbp. Figure 2 gives an overview of the sequenced region. Unexpectedly, the third gene of the oadh gene cluster (oadh2ld) was found to be only 258 nucleotides long. It encodes an 86-amino-acid polypeptide with similarity to the N-terminal part of an E2 component. As the N-terminal about 80 amino acids of E2 components form a lipoyl domain, oadh2ld has coding capacity for an unattached “lipoyl domain” which is not part of a larger protein. Comparison of the deduced sequence of the unattached lipoyl domain with a variety of lipoyl domains of E2 proteins revealed that (i) the highly conserved lysine to which the lipoamide is covalently bound is present, (ii) all but one of the amino acids which were found to form the hydrophobic core of the lipoyl domain from the Bacillus stearothermophilus pyruvate dehydrogenase (10) are conserved, and (iii) the overall degree of similarity to other lipoyl domains is high. Therefore, there is no indication that the isolated domain may have lost functionality.
FIG. 2.
Overview of the genomic region of H. volcanii containing the new oadh2 gene cluster. The sequence is based on two clones containing overlapping genomic fragments (see the text). Open reading frames are boxed, the three genes of the oadh2 gene cluster are shaded, and the names of genes and deduced polypeptides are included. The proposed polycistronic transcript is indicated. In the upper part of the figure the sequence directly upstream of oadh2A1 is shown, possible start codons are underlined, the proposed BRE and TATA box sequences are shown in bold, and the putative transcriptional start point is indicated by an arrow.
The gene cluster seems to be confined to the three genes, because (i) the region of about 400 bp upstream of the first gene does not contain any further open reading frames, and (ii) 15 bp downstream of the third gene an open reading frame (denoted as ppk) was found which is transcribed in the opposite direction and is based on the sequence of the deduced polypeptide codes for a polyphosphate kinase, which has no obvious functional relationship to the dehydrogenase complex. Since Jolley et al. (20) have recently published the genes for the first OADH of H. volcanii, the newly cloned genes code for subunits of a second OADH and therefore were tentatively named oadh2A1 and oadh2A2, encoding the subunits E1α and E1β, and oadh2ld, encoding the unattached lipoyl domain. A final name will be given when the substrate of the OADH is identified.
Primary sequence analyses and phylogeny.
H. volcanii has a high GC content, and therefore stop codons, which are AT rich, are very rare, and it is not easy to predict the correct translational start point. Three arguments indicate that not the first but the second possible ATG codon is the real start codon for oadh2A1 translation: (i) the N terminus of the deduced protein compares much better to E1α subunits of OADHs from other species in a multiple sequence alignment when the second ATG is used; (ii) half of the codons between the first and second ATG are very rare codons for protein-coding genes of haloarchaea (39); and (iii) no basal transcription elements could be detected more than 300 nucleotides upstream of the first possible ATG. In contrast, sequences which perfectly fit the consensus sequences of basal promoter elements (BRE and TATA box) (37) are located 30 to 40 nucleotides upstream of the second possible ATG (compare Fig. 2). Transcription is predicted to start seven nucleotides upstream of the ATG, resulting in an mRNA with a very short 5′ untranslated region, which is typical for the transcripts of haloarchaea (e.g., see references 33 and 34) and other archaeal species (36). The first two genes of the gene cluster, oadh2A1 and oadh2A2, overlap by one nucleotide and thus seem to be cotranscribed. There is a gap of 31 nucleotides between the second and third genes. The gap is too short to include promoter elements for independent transcription of oadh2ld, and indeed neither a BRE nor a TATA box could be found. A T-rich sequence, TTTTTTCTT, is located seven nucleotides downstream of oadh2ld. T-rich sequences are often involved in transcriptional termination in archaea (5), and the sequence TTATTCTTT was shown in vivo to be an efficient terminator of transcription for H. volcanii (40). Analysis of the DNA sequence thus makes it likely that a common transcript of the three oadh2 genes with a length of 2,417 nucleotides is formed and that the three oadh2 genes form an operon.
