Abstract
During prolonged incubation in stationary phase Escherichia coli undergoes starvation-induced differentiation, resulting in highly resistant cells. In rich medium with high amino acid content further incubation of cultures at high cell density leads to the generation of a population of cells no longer able to form colonies. The viability loss is due to some component of spent medium, active at high pH and high cell density, and can be prevented either by keeping the pH close to neutrality, by washing off the nonsalt components of the medium, or by keeping the saturating cell density low. Exposure to short-chain n-alcohols within a specific time window in stationary phase also prevents viability loss, in an rpoS-dependent fashion. The development of stress resistance, a hallmark of stationary-phase cells, is affected following alcohol treatment, as is the response to extracellular factors in spent medium. Alcohols seem to block cells in an early phase of starvation-induced differentiation, most likely by interfering with processes important for regulation of σs such as cell density signals and sensing the nutrient content of the medium.
The cessation of growth, a part of the typical feast and famine bacterial lifestyle, follows nutrient exhaustion and attainment of high cell density. As they progress into stationary phase, bacteria typically undergo a developmental program bringing about a number of morphological and physiological changes, resulting in cells strikingly different from vegetatively growing ones. These differentiated cells display increased resistance to stresses and are able to survive prolonged nutrient deprivation.
Upon prolonged incubation in stationary phase viable counts start to decline unless fresh nutrients are provided. The crucial processes and cell components whose malfunction underlies cellular death are still unclear. Even though the term senescence (aging) is often used to describe this phase of the bacterial life cycle, it is not clear whether the observed dynamic of viable counts in standard laboratory media is indeed consistent with the definition of senescence (18). One question that follows is whether the progression into stationary phase and ultimately death is the result of mere nutrient and energy exhaustion or whether it is the result of an active process, i.e., a genetic program. The presence of genes coding for pairs of toxin and antidote molecules (termed addiction molecules) in the Escherichia coli chromosome (1) hints at the possibility that programmed cell death could occur in a subpopulation of cells. Nutrients released from those cells could provide an energy source for the cells that remain viable. For E. coli populations in stationary phase, cell debris can indeed serve as a nutrient source supporting survival and even growth as shown by cells exhibiting the growth advantage in stationary phase (GASP) phenotype, which resume growth in stationary phase, scavenging nutrients released from the dead cells (34, 35). However, the growth resumption underlying the GASP phenomenon is due to mutation(s) and is a case of evolutionary cheating rather than cooperation in ensuring the survival of the clone (31). Another possibility is that programmed death of part of a starved E. coli population would be a parallel to the fate of stalk cells in fruiting bodies of bacteria such as Myxococcus xanthus and of slime molds such as Dictyostelium discoideum, some of whose cells die during multicellular development to ensure the survival of the clone. In order to understand the processes underlying survival and/or cell death during starvation conditions, a detailed knowledge of the transition into stationary phase is needed.
Here we attempted to gain further insights into the transition of E. coli into stationary phase by identifying the condition(s) under which the typical decline in viability is altered, i.e., in which the typical life span of nondividing bacteria is prolonged or reduced. Identifying such a condition would be instrumental in understanding the process underlying the loss of viability in general. Taking this approach, we have found that at least in rich medium (Luria-Bertani [LB]), pH and cell density are crucial parameters underlying viability loss, suggesting an important role for self-generated extracellular signals. The production and/or sensing of such signal(s) can be interrupted by the addition of a relatively low concentration of several n-alcohols, resulting in delay of viability loss for extended periods of time. In other well-studied cases of starvation-induced developmental processes, such as sporulation in Bacillus subtilis and filamentous growth in Saccharomyces cerevisiae, 1-alcohols are known to have profound effects on cells upon nutrient exhaustion. Ethanol completely blocks sporulation in B. subtilis (4), whereas ethanol and 1-butanol stimulate hyperfilamentation in diploid cells and filamentous growth of haploid cells, leading to a pseudohyphal morphotype in S. cerevisiae (21). Also worth noting is the effect of ethanol in Lactobacillus coryniformis, where it prevents the decline of antifungal activity production during prolonged incubation in stationary phase (23). All together these results indicate that short-chain alcohols can interfere with key processes during starvation-induced development. Elucidating their mechanisms of action will advance our understanding of the processes they affect.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The E. coli strains used in this work were MG1655 (from the J. Beckwith collection, Harvard Medical School) and its derivative with a mutation in rpoS constructed by P1 transduction from ZK1000 (3). The effect of alcohol was also tested with other strains (data not shown) such as MG1655 from the E. coli Genetic Stock Center, ZK126 (W3110 derivative, lab collection), and ECOR strains 13, 29, 37, 38, 51, and 71 (27). The indicator strain used in bioassays for measuring ampicillin and chloramphenicol concentrations was BAS901, carrying the imp4213 allele (28) (from Spencer Benson). Cultures were grown in LB (10 g of tryptone per liter, 5 g of yeast extract per liter, and 5 g of NaCl per liter) rich medium unless stated otherwise (5 ml) in 180- by 15-mm glass test tubes at 37°C and 60% humidity, with aeration. CFU counts were monitored for 5 to 7 days by plating appropriate dilutions onto LB agar plates and counting colonies after 24 h of incubation at 37°C (plates were also checked after a further 24-h incubation for additional colonies). Shown in the figures are data from single representative experiments, but all experiments were repeated multiple times to ensure the reproducibility of results. Unless indicated otherwise, alcohols and other compounds tested were added at 24 h after inoculation. The typical ethanol concentration used was 125 mM (0.75%). Biological buffers used were HEPES/KOH (pH 7) (N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid]) and AMPSO/KOH (pH 9) {3-[(1,1-dimethyl-2-hydroxyethyl)amino]-2-hydroxy-propanesulfonic acid}, at a final concentration of 100 mM.
Ampicillin and chloramphenicol treatments.
In ampicillin-treated cultures the antibiotic was added 24 h after inoculation at a final concentration of 150 μg/ml. An additional 80 μg/ml was added at 36, 48, 72, 96, and 120 h after inoculation in order to compensate for ampicillin degradation and to keep the concentration higher than 25 μg/ml. The concentration was monitored as follows. Sterile paper disks 13 mm in diameter were soaked with 100 μl of culture supernatant (cells were removed by centrifugation) and applied onto a lawn of BAS901 (the imp4213 allele renders the strain hypersensitive to ampicillin and other antibiotics). After 24 h of incubation at 37°C, the zone of growth inhibition was measured and compared to those obtained with antibiotic solutions of known concentrations. In chloramphenicol-treated cultures the antibiotic, dissolved in 100% ethanol or isopropanol, was added at 24 h after inoculation. Resulting concentrations of chloramphenicol, ethanol, and isopropanol were 200 μg/ml, 125 mM, and 125 mM, respectively. An additional 30 μg/ml (dissolved in isopropanol) was added at 48, 72, 96, and 120 h after inoculation in order to compensate for degradation and to keep the concentration higher than 30 μg/ml. Control cultures for these experiments were treated with only ethanol or isopropanol at 24 h and with isopropanol at 48, 72, 96, and 120 h after inoculation. The concentration of chloramphenicol was monitored daily as described above.
Measurements of β-galactosidase activity.
Alcohols were added to 24-h-old LB cultures. After 3 h of incubation chloramphenicol, dissolved in isopropanol, was added at a final concentration of 200 μg/ml. Following an additional 1-h incubation, 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added to the cultures. The first β-galactosidase measurement was done 1 h after the addition of chloramphenicol, and the second one was done 1.5 h after the addition of IPTG. Prior to measurements, culture samples were centrifuged, the supernatant was discarded, and the cell pellet was resuspended in Z buffer (24). β-Galactosidase levels were measured as described by Miller (24), except that samples were centrifuged prior to the measurement of optical density at 420 nm. Measurements were also performed on control cultures not treated with alcohols, chloramphenicol (the amount of isopropanol corresponding to that of the chloramphenicol stock solution used in parallel cultures was included), or IPTG.
Acid resistance assay.
Cultures of the wild type and the rpoS mutant in LB medium were set up as described above. Ethanol was added 24 h after inoculation. Twenty-four, 48, and/or 72 h afterwards, viable counts were determined by plating appropriate dilutions onto LB agar plates. To measure resistance to acid stress, culture samples were diluted 100-fold in 2 ml of fresh LB-HCl (pH 2.5); serial dilutions were plated onto LB agar plates 2 h after incubation at 37°C, and CFU counts were calculated.
RESULTS AND DISCUSSION
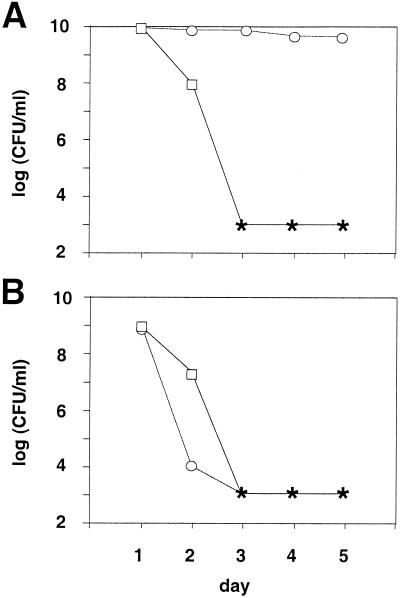
After prolonged incubation in stationary phase, E. coli generates a population of cells that show no metabolic activity of any sort and are no longer able to form colonies, which is reflected as a drop in CFU. Different conditions of starvation lead to different kinetics of decline in viable cell counts. After the initial drop, the further loss of viability typically slows down, after which the viable counts stabilize. However, this apparent dynamic does not necessarily reflect a decreased mortality rate because by the time the decline in viability slows down the population is no longer homogeneous. Prolonged incubation in stationary phase inevitably selects for mutants exhibiting the GASP phenotype, which are able to reinitiate growth under this particular condition by scavenging nutrients released by dead cells (34, 35). The growth of this subpopulation of mutant cells and its effect on the survival of the original population impede the analysis of mortality rates by monitoring overall CFU counts. In order to circumvent such an interference, we monitored the CFU counts in cultures treated with ampicillin, which prevents growth but does not have any effect on nondividing cells. Under such conditions, in which the growth of GASP mutants is blocked, the initial exponential loss of viability continues; i.e., the mortality rate stays nearly constant for at least 3 days, after which it increases (Fig. 1). This observed loss of viability is not caused by ampicillin itself, as shown by the viability profile of ampicillin-treated cultures grown in medium buffered to pH 7 (neutral pH prevents viability loss typically observed in nonbuffered LB medium; see below). Given that one of the criteria for defining the senescence is an increase in the mortality rate over time (18), it appears that in rich LB medium, after an initial phase in which it undergoes stochastic death, E. coli indeed senesces. The question remains as to which factors influence the observed loss of viability. In other words, what are the parameters that define its onset and, once started, its dynamic, i.e., the slope of the survival curve?
FIG. 1.
Viability of Escherichia coli K-12 in stationary phase in the absence of the GASP phenotype. (A) The growth of GASP mutants was prevented by treatment with ampicillin. Circles represent viable counts from 20 independent, ampicillin-treated cultures. Closed symbols represent values of less than 104 or 103 CFU/ml, which were found in the majority of cases at days 4 and 5. Horizontal bars represent median values. Squares represent viable counts from three independent ampicillin-treated cultures in which LB medium was buffered at pH 7. (B) Squares represent ampicillin concentration in a representative culture, measured as described in Materials and Methods.
LB medium is a complex mixture of nutrients, containing large amounts of amino acids and peptides as well as a relatively high concentration of sodium chloride. Growth of E. coli in LB medium results in alkalinization of the medium, presumably due to the release of basic waste products from amino acid metabolism. After 24 h the pH reaches 8.5 and increases to 9 within the next 24-h period. If LB medium is buffered at pH 7 either before or 24 h after inoculation, there is no loss of viable counts during at least the first 4 to 5 days, showing that the alkalinization of the medium is an important parameter underlying stationary-phase viability loss (Fig. 2 A). At high pH, membrane proteins can become denatured to various degrees, which in turn can affect their function. Because the effect of alkalinization on cell physiology is pleiotropic, it is not clear which pH-sensitive process is responsible for the viability loss. However, cells from 24-h-old LB cultures washed and transferred to 86 mM NaCl (at the same concentration as in LB medium) or to 10 mM MgSO4, both buffered to pH 9, do not show significant viability loss for at least 5 days (Fig. 2B). This suggests that the viability loss at high pH is mainly due to the adverse effect of some component(s) of the spent LB medium instead of high pH per se.
FIG. 2.
Influence of pH and cell density on viability of Escherichia coli K-12 in stationary phase. (A) Viability in nonbuffered LB medium (▿) or LB medium buffered at pH 7 at inoculation (○) or at 24 h after inoculation (□). (B) Viability in 86 mM NaCl (○) or 10 mM MgSO4 (□) solution buffered to pH 9. Cells were grown in LB medium for 24 h before transfer to salt solutions. (C) Viability in diluted LB medium. Cells were grown in media with 1/10 (circles), 1/100 (squares), and 1/1,000 (triangles) the amount of tryptone and yeast extract present in LB medium. Open symbols represent nonbuffered media, closed symbols represent media buffered to pH 9, and hatched symbols represent media buffered to pH 9 24 h after inoculation. (D) Viability in concentrated (squares) or standard (triangles) LB medium at different cell densities. Closed symbols represent cultures in which the majority of cells were removed at 24 h, and open symbols represent control cultures.
Cells grown in diluted LB medium in which the salt concentration is kept at 86 mM saturate at lower cell densities, and in that case viable counts stay constant for at least 5 days at both neutral and high pHs (Fig. 2C). On the other hand, if the number of cells in high-density cultures is decreased by removing a fraction of the cells, the remaining population still undergoes viability loss (Fig. 2D). This means that the medium component affecting viability is present only after growth to high cell densities (>108 CFU/ml). In addition, if the concentration of yeast extract and tryptone is doubled and cell density is increased, viability loss is more pronounced (Fig. 2D).
Another condition that prevents viability loss for extended periods of time is the exposure of the stationary-phase cells to short-chain n-alcohols. The addition of ethanol, 1-propanol, or 1-butanol to stationary-phase cultures of E. coli completely prevents the loss of viability during prolonged incubation (Fig. 3A). This effect is not specific to laboratory strains, as several clinical and environmental isolates respond in the same way (data not shown). It is observed to occur in different media (all with high amino acid content) and at different temperatures. There is a specific time window during which the exposure to alcohols has to occur for the delay in viability loss to be observed. The effect is reversible, as viability loss resumes within a 24-h period if the alcohol is removed from the medium (Fig. 3B).
FIG. 3.
Influence of n-alcohols on the viability of E. coli K-12 in stationary phase. (A) Ethanol (○), 1-propanol (□), and 1-butanol (⋄) were added at 24 h into LB cultures at 125, 80, and 50 mM final concentrations, respectively. ▿, nontreated culture. (B) Viability loss resumes upon removal of alcohol. Ethanol was added to 24-h-old LB cultures, and 24 h later they were centrifuged and cells were resuspended in the supernatant of a parallel untreated culture (○) or in the same supernatant (□). (C) Addition of alcohols to LB cultures buffered to pH 9 at 24 h. Ethanol (○), 1-propanol (□), and 1-butanol (⋄) were added to 24-h-old cultures at 125, 80, and 50 mM final concentrations, respectively. (D) Ethanol (125 mM) was added to a 24-h-old LB culture, which was subsequently treated with ampicillin.
Short-chain alcohols are biologically very versatile compounds. Due to their physical and chemical properties, they are able to interact with many cellular components and processes. Being soluble in both water and lipids, they readily influence biological systems, affecting lipid-lipid and lipid-protein as well as protein-protein interactions. E. coli can metabolize ethanol, oxidizing it to acetate (7), but exposure to high concentrations of ethanol impedes its growth. Growing E. coli counteracts the effects of alcohols on its membranes by changing the fatty acid composition in membrane lipids (17). Concentrations which impede growth are known to induce several stress responses such as the heat shock, psp and usp regulons, which are diagnostic of protein denaturation (14, 25, 32).
The oxidation of ethanol to acetate ultimately results in the excretion of protons from the cell (7); therefore, it is possible that the delay in the viability loss after the addition of ethanol is due to the decreasing pH of the medium. Indeed, the pH of the medium decreases by 1 to 1.5 units after 36 to 48 h following the addition of alcohol. In medium buffered to pH 9, either prior to inoculation or after reaching stationary phase, alcohols still delay viability loss; therefore, their effect is only partially due to the counteracting of the alkalinization of the medium (Fig. 3C). Consistent with this, the addition of other compounds to the stationary-phase cultures that also decrease the pH of the medium, such as acetate, glucose, trehalose, mannose, mannitol, or dulcitol, does not extend viability. Furthermore, the ethanol effect is observed even in a strain lacking alcohol dehydrogenase (AdhE), the principal enzyme carrying out the first two steps of ethanol oxidation. Although AdhE is the main enzyme that reduces alcohols, other enzymes might be able to use them as a substrate, though not efficiently, such as propane-diol dehydrogenase (7). Even if alcohols are used as an energy and carbon source, their effect on viability is not due to growth and cell turnover because the effect is not abolished by the addition of ampicillin (Fig. 3D).
Short-chain alcohols can change the fluidity of the membrane and hence its permeability (10, 13). Growing cells resist these changes by adjusting the composition of fatty acids in membrane lipids (17). This is accomplished by mobilization of membrane lipids and their selective breakdown and resynthesis. The transition into stationary phase also involves a change in the fatty acid composition of the inner membrane lipids, and it has been suggested that the breakdown of membrane lipids could be the main energy source in stationary phase (9). Alcohols could interfere with this process by mobilizing lipids and providing energy, or by rendering membranes more permeable, allowing utilization of nutrients still present in the medium. fad and cfa mutants, however, respond to ethanol (data not shown), meaning that the breakdown and resynthesis of fatty acids and their conversion to cyclopropane derivatives are not a major route of the alcohol effect on viability. Furthermore, if fluidization of the membrane were the key event, it would be difficult to explain the inability of methanol and isopropanol to elicit the same effect.
During vegetative growth ethanol and other alcohols can induce several stress responses in a concentration-dependent manner. For example, exposure to alcohols induces the heat shock, usp and psp operons (14, 25, 32). In each case, a specific set of proteins is induced and their actions counteract the initial perturbation. It is possible that by exposing cells to alcohols early in stationary phase the induction of these responses makes cells more resistant to subsequent stresses and therefore they survive longer than untreated ones. However, the effective concentrations needed for the full induction of these responses are much higher than those preventing viability loss (4 to 10% and <1%, respectively). uspB mutants do respond to stationary-phase ethanol exposure by a delay in viability loss (data not shown), showing that this effect is at least not dependent on usp functions at the ethanol concentration used here. Heat shock is normally induced in stationary phase (11), and exposure to alcohols could reinforce or extend this response.
Finally, the effect of alcohols on viability is independent of protein synthesis, as shown by its resistance to chloramphenicol (Fig. 4A). To exclude the possibility that this result is due to the inability of chloramphenicol to enter stationary-phase cells, we measured the induction of native β-galactosidase by IPTG in 1-day-old cultures. This induction was completely suppressed in the presence of chloramphenicol, showing that it effectively inhibits protein synthesis even under stationary-phase conditions (Fig. 4B). The exposure of cells to ethanol, butanol, or isopropanol prior to induction did not have any effect on the action of chloramphenicol.
FIG. 4.
The effect of alcohol is independent of protein synthesis. (A) Ethanol was added to 24-h-old LB cultures with (□) or without (○) 200 μg of chloramphenicol per ml. Isopropanol was added to control cultures with (▵) or without (⋄) chloramphenicol (200 μg/ml). An additional 30 μg/ml (dissolved in isopropanol) was added at 48, 72, 96, and 120 h after inoculation in order to compensate for degradation. Asterisk, CFU counts below 105/ml; ▪, chloramphenicol concentration (right-hand axis), measured by a bioassay described in Materials and Methods. (B) Induction of native β-galactosidase by IPTG in 1-day-old LB cultures. Shown are induction levels before (hatched bars) and 1.5 h after the addition of IPTG (open bars). Indicated cultures were treated with chloramphenicol (200 μg/ml) and alcohols before the addition of IPTG (see Materials and Methods for details).
Taken together these results suggest that the alcohols act directly, as opposed to acting through indirect effects such as decreasing pH, supporting cell turnover, or inducing some protective stress response(s). Increasing evidence from studies with eukaryotic cells suggests that the alcohols alter cell functions by interacting directly with selective proteins, including ion channels, kinases, and transporters (8, 15). Studies with 1-alcohols of increasing chain length established different cutoffs for alcohol effects on diverse proteins in vitro and in vivo. The inactivity of 1-alcohols of greater chain length and hence greater hydrophobicity than those below the cutoff is consistent with the view that the active 1-alcohols interact with proteins rather than lipid sites. In the case of stationary-phase E. coli cells, viability loss is delayed by ethanol, propanol, butanol, and to a limited extent by pentanol (Table 1). Among other 1-alcohols, methanol, hexanol, and octanol have no effect. Isopropanol, 1-butenol, and 3,3-dimethyl-1-butanol have no effect either, demonstrating that there are structural requirements for alcohol-delayed viability loss.
TABLE 1.
Effects of different compounds on viability in stationary phasea
| Compound | Concentration (mM) | Effect on viability |
|---|---|---|
| Methanol | 75-200 | 0 |
| Ethanol | 20-130 | + |
| 1-Propanol | 75-80 | + |
| 2-Propanol | 95 | 0 |
| 1-Butanol | 50-60 | + |
| 1-Pentanol | 10-20 | ± |
| 50 | − | |
| 1-Hexanol | 1-5 | 0 |
| 1-Octanol | 0.25-0.5 | 0 |
| 1 | − | |
| 1-Butenol | 5-15 | − |
| 3,3-Dimethyl-1-butanol | 1-3 | − |
| Hexane | 1.5-5 | 0 |
| 120 | − |
Indicated compounds were added to 24-h-old LB cultures of the wild type, and CFU counts were monitored during the next 5 days. Preventing viability loss, exacerbating viability loss, and no effect on viability loss were scored as +, −, and 0, respectively.
Since the alcohol effect is independent of protein synthesis, it follows that a putative protein target is already present in the cell, most likely in the membrane. Because the alcohols are added to stationary-phase cultures, we tested whether the same effect is observed in mutants of the stationary-phase-specific sigma factor, σs, encoded by the rpoS gene. rpoS mutants lose viability even after exposure to alcohols, demonstrating that this is a genuine stationary-phase phenomenon; i.e., the target of alcohol's effect is specific to the stationary-phase cell (Fig. 5A).
FIG. 5.
The effect of alcohol is dependent on RpoS function(s). (A) Ethanol (○) was added at 24 h into LB cultures of an rpoS mutant strain. □, nontreated culture. (B) Survival of wild-type and rpoS mutant strains following acid stress. Shown are median values of CFU counts in LB cultures of indicated strains (with and without ethanol) at days 2, 3, and 4 from four independent cultures before (open bars) and after (hatched bars) 2.5-h exposure to pH 2.5 in fresh medium. Asterisk, CFU counts below 104/ml.
In E. coli, progression into stationary phase is accomplished by a complex genetic program governed in large part by the transcriptional regulator σs (16, 20). It is a response to several environmental parameters such as a drop in nutrient concentration (carbon, nitrogen, phosphate), quorum-sensing signals, osmolarity, and temperature. Subsequently, the expressions of more than 50 genes are affected, positively or negatively, resulting in cells quite different from vegetatively growing ones, able to resist various environmental stresses with drastically reduced metabolic activity. The action of 1-alcohols seems to specifically interfere with this starvation-induced differentiation. Since stress resistance is a hallmark of the stationary-phase cell, we measured resistance of stationary-phase cultures to acid stress in order to establish whether the exposure to alcohols can change this process. As shown in Fig. 5B, the acid stress resistance profile of ethanol-treated cultures is different from that of nontreated ones. Resistance to acid stress is primarily under σs control (29), and because σs is also a master regulator of the starvation response, the acid stress profile as a function of time is particularly revealing: the resistance of untreated cells increases over time, whereas the resistance of ethanol-treated ones stays the same throughout. rpoS mutants fail to develop this resistance with or without alcohol treatment.
Taken together, these results suggest that alcohols block starvation-induced development and cells are hence locked in a specific phase of that process. Consistent with this are the specific timings of alcohol exposure needed for observable effect and the reversibility of the effect by removal of alcohol. If this is so, then functions known to be important for long-term survival in spent LB medium, i.e., for very late stationary phase, should be dispensable in cells treated with alcohols. Indeed, one such mutant, surA, which poorly survives long-term incubation in stationary phase, survives as well as the wild type when exposed to ethanol, even in spent LB medium buffered to high pH (data not shown).
Several genes in E. coli are known to be induced as a response to different self-produced extracellular factors after growth in LB medium (2). The expression of rpoS itself is stimulated by such extracellular signal(s) (19). It is possible that alcohols, or the metabolites they are converted into, interfere with the production and/or response to these extracellular factors. The nature of these compounds is still not known, but the composition of signaling molecules identified so far in E. coli and other bacteria includes amino acids, peptides, fatty acids, and acylated homoserine lactones. Genes involved in the uptake, synthesis, or degradation of amino acids that yield pyruvate and succinate are highly induced in spent LB medium at high cell densities (2). One of them, tna, encoding a tryptophanase, suggests a link between tryptophan metabolism and stationary-phase survival. Indeed, the addition of tryptophan to the stationary-phase cultures greatly exacerbates the viability loss (Fig. 6). This adverse effect of tryptophan is completely abolished if the culture is exposed to ethanol prior to the addition of tryptophan. In rpoS mutant cells, unable to respond to ethanol, tryptophan's effect on viability cannot be reversed by the presence of ethanol (Fig. 6B). This suggests that ethanol can interfere with specific signaling inputs able to influence typical stationary-phase development. Lending support to this view is the finding that the expression of cma lacZ fusions (conditioned medium activated) in media conditioned by alcohol-treated cells is different from that observed with untreated cells (data not shown).
FIG. 6.
Effects of tryptophan on stationary-phase viability loss.( A) Addition of tryptophan (2 mM) at 24 h into an LB culture of the wild type exacerbates viability loss (□). Ethanol treatment abolishes the adverse effect of tryptophan (○). (B) Effects of tryptophan on an rpoS mutant strain with (○) or without (□) ethanol treatment. Asterisk, CFU counts below 103/ml.
Similar effects resulting from exposure to alcohols have been observed with other organisms. Nutrient depletion induces development of B. subtilis into highly resistant spores, and exposure to ethanol within a specific time frame after logarithmic growth blocks sporulation altogether, locking the cells in the early phase of this process (4). The antifungal activity of L. coryniformis is at its peak at the onset of stationary phase and decreases during further incubation, but the addition of ethanol to the medium prevents this decline, effectively locking cells at this early stage in stationary phase (23). In S. cerevisiae, nitrogen starvation and poor carbon sources induce differentiation into a filamentous growth form. Ethanol and 1-butanol interfere with this process and stimulate hyperfilamentation in diploid cells and filamentous growth of haploid cells leading to a pseudohyphal morphotype, respectively (21). In higher eukaryotes, exposure to ethanol is associated with different impairments of developing and mature nervous systems (6). In human cell lines various effects of ethanol and other alcohols on cell development have been described, ranging from inhibition of cell-cell adhesion to delays in cell cycle and perturbation of energy metabolism (5, 12, 22).
How alcohols induce these changes remains largely unknown. For yeast, genetic analyses of alcohol-induced changes suggest that alcohols interfere with sensing of nutrient limitation and metabolic by-products that regulate differentiation (21). Several proteins in human cell lines have been identified as directly inhibited by 1-alcohols, such as the L1 immunoglobulin cell adhesion molecule and protein kinase C (30, 33), but the sequence of events leading to the observed effects is still poorly understood (33). The mechanism of ethanol-induced sporulation block in Bacillus is also unknown.
The results presented here show that in E. coli alcohols interfere with starvation-induced differentiation. In LB and other media with high amino acid content this differentiation, happening at high cell densities, leads to cell death unless the increase in pH is prevented. Therefore, death under these conditions is the result of an active process, i.e., a genetic program, rather than simple exhaustion of energy and nutrients. By blocking differentiation, alcohols prevent the viability loss in these media. They cause this developmental block by affecting one or several processes carried out by the products of genes under the control of σs. The regulation of σs, and hence of the entire stationary-phase differentiation, is complex and involves the integration of a multitude of intracellular and extracellular signals. The amounts of nutrients and cell density signals seem to be crucial for the induction of σs (19, 26). Alcohols, as they are able to interfere with many cellular processes, most likely interfere with several of these inputs and therefore block much of the σs-regulated cascade. The identification of the specific target processes as well as proteins and genes will be helpful in elucidating starvation-induced differentiation and ultimately cell death.
Acknowledgments
We thank Michael Bianchetta for help in writing the manuscript.
This work was supported by grants from NIH (GM55199) and NSF (MCB9728936) to R.K. and by a Bernard Fields Postdoctoral Award and Charles E. Culpeper grant (Rockefeller Brothers Fund) awarded to M.V.
REFERENCES
- 1.Aizenman, E., H. Engelberg-Kulka, and G. Glaser. 1996. An Escherichia coli chromosomal “addiction module” regulated by 3′,5′-bispyrophosphate: a model for programmed bacterial cell death. Proc. Natl. Acad. Sci. USA 93:6059-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baca-DeLancey, R. R., M. M. T. South, X. Ding, and P. N. Rather. 1999. Escherichia coli genes regulated by cell-to-cell signaling. Proc. Natl. Acad. Sci. USA 96:4610-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohannon, D. E., N. Connell, J. Keener, A. Tormo, M. Espinosa-Urgel, M. M. Zambrano, and R. Kolter. 1991. Stationary-phase-inducible “gearbox” promoters: differential effects of katF mutations and the role of σ70. J. Bacteriol. 173:4482-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohin, J. P., D. Rigomier, and P. Schaeffer. 1976. Ethanol sensitivity of sporulation in Bacillus subtilis: a new tool for the analysis of sporulation process. J. Bacteriol. 127:934-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charness, M. E., R. M. Safran, and G. Perides. 1994. Ethanol inhibits neural cell-cell adhesion. J. Biol. Chem. 269:9304-9309. [PubMed] [Google Scholar]
- 6.Charness, M. E., R. P. Simon, and D. A. Greenberg. 1989. Ethanol and the nervous system. N. Engl. J. Med. 321:442-454. [DOI] [PubMed] [Google Scholar]
- 7.Clark, D. P., and J. E. Cronan, Jr. 1996. Two-carbon compounds and fatty acids as carbon sources, p. 343-357. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 8.Diamond, I., and A. S. Gordon. 1997. Cellular and molecular neuroscience of alcoholism. Physiol. Rev. 77:1-20. [DOI] [PubMed] [Google Scholar]
- 9.DiRusso, C. C., and T. Nyström. 1998. The fats of Escherichia coli during infancy and old age: regulation by global regulators, alarmones and lipid intermediates. Mol. Microbiol. 27:1-8. [DOI] [PubMed] [Google Scholar]
- 10.Dombek, K. M., and L. O. Ingram. 1984. Effects of ethanol on the Escherichia coli plasma membrane. J. Bacteriol. 157:233-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dukan, S., and T. Nyström. 1998. Bacterial senescence: stasis results in increased and differential oxidation of cytoplasmic proteins leading to developmental induction of the heat shock regulon. Genes Dev. 12:3431-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dwyer, D. S., Y. Liu, and R. J. Bradley. 1999. An ethanol-sensitive variant of the PC12 neuronal cell line: sensitivity to alcohol is associated with increased cell adhesion and decreased glucose accumulation. J. Cell. Physiol. 178:93-101. [DOI] [PubMed] [Google Scholar]
- 13.Eaton, L. C., T. F. Tedder, and L. O. Ingram. 1982. Effects of fatty acid composition on the sensitivity of membrane functions to ethanol in Escherichia coli. Subst. Alcohol Actions Misuse 3:77-87. [PubMed] [Google Scholar]
- 14.Farewell, A., K. Kvint, and T. Nyström. 1998. uspB, a new σS-regulated gene in Escherichia coli which is required for stationary-phase resistance to ethanol. J. Bacteriol. 180:6140-6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris, R. A. 1999. Ethanol actions on multiple ion channels: which are important? Alcohol Clin. Exp. Res. 23:1563-1570. [PubMed] [Google Scholar]
- 16.Hengge-Aronis, R. 2000. The general stress response in Escherichia coli, p. 161-178. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 17.Ingram, L. O. 1976. Adaptation of membrane lipids to alcohols. J. Bacteriol. 125:670-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, F. B., D. A. Sinclair, and L. Guarente. 1999. Molecular biology of aging. Cell 96:291-302. [DOI] [PubMed] [Google Scholar]
- 19.Liu, X., C. Ng, and T. Ferenci. 2000. Global adaptations resulting from high population densities in Escherichia coli cultures. J. Bacteriol. 182:4158-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loewen, P. C., B. Hu, J. Strutinsky, and R. Sparling. 1998. Regulation in the rpoS regulon of Escherichia coli. Can. J. Microbiol. 44:707-717. [DOI] [PubMed] [Google Scholar]
- 21.Lorenz, M. C., N. Shane Cutler, and J. Heitman. 2000. Characterization of alcohol-induced filamentous growth in Saccharomyces cerevisiae. Mol. Biol. Cell 11:183-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo, J., J. R. West, R. T. Cook, and N. J. Pantazis. 1999. Ethanol induces cell death and cell cycle delay in cultures of pheochromocytoma PC12 cells. Alcohol Clin. Exp. Res. 23:644-656. [PubMed] [Google Scholar]
- 23.Magnusson, J., and J. Schnürer. 2001. Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ. Microbiol. 67:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Neidhardt, F. C., and R. A. VanBogelen. 1987. Heat shock response, p. 1334-1345. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 26.Notley, L., and T. Ferenci. 1996. Induction of RpoS-dependent functions in glucose-limited continuous culture: what level of nutrient limitation induces stationary phase of Escherichia coli? J. Bacteriol. 178:1465-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochman, H., and R. K. Selander. 1984. Standard reference strains of Escherichia coli from natural populations. J. Bacteriol. 157:690-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampson, B. A., R. Misra, and S. A. Benson. 1989. Identification and characterization of a new gene of Escherichia coli K-12 involved in outer membrane permeability. Genetics 122:491-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Small, P., D. Blankenhorn, D. Welty, E. Zinser, and J. L. Slonczewski. 1994. Acid and base resistance in Escherichia coli and Shigella flexneri: role of rpoS and growth pH. J. Bacteriol. 176:1729-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stubbs, C. D., and S. J. Slater. 1999. Ethanol and protein kinase C. Alcohol Clin. Exp. Res. 23:1552-1560. [PubMed] [Google Scholar]
- 31.Vulić, M., and R. Kolter. 2001. Evolutionary cheating in Escherichia coli stationary phase cultures. Genetics 158:519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiner, L., J. L. Brissette, and P. Model. 1991. Stress-induced expression of the Escherichia coli phage shock protein operon is dependent on σ54 and modulated by positive and negative feedback mechanisms. Genes Dev. 5:1912-1923. [DOI] [PubMed] [Google Scholar]
- 33.Wilkemeyer, M. F., A. B. Sebastian, S. A. Smith, and M. E. Charness. 2000. Antagonists of alcohol inhibition of cell adhesion. Proc. Natl. Acad. Sci. USA 97:3690-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zambrano, M. M., D. A. Siegele, M. Almirón, A. Tormo, and R. Kolter. 1993. Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science 259:1757-1760. [DOI] [PubMed] [Google Scholar]
- 35.Zinser, E. R., and R. Kolter. 1999. Mutations enhancing amino acid catabolism confer a growth advantage in stationary phase. J. Bacteriol. 181:5800-5807. [DOI] [PMC free article] [PubMed] [Google Scholar]