Abstract
In the presence of urea the neutrophilic human pathogen Helicobacter pylori survives for several hours at pH 1 with concomitant cytoplasmic pH homeostasis. To study this effect in detail, the transmembrane proton motive force and cytoplasmic urease activity of H. pylori were determined at various pH values. In the absence of urea, the organism maintained a close-to-neutral cytoplasm and an internally negative membrane potential at external pH values greater than 4 to 5. In the presence of urea, H. pylori accomplished cytoplasmic pH homeostasis down to an external pH of 1.2. At this external pH, the cytoplasmic pH was 4.9 and the membrane potential was slightly negative inside. The latter finding is in contrast to the situation in acidophiles, which develop inside-positive membrane potentials under similar conditions. Measurements of the time course of the membrane potential confirmed that addition of urea to the cells led to hyperpolarization. Most likely, this effect was due to electrogenic export of ammonium cations from the cytoplasm. The urease activity of intact cells increased nearly exponentially with decreasing external pH. This activation was not due to enhanced gene expression at low external pH values. In cell extracts the pH optimum of urease activity was dependent on the buffer system and was about pH 5 in sodium citrate buffer. Since this is the cytoplasmic pH of the cells at pH 1 to 2, we propose that cytoplasmic pH is a factor in the in vivo activation of the urease at low external pH values. The mechanism by which urease activity leads to cytoplasmic pH homeostasis in H. pylori is discussed.
As a neutrophilic bacterium capable of growth at pH values of >5.5 (21), Helicobacter pylori is unique with respect to its acid tolerance and long-term persistence in the human stomach. Mechanisms enabling H. pylori to cope with fluctuating pH must be essential, particularly during primary infection, in order to overcome the gastric acid barrier. Recently, we have shown that in the presence of urea and without any previous adaptation, growing cells of H. pylori are capable of survival and cytoplasmic pH (pHin) homeostasis for several hours after a shift of the medium pH (pHout) to pH 1 (30), a physiologically relevant condition frequently found in the gastric lumen. At this pH, acidophiles exhibit a positive inside membrane potential (ΔΨ) (2, 22, 33). For H. pylori, the sign and value of ΔΨ at low pHout values is still a matter of debate, and data are lacking for pHout values of <3. An inside positive ΔΨ has been reported at pH 3, which was, however, not sensitive to addition of a protonophore (14). In other studies ΔΨ remained negative inside down to a pH of 3 (28). Because of these conflicting data, we reexamined the ΔΨ of H. pylori cells at low pHout values and extended the studies to pH 1 to 2. We observed that in the presence of urea ΔΨ remained inside negative at all pHout values between 1.2 and 7. Therefore, we propose that this phenomenon is associated with the electrogenic export of ammonium cations from the cytoplasm.
Urease is a virulence factor of H. pylori, and this enzyme is thought to confer acid resistance to H. pylori by cleavage of urea and elevation of the microenvironmental pH (12). However, the mechanism by which urease contributes to survival under acidic conditions is highly controversial. Originally, it was assumed that the enzyme activity is extracytoplasmic and that in the stomach protection occurs due to the creation of a cloud of ammonia around the cells (9). This hypothesis, that external urease activity protects H. pylori from acid stress, was recently put forward again, based on the observation that in a nonstirred solution urease exhibits some residual activity at pH 3 (8). However, it has been shown that urease is cytoplasmic (28) and that the urea porter UreI influences urease activity by mediating acid-triggered urea uptake (24, 27, 36). According to the authors of these papers the NH3 product of the urease reaction leaves the cytoplasm in its neutral form and neutralizes the periplasmic pH by binding protons in that environment. Finally, our preliminary data suggest that urease activity leads to cytoplasmic rather than periplasmic pH homeostasis of H. pylori cells and that this process is sufficient for survival at pH 1 (30). The results of the experiments reported here fully support this notion, and a hypothesis for the mechanism of this process (30, 37) is discussed.