An attempt was made to use the deduced protein sequences for the identification of a putative substrate of OADH2. Since the annotated function of an increasing fraction of database entries relies only on primary sequence comparisons and therefore might not always be true, sequences were collected which encode OADHs with experimentally determined substrate specificity. Sequences from five species were selected for each of the following four substrates: acetoin, branched chain 2-oxo acids, 2-oxoglutarate, and pyruvate. In addition, one experimentally characterized 2-oxobutyrate dehydrogenase was found. A multiple sequence alignment was constructed comprised of these 21 experimentally verified E1α sequences, the two sequences from H. volcanii, and seven sequences found in fully sequenced archaeal genomes including two deduced E1αs from Halobacterium salinarum. The same was done with the E1β sequences, except that H. salinarum contains only one E1β gene. Phylogenetic trees were calculated for both subunits using parsimony, distance matrix, and most-likelihood methods. As an example, a parsimony tree of E1α is shown in Fig. 3. The following results were consistently obtained for both subunits with all different methods. (i) Archaeal OADH subunits do not form a coherent group but branch off at different places within the bacterial OADH tree. (ii) The OADH2 E1α and E1β subunits of H. volcanii are found in a group together with the five acetoin dehydrogenases, the pyruvate dehydrogenases of Homo sapiens and Zymomonas mobilis, and two different E1s of Sulfolobus solfataricus. The closest relationship is to one of the two Sulfolobus enzymes. Notably, the OADH2 subunits do not group with OADH1 subunits or OADHs from H. salinarum. (iii) OADH1 of H. volcanii is closely related to OADH1 and OADH2 (only E1α present) of H. salinarum.
FIG. 3.
Phylogeny of E1α subunits of OADHs. The tree was constructed using the parsimony program ProtPars (15) from 21 sequences of OADHs with experimentally determined substrate specificity and nine deduced archaeal sequences. Other tree construction methods and bootstrap analyses yielded similar results (see the text). Names of archaeal species are shown in bold, names of species with pyruvate dehydrogenases are boxed, names of species with 2-oxoglutarate dehydrogenases are underlined, names of species with acetoin dehydrogenases are surrounded by ovals, and names of species with branched-chain OADHs are shown in italic. The 2-oxobutyrate dehydrogenase (2-OBDH) of Pseudomonas putida (P. putida) is labeled accordingly. Other species abbreviations include the following: A. pernix, Aeropyrum pernix; A. vinelandii, Azotobacter vinelandii; C. magnum, Clostridium magnum; K. pneumoniae, Klebsiella pneumoniae; P. carbinolyticus, Pelobacter carbinolyticus; R. eutropha, Ralstonia eutropha; R. capsulatus, Rhodobacter capsulatus; T. acidophilum, Thermoplasma acidophilum; T. volcanium, Thermoplasmo volcanium.
The phylogenetic trees of both subunits indicate that the two different oadhA gene groups of H. volcanii were not derived from a common precursor gene by gene duplication; rather, they indicate that H. volcanii obtained the oadh2 genes and the oadh1 genes independently, probably by lateral transfer from bacteria. The phylogenetic analysis further indicated that the bacterial precursor of OADH2 was most likely acetoin specific.
The phylogenetic analysis also indicated that the ancestor of all recent archaea was devoid of oadh genes and that therefore all archaeal oadh genes were later acquisitions, because (i) only a minority of fully sequenced archaeal genomes contain oadh genes, (ii) the archaeal OADH sequences do not group together but branch off at diverse positions within the bacterial OADH tree, and (iii) even in cases when two protein sequences are closely related, e.g., OADH2 of H. volcanii and OADH1 of S. solfataricus, this relationship does not reflect a common history of the organisms, since many data including the trees of informational genes show that the euryarchaeon H. volcanii and the crenarchaeon Sulfolobus shibatae are phylogenetically distant.
A role of the OADH2 in the degradation of amino acids or peptides.
Based on the phylogenetic analyses, it was first evaluated whether OADH2 could be an acetoin dehydrogenase. However, neither the wild type nor the mutant could grow on acetoin anaerobically in synthetic medium in the presence or in the absence of nitrate. The same results were obtained in complex medium. In addition, no acetoin dehydrogenase activity could be detected in cell extracts of the wild type grown aerobically or grown via nitrate respiration. Taken together, an acetoin dehydrogenase activity was not detected in H. volcanii.
Next, whether OADH2 could be involved in glucose degradation was tested, because it was found earlier that H. volcanii can grow via nitrate respiration in synthetic medium with glucose, that a mutation of a glucose transporter abolishes growth on glucose, and that glucose oxidation seemed to be essential during nitrate respiration in complex medium (43). The wild type and the mutant were grown anaerobically on different glucose concentrations and a saturating nitrate concentration. However, it turned out that growth yields of both strains are identical for all glucose concentrations tested (Fig. 4A). These results exclude a role for OADH2 in glucose degradation and specifically show that it is neither a pyruvate dehydrogenase nor a 2-oxoglutarate dehydrogenase.
FIG. 4.
Growth of the wild type and the OADH2− mutant 170/29 in synthetic medium. The wild type is represented by filled symbols, and the mutant is represented by open symbols. (A) Anaerobic growth on glucose and nitrate. (B) Anaerobic growth on Casamino Acids ± nitrate. The presence and absence of nitrate are indicated. (C) Aerobic growth on Casamino Acids as sole carbon and energy sources. The concentrations are indicated.
In a further search for a possible substrate of OADH2, the wild type and the mutant were grown comparatively in a variety of different media. As shown in Fig. 4B, a clear phenotype of the mutant was detected during anaerobic growth on Casamino Acids and nitrate. The growth yield of the mutant was 50% of that of the wild type, indicating that OADH2 is involved in the oxidation of an amino acid or a peptide. No growth was possible when nitrate was omitted, showing that Casamino Acids cannot be fermented, thereby proving that degradation is possible only via anaerobic respiration and that OADH2 is involved in oxidation of a component of Casamino Acids (Fig. 4B). Figure 4C shows that growth yields of the wild type and the mutant were identical when they were grown on Casamino Acids aerobically, indicating that OADH2 has an important role solely during anaerobic respiration, not during aerobic respiration.
For further identification of the substrate of OADH2, an attempt was made to grow the wild type and the mutant comparatively in synthetic medium with nitrate as an electron acceptor and branched-chain amino acids or methionine as electron donors, but no growth could be detected with these amino acids alone or in different combinations. As Enterococcus faecalis grows only on branched-chain 2-oxo acids but fails to grow on branched-chain amino acids (44, 45), the 2-oxo acids also were tested, but they could not enable nitrate-respirative growth of H. volcanii. Furthermore, all 20 amino acids and many different combinations of 6 out of the 20 amino acids were tested, but without success. The growth experiments were complemented with a biochemical approach: cell extracts of the wild type grown aerobically or by nitrate respiration were used to search for OADH activity using all known potential substrates. In none of the cases could any enzymatic activity be detected.
Taken together, OADH2 is involved in the oxidation of a component of Casamino Acids, but no indication for the usage of any of the 20 amino acids or for the oxidation of any of the known substrates of OADHs could be found. While the failure to detect an enzymatic activity could be caused by the inability to find the suitable conditions, we regard the lack of growth of the wild type with any of the known precursors of OADH substrates as a strong argument in favor of the possibility that a novel substrate might be used.
Indications for a complex regulation of different degradative pathways.
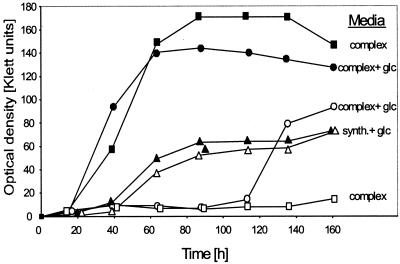
The OADH2 mutant was isolated as a loss-of-function mutant during a 2-bromo-3′-deoxyuridine selection (38). The principle of this method is that cells which are able to grow during incubation under selective conditions, e.g., nitrate-respirative conditions, are killed, while resting cells survive. The discovery that the OADH2 mutant can grow both with glucose and with Casamino Acids as electron donors (Fig. 4A and B) raised the question of why it was possible to isolate it using the BrdU selection. The explanation was found as combinations of substrates were used. Figure 5 shows that the wild type and the mutant grow indistinguishably on glucose and nitrate but that the addition of a complex carbon source, i.e., yeast extract and tryptone, inhibits glucose-mediated growth of the mutant for about 4 days, while no such effect can be seen for the wild type. The mutant was found to be unable to grow in complex medium for at least 5 days in contrast to the wild type, explaining its selection (Fig. 5). In addition, the mutant is readily able to grow in synthetic medium both with glucose and with Casamino Acids as sole electron donors during anaerobic respiration, but its growth is inhibited for about 4 days when a mixture of both donors is used (data not shown). These experiments indicate a complex cross-regulation of the expression of genes encoding enzymes for either of the two degradative pathways.
FIG. 5.
Nitrate-respirative growth of the wild type and the mutant 170/29 on single versus mixed carbon sources. The wild type is represented by filled symbols, and the mutant is represented by open symbols. The following media were used: complex, medium containing tryptone and yeast extract; complex + glc, complex medium containing additional 5 mM glucose; synth. + glc, synthetic medium containing 5 mM glucose as the sole electron donor and carbon source. All media contained 50 mM nitrate as an electron acceptor.
Conclusions.
Taken together, characterization of a nitrate respiration-deficient mutant of H. volcanii has for the first time allowed a functional role for an OADH in an archaeon to be defined. While the substrate specificity could not be elucidated, it is clear that OADH2 has an important role during anaerobic but not during aerobic oxidation of a 2-oxo acid derived from a component of Casamino Acids. The oxidation is linked to nitrate reduction. The results underscore the power of a discovery-based approach and the importance of usage of a genetically tractable model species like H. volcanii. Analysis of the oadh2 gene cluster and its deduced products uncovered several unusual features and led to a variety of interesting questions which future work will focus on, e.g., whether the unattached lipoyl domain is produced and which role it plays during catalysis, whether the E1 subunits of OADH2 functionally and/or physically interact with E2 of OADH1, and the nature of the substrate recognized by OADH2.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft through grant So264/8.
REFERENCES
- 1.Adachi, J., Y. Cao, and M. Hasegawa. 1993. Tempo and mode of mitochondrial DNA evolution in vertebrates at the amino acid sequence level: rapid evolution in warm-blooded vertebrates. J. Mol. Evol. 36:270-281. [DOI] [PubMed] [Google Scholar]
- 2.Aevarsson, A., K. Seger, S. Turley, J. R. Sokatch, and W. G. J. Hol. 1999. Crystal structure of 2-oxoisovalerate dehydrogenase and the architecture of 2-oxo acid dehydrogenase multienzyme complexes. Nat. Struct. Biol. 6:785-792. [DOI] [PubMed] [Google Scholar]
- 3.Bairoch, A., and R. Apweiler. 1999. The SWISS-PROT protein sequence data bank and its supplement TrEMBL in 1999. Nucleic Acids Res. 27:49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg, A., and A. de Kok. 1997. 2-Oxo acid dehydrogenase multienzyme complexes. The central role of the lipoyl domain. Biol. Chem. 378:617-634. [PubMed] [Google Scholar]
- 5.Brown, J. W., C. J. Daniels, and J. N. Reeve. 1989. Gene structure, organization, and expression in archaebacteria. Crit. Rev. Microbiol. 16:287-338. [DOI] [PubMed] [Google Scholar]
- 6.Cline, S. W., W. L. Lam, R. L. Charlebois, L. C. Schalkwyk, and W. F. Doolittle. 1989. Transformation methods for halophilic archaebacteria. Can. J. Microbiol. 35:148-152. [DOI] [PubMed] [Google Scholar]
- 7.Cline, S. W., L. C. Schalkwyk, and W. F. Doolittle. 1989. Transformation of the archaebacterium Halobacterium volcanii with genomic DNA. J. Bacteriol. 171:4987-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuff, J. A., M. E. Clamp, A. S. Siddiqui, M. Finlay, and G. J. Barton. 1998. Jpred: a consensus secondary structure prediction server. Bioinformatics 14:892-893. [DOI] [PubMed] [Google Scholar]
- 9.Danson, M. J., R. Eisenthal, S. Hall, S. R. Kessel, and D. L. Williams. 1984. Dihydrolipoamide dehydrogenase from halophilic archaebacteria. Biochem. J. 218:811-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dardel, F., A. L. Davis, E. D. Laue, and R. N. Perham. 1993. Three-dimensional structure of the lipoyl domain from Bacillus stearothermophilus pyruvate dehydrogenase multienzyme complex. J. Mol. Biol. 229:1037-1048. [DOI] [PubMed] [Google Scholar]
- 11.Denoya, C. D., R. W. Fedechko, E. W. Hafner, H. A. McArthur, M. R. Morgenstern, D. D. Skinner, K. Stutzman-Engwall, R. G. Wax, and W. C. Wernau. 1995. A second branched-chain alpha-keto acid dehydrogenase gene cluster (bkdFGH) from Streptomyces avermitilis: its relationship to avermectin biosynthesis and the construction of a bkdF mutant suitable for the production of novel antiparasitic avermectins. J. Bacteriol. 177:3504-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodd, I. B., and J. B. Egan. 1990. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 18:5019-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Downard, J., and D. Toal. 1995. Branched-chain fatty acids: the case for a novel form of cell-cell signalling during Myxococcus xanthus development. Mol. Microbiol. 16:171-175. [DOI] [PubMed] [Google Scholar]
- 14.Etzold, T., A. Ulyanov, and P. Argos. 1996. SRS: information retrieval system for molecular biology data banks. Methods Enzymol. 266:114-128. [DOI] [PubMed] [Google Scholar]
- 15.Felsenstein, J. 1996. Inferring phylogenies from protein sequences by parsimony, distance, and likelihood methods. Methods Enzymol. 266:418-427. [DOI] [PubMed] [Google Scholar]
- 16.Frishman, D., and H. W. Mewes. 1997. PEDANTic genome analysis. Trends Genet. 13:415-416. [Google Scholar]
- 17.Hanahan, D. 1985. Techniques for transformation of E. coli. In D. Glover (ed.), DNA cloning—a practical approach. IRL Press, Washington, D.C.
- 18.Hofmann, K., P. Bucher, L. Falquet, and A. Bairoch. 1999. The PROSITE database, its status in 1999. Nucleic Acids Res. 27:215-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmes, M. L., and M. L. Dyallsmith. 1990. A plasmid vector with a selectable marker for halophilic archaebacteria. J. Bacteriol. 172:756-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jolley, K. A., D. G. Maddocks, S. L. Gyles, Z. Mullan, S. L. Tang, M. Dyall-Smith, D. W. Hough, and M. Danson. 2000. 2-Oxoacid dehydrogenase multienzyme complexes in the halophilic Archaea? Gene sequences and protein structural predictions. Microbiology 146:1061-1069. [DOI] [PubMed] [Google Scholar]
- 21.Jolley, K. A., E. Rapaport, D. W. Hough, M. J. Danson, W. G. Woods, and M. L. Dyall-Smith. 1996. Dihydrolipoamide dehydrogenase from the halophilic archaeon Haloferax volcanii: homologous overexpression of the cloned gene. J. Bacteriol. 178:3044-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerscher, L., and D. Oesterhelt. 1981. Purification and properties of two 2-oxoacid:ferredoxin oxidoreductases from Halobacterium halobium. Eur. J. Biochem. 116:587-594. [DOI] [PubMed] [Google Scholar]
- 23.Kerscher, L., and D. Oesterhelt. 1977. Ferredoxin is the coenzyme of á-ketoacid oxidoreductases in Halobacterium halobium. FEBS Lett. 83:197-201. [DOI] [PubMed] [Google Scholar]
- 24.Lupas, A. 1996. Prediction and analysis of coiled-coil structures. Methods Enzymol. 266:513-525. [DOI] [PubMed] [Google Scholar]
- 25.Mevarech, M., and R. Werczberger. 1985. Genetic transfer in Halobacterium volcanii. J. Bacteriol. 162:461-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oku, H., and T. Kaneda. 1988. Biosynthesis of branched-chain fatty acids in Bacillus subtilis. A decarboxylase is essential for branched-chain fatty acid synthase. J. Biol. Chem. 263:18386-18396. [PubMed] [Google Scholar]
- 27.Pearson, W. R. 1990. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 183:63-98. [DOI] [PubMed] [Google Scholar]
- 28.Perham, R. N. 2000. Swinging arms and swinging domains in multifunctional enzymes: catalytic machines for multistep reactions. Annu. Rev. Biochem. 69:961-1004. [DOI] [PubMed] [Google Scholar]
- 29.Perham, R. N. 1991. Domains, motifs, and linkers in 2-oxo acid dehydrogenase multienzyme complexes: a paradigm in the design of a multifunctional protein. Biochemistry 30:8501-8512. [DOI] [PubMed] [Google Scholar]
- 30.Perham, R. N., and P. A. Reche. 1998. Swinging arms in multifunctional enzymes and the specificity of post-translational modification. Biochem. Soc. Trans. 26:299-303. [DOI] [PubMed] [Google Scholar]
- 31.Plaga, W., F. Lottspeich, and D. Oesterhelt. 1992. Improved purification, crystallization and primary structure of pyruvate:ferredoxin oxidoreductase from Halobacterium halobium. Eur. J. Biochem. 205:391-397. [DOI] [PubMed] [Google Scholar]
- 32.Pratt, K. J., C. Carles, T. J. Carne, M. J. Danson, and K. J. Stevenson. 1989. Detection of bacterial lipoic acid. A modified gas-chromatographic-mass-spectrometric procedure. Biochem. J. 258:749-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price, L. B., and R. F. Shand. 2000. Halocin S8: a 36-amino-acid microhalocin from the haloarchaeal strain S8a. J. Bacteriol. 182:4951-4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruepp, A., and J. Soppa. 1996. Fermentative arginine degradation in Halobacterium salinarium (formerly Halobacterium halobium): genes, gene products, and transcripts of the arcRACB gene cluster. J. Bacteriol. 178:4942-4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Slupska, M. M., A. G. King, S. Fitz-Gibbon, J. Besemer, M. Borodovsky, and J. H. Miller. 2001. Leaderless transcripts of the crenarchaeal hyperthermophile Pyrobaculum aerophilum. J. Mol. Biol. 309:347-360. [DOI] [PubMed] [Google Scholar]
- 37.Soppa, J. 1999. Transcription initiation in Archaea: facts, factors and future aspects. Mol. Microbiol. 31:1295-1305. [DOI] [PubMed] [Google Scholar]
- 38.Soppa, J. 1998. Optimization of the 5-bromo-2′-deoxyuridine selection and its application for the isolation of nitrate respiration-deficient mutants of Haloferax volcanii. J. Microbiol. Methods 34:41-48. [Google Scholar]
- 39.Soppa, J. 1994. Compilation of halobacterial protein-coding genes, the halobacterial codon usage table and its use. Syst. Appl. Microbiol. 16:725-733. [Google Scholar]
- 40.Thompson, D. K., and C. J. Daniels. 1998. Heat shock inducibility of an archaeal TATA-like promoter is controlled by adjacent sequence elements. Mol. Microbiol. 27:541-551. [DOI] [PubMed] [Google Scholar]
- 41.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vettakkorumakankav, N. N., and K. J. Stevenson. 1992. Dihydrolipoamide dehydrogenase from Haloferax volcanii: gene cloning, complete primary sequence and comparison to other dihydrolipoamide dehydrogenases. Biochem. Cell. Biol. 70:656-663. [DOI] [PubMed] [Google Scholar]
- 43.Wanner, C., and J. Soppa. 1999. Genetic identification of three ABC transporters as essential elements for nitrate respiration in Haloferax volcanii. Genetics 152:1417-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward, D. E., C. C. van der Weijden, M. J. van der Merwe, H. V. Westerhoff, A. Claiborne, and J. L. Snoep. 2000. Branched-chain alpha-keto acid catabolism via the gene products of the bkd operon in Enterococcus faecalis: a new, secreted metabolite serving as a temporary redox sink. J. Bacteriol. 182:3239-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ward, D. E., R. P. Ross, C. C. van der Weuden, J. L. Snoep, and A. Claiborne. 1999. Catabolism of branched-chain α-keto acids in Enterococcus faecalis: the bkd gene cluster, enzymes, and metabolic route. J. Bacteriol. 181:5433-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]