A further controversial issue concerns the pH optimum of the H. pylori urease. In cell extracts diluted with citrate-phosphate buffer the enzyme exhibits a pH optimum of 7.4 (28). In whole cells urease activity is maximal at a low pHout (28). This phenomenon is attributed to a controlled urea supply determined by acid activation of UreI. However, at a pHout of 3 urease activity in the cytoplasm is expected to be severely inhibited, since under these conditions the pHin is only 5.5 to 5.7 (30), a value substantially different from the pH optimum of the urease (28). The urease activities of Yersinia enterocolitica and Morganella morganii have a pH optimum of 5.5 in citrate-based buffers (37). Moreover, the activity of the urease from Klebsiella aerogenes has been reported to be inhibited by acid forms of phosphate (31). These conflicting data led us to reexamine the pH optimum of urease activity in H. pylori cell extracts. Various buffer systems were used, since it is known that at acidic pH values the pH optimum of an enzyme may depend strongly on the buffer used (15). We observed that in sodium citrate buffer the pH optimum of H. pylori urease is around pH 5. This finding was combined with the results of determinations of pHin and cytoplasmic urease activity of cells suspended at pHout values between 1.2 and 7. We concluded that lowering the pHin to values around 5 after an acid shift of H. pylori cells may contribute to the activation of urease activity observed under these conditions.
MATERIALS AND METHODS
Strain and growth conditions.
H. pylori wild-type strain DSM 4867 was cultivated as described before (30).
Buffers.
Citrate-phosphate buffers were prepared at 37°C as described previously (16, 18). For pH 3 to 7 buffers 100 mM citric acid and 200 mM Na2HPO4 were mixed to obtain the appropriate pH. For the pH 1 buffer, the pH of 100 mM citric acid was adjusted with concentrated HCl. For pH 2, the 100 mM citric acid buffer was used without any further addition. Buffer containing 100 mM MES [2-(N-morpholino)ethanesulfonic acid] or 100 mM citric acid was titrated with concentrated NaOH to obtain the appropriate pH. Sodium phosphate buffer was composed of a mixture of 100 mM Na2HPO4 and 100 mM NaH2PO4.
Urease activity.
The in vivo urease activity of H. pylori cells was monitored by determining the decrease in the urea concentration in APM (acid-precipitated brucella broth supplemented with 5% fetal calf serum, as described previously [30]). Samples (200 μl) were withdrawn at different times. Fluorofamide (10 μM) was added immediately, and the samples were stored frozen in liquid nitrogen. Subsequently, samples were thawed in a sonication bath for 3 min and centrifuged at a low speed (5 min, 16,000 × g). The concentration of urea in the supernatant was determined as described by Rahmatullah and Boyde (23).
For determination of urease activity in cell extracts, cells from 2 ml of an H. pylori culture were harvested by centrifugation (16,000 × g) and washed twice with 500 μl of 350 mM Tris-HCl (pH 8.0). The cell pellet was frozen in liquid N2. After thawing, the cells were resuspended in 500 μl of 20 mM Tris-HCl (pH 8.0) and sonicated three times (30 s each) on ice by using a microtip (Branson cell disrupter B 15; output control 3, 50% pulsed). After centrifugation at 16,000 × g for 5 min, the urease activity in the supernatant was determined by measuring the released ammonia by the phenol-hypochlorite assay (35). The assay was started by addition of 1 μg of protein to 1 ml of buffer supplemented with 10 mM urea. After 5, 10, and 15 min of incubation at 37°C, a 20- to 40-μl aliquot was added to 400 μl of 100 mM sodium phosphate buffer (pH 7.4) plus 200 μl of 3% phenol-0.003% sodium nitroprusside; this was followed by addition of 200 μl of 2% NaOH-0.05% NaClO. After incubation for 45 min in the dark at room temperature, absorption was determined at 635 nm. NH4Cl was used as a standard.
Protein concentration determination.
Protein concentrations in cell extracts were determined by the method of Bradford (5) by using the Bio-Rad protein assay (Bio-Rad, Munich, Germany) and bovine serum albumin as a standard.
Determination of pHin.
For the acid shock experiments, cells were first collected by centrifugation (10 min, 5,000 × g, 37°C), resuspended to an optical density at 578 nm (OD578) of about 2 in 150 mM NaCl, and diluted 10-fold with citrate-phosphate buffer at the appropriate pH supplemented with 20 mM urea, as indicated below. The pHin was calculated from the distribution of [14C]salicylate across the cytoplasmic membrane (14, 25). Separation of the supernatant and the pellet was performed by centrifugation through silicone oil as described by Michels and Bakker (19). For nonenergized cells, 1 mM 2,4-dinitrophenol (2,4-DNP) was added. After 5 min of incubation in the appropriate citrate-phosphate buffer, 5 ml of the cell suspension was incubated with 2 μCi of 3H2O (1 mCi/g) per ml and 50 nCi of [7-14C]salicylic acid (55.5 mCi/mmol; New England Nuclear Corp.) per ml for 3 min at 37°C with shaking. Cells were separated from the medium by centrifugation in a 12-ml conical polypropylene centrifuge tube (Sarstedt) through 0.5 ml of a 1:2 mixture of AR20 and AR200 silicon oils (Serva) for 10 min at 5,400 × g and room temperature, followed immediately by centrifugation for 10 min at 9,500 × g and 4°C to improve phase separation. A 50-μl aliquot of the supernatant was added to 0.5 ml of 0.4 M NaOH in a 6-ml miniature scintillation vial (Canberra-Packard). The remainder of the supernatant was discarded. The bottom part of the centrifuge tube containing the complete cell pellet and some oil was separated with a scalpel and added to 0.5 ml of 0.4 M NaOH in a scintillation vial, and this was followed by overnight incubation at room temperature. Subsequently, 5 ml of Ultima Gold scintillation fluid (Canberra-Packard) was added to each sample. The radioactivity of the two isotopes in each sample was determined with a Tri-Carb 2300TR liquid scintillation counter (Canberra-Packard) in the dual-label disintegrations per minute mode with automatic correction for spill-over and quenching with the aid of an external standard. pHin values were calculated from the measured distribution of [14C]salicylate between the sediment and the supernatant by using the equation: ATin/ATout = Cr (1 + Vr) − Vr = (1/Ka + 1/H+in)/(1/Ka + 1/H+out), where ATin and ATout are the total 14C concentrations in the cells and in the medium, respectively; Cr is the 14C/3H accumulation ratio between the sediment and the supernatant; Vr is the ratio of the external water space of the cell pellet to the internal water space of the cell pellet, which was taken to be 1.0, a value observed for many other prokaryotes with this method (3, 4, 19); and 1/Ka is 933 for salicylic acid (7). Binding of [14C]salicylate to cellular components was neglected for apparent values. Corrected values were corrected for binding by the exponential mean method (38).
Fluorimetric determination of ΔΨ.
Cells of H. pylori (OD578, ∼0.2) were harvested by centrifugation and resuspended in 0.1 volume of prewarmed 0.9% NaCl. Continuous fluorimetric measurements were obtained with citrate-phosphate buffers at the appropriate pH values by using 1 μM 3,3′-dipropylthiadicarbocyanine iodide (Disk3) (Molecular Probes Inc.) (5) as a ΔΨ-sensitive cationic dye (18, 28). The bacterial suspension was added to the dye solution at a final OD578 of about 0.03. Fluorescence was measured at 37°C at an excitation wavelength of 600 nm and an emission wavelength of 665 nm. ΔΨ was eliminated by addition of 150 nM 3,3′,4′,5-tetrachlorosalicylanilide (TCS) or 2% n-butanol.
Determination of ΔΨ with 14C-labeled lipophilic ions.
ΔΨ was calculated from the distribution of [14C]tetraphenylphosphonium ([14C]TPP+) or S14CN− across the cytoplasmic membrane of cells centrifuged through silicone oil as described above for the determination of pHin. For estimation of binding of the radiochemicals to cellular components, 2% butanol was added. Each cell suspension was incubated with 2 μCi of 3H2O (1 mCi/g) per ml and 50 nCi of [14C]TPP+ (20 mCi/mmol; American Radiolabeled Chemicals Inc.) per ml or 100 nCi of S14CN− (4.2 mCi/mmol; Sigma) per ml for 3 min at 37°C with shaking. ΔΨ was determined by using the equations: ATin/ATout = Cr × (1 + Vr) − Vr and ΔΨ = −ln(ATin/ATout) × (RT/zF). Binding of [14C]TPP+ to cellular components was neglected for apparent values. Corrected values were corrected for binding by the exponential mean method (38).
Sign of bioenergetic parameters.
Transmembrane parameters are determined by subtracting outside values from inside values. Hence, ΔΨ, ΔpH (pHin − pHout), and the proton motive force (PMF) are negative when the values inside are negative.
RESULTS
pHin in the presence of urea.
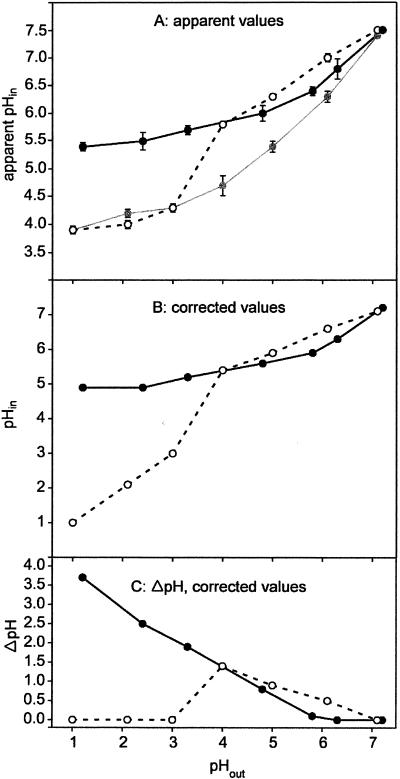
Figure 1 shows the profiles for pHin (Fig. 1A and B) and ΔpH (Fig. 1C) of H. pylori cells suspended in citrate-phosphate buffers having pH values between 1 and 7.2. The data were obtained by measuring the distribution of the anionic form of the weak acid [14C]salicylic acid across the cell membrane by an established procedure (19, 25) adapted for H. pylori cells (30). In the presence of urea, the pHout increased by 0.1 to 0.8 pH unit during the experiment depending on the cell urease activity and the buffering capacity of the medium at each pH (see Table 2). These changes in pHout were taken into account for calculation of all data. Figure 1A shows that the apparent pHin of urea-degrading cells decreased from 7.5 to 5.4 when the pHout decreased from 7.2 to 1.2. At a pHout of 2 the apparent pHin (pH 5.5) was the same as the value previously determined after 1 h of incubation in the same buffer (30), indicating that after 8 min of acid stress a steady-state pHin was reached. In the presence of 1 mM 2,4-DNP (a protonophore) at a low pHout, the apparent pHin decreased to around 4 (Fig. 1A). A residual pHin of 4 has also been reported for nonenergized acidophilic organisms (19, 39) and has been attributed to several phenomena (13, 19), including nonspecific binding of the radiochemical to cellular components (11, 19). Support for the notion that the latter phenomenon also occurs in H. pylori comes from the observation that permeabilization of the cells by treatment with 2% n-butanol gave values virtually identical to those obtained after treatment with 1 mM 2,4-DNP (data not shown). By using data obtained in the presence of 2,4-DNP, pHin values were corrected for binding by the exponential mean method (Fig. 1B) (38). At pHout values of 1.2 and 2.4, a corrected pHin of 4.9 was maintained with urea, whereas the pHin was 5.2 for a pHout of 3.3. At pHout values of >4 and in the presence of urea, ΔpH decreased continuously to zero at a neutral pHout (Fig. 1C).
FIG. 1.
pHin of H. pylori measured after 8 min of incubation in citrate-phoshate buffer at the appropriate pHout. (A) Apparent pHin; (B) pHin after correction for binding as described by Zaritsky et al. (38); (C) ΔpH (corrected values). Open circles, cells in the absence of urea; solid circles, cells in the presence of 20 mM urea; gray circles, cells with urea and 1 mM 2,4-DNP. The ratio of accumulation of 14C in the cells to accumulation of 14C in the medium (ATin/ATout) was 280 ± 67 for pH 1 in the presence of urea, while it was only 10 ± 1 at pH 1 in the presence of urea and 2,4-DNP. At pH 7 ATin/ATout was around 2 ± 0.5, irrespective of the presence of urea or 2,4-DNP, indicating that the pHin approximately matched the pHout.
TABLE 2.
ΔpH, ΔΨ, and PMF determined for H. pylori at different pHout values in the presence and absence of ureaa
| pHout | In the presence of urea
|
In the absence of urea
|
||||
|---|---|---|---|---|---|---|
| ΔpH (mV) | ΔΨ (mV) | PMF (mV) | ΔpH (mV) | ΔΨ (mV) | PMF (mV) | |
| 1.2 | −228 | −26 | −254 | |||
| 2.4 | −154 | −53 | −207 | |||
| 3.3 | −117 | −64 | −181 | |||
| 4.8 | −49 | −47 | −96 | |||
| 5.8 | −6 | −56 | −62 | |||
| 6.3 | 0 | −108 | −108 | |||
| 7.2 | 0 | −143 | −143 | |||
| 1 | 0 | 0 | 0 | |||
| 2.1 | 0 | 0 | 0 | |||
| 3 | 0 | 0 | 0 | |||
| 4 | −86 | 0 | −86 | |||
| 5 | −55 | 0 | −55 | |||
| 6.1 | −31 | −102 | −133 | |||
| 7.1 | 0 | −132 | −132 | |||
pHin in the absence of urea.
In the absence of urea, pHout remained constant regardless of the starting pH of the buffer used. At pHout values of ≥4 the cells maintained an alkaline inside ΔpH across the cytoplasmic membrane, indicating that they performed pH homeostasis (Fig. 1). In contrast, at pHout values between 1 and 3 the cells did not accomplish pH homeostasis, since the pHin was close to that of cells treated either with the protonophore 2,4-DNP (Fig. 1A) or with n-butanol (data not shown). Hence, these cells did not maintain a ΔpH across the cytoplasmic membrane at pHout values of <4 (Fig. 1C). At pHout values of 4 to 7, the pHin was lower in the presence of urea than in its absence. Possible explanations for this effect are discussed below.
Taken together, the data in Fig. 1 indicate that urease activity is essential for pH homeostasis at pHout values of <4 and that at pHout values of >4, at which urease activity apparently was not essential for the pathogen, the pHin increased gradually from 5.8 to 7.5 with increasing pHout, irrespective of the presence of urea.
Continuous fluorimetric determination of ΔΨ.
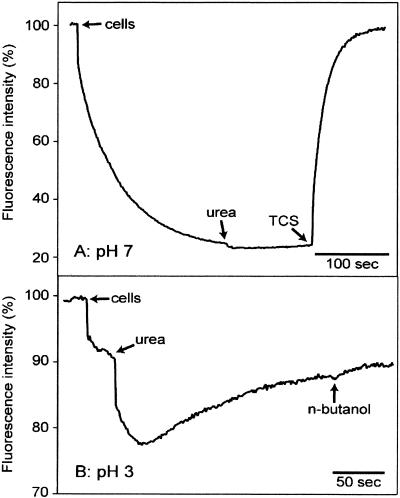
The potentiometric fluorescent probe DiSC3(5) can be used for qualitative, continuous detection of ΔΨ of H. pylori (18, 28). This cationic fluorophore accumulates on hyperpolarized membranes and is translocated into the lipid bilayer, resulting in a decrease in fluorescence and absorption shifts (6). At pH 7, addition of H. pylori cells (final OD578, ∼0.03) led to a 75% quenching of fluorescence intensity caused by uptake of the fluorescent dye driven by ΔΨ (Fig. 2A). Addition of 5 mM urea resulted in a marginal further decrease in fluorescence (i.e., increase in ΔΨ). The ΔΨ was stable until its collapse was induced by the addition of 150 nM TCS, a protonophore. For determination of ΔΨ of H. pylori cells at lower pHout values, the fluorimetric method turned out to have limited value. At pH 3, addition of urea did result in a significant quenching of fluorescence, suggesting that there was hyperpolarization (internally negative) (Fig. 2B). However, fluorescence intensity was not stable and increased with time to a value hardly lower than that prior to addition of urea, whereas the pHout remained constant at the low cell density used. Collapse of ΔΨ was implemented with 2% butanol, since TSC was not suitable for uncoupling the cells at a pH of <4 (data not shown). n-Butanol caused only a small further increase in fluorescence intensity. Similar results were obtained at pHout values of 4 and 5, whereas at pHout values of 1 to 2 no reproducible data were obtained (results not shown). As a control, cells of the acidophilic gram-positive bacterium Alicyclobacillus acidocaldarius were used, which do not possess significant urease activity. Addition of urea to these cells at pH 3 had no effect on ΔΨ when the fluorimetric method was used (results not shown). Taken together, these data confirm that addition of urea to H. pylori cells at a low pHout leads to rapid and significant hyperpolarization (28). We discuss this effect below.
FIG. 2.
Fluorimetric determination of ΔΨ of H. pylori at pH 7 (A) and pH 3 (B). Cells were added at an OD578 of ∼0.03 to citrate-phosphate buffer containing 1 μM DiSC3(5). The times when 5 mM urea, 150 nM TCS, and 2% n-butanol were added are indicated. Excitation was at 600 nm, and emission was at 665 nm.
Determination of ΔΨ via distribution of the lipophilic ions TPP+ and SCN−.
A more quantitative analysis of ΔΨ was performed by using the radioactive lipophilic ions [14C]TPP+ and S14CN− (19, 25, 33). The principle for this measurement is the movement of a permeable ion across the membrane in response to ΔΨ until electrochemical equilibrium is established. An additional advantage over the fluorimetric method is the possibility of obtaining simultaneous measurements for several samples, which is especially important for H. pylori as aging of cells rapidly leads to a loss of viability.
Figure 3 shows that when [14C]TPP+ was the ΔΨ probe, H. pylori maintained a negative internal ΔΨ in the presence of urea over the whole pHout range used, pH 1 to 7. Binding of the radiochemical was estimated after addition of n-butanol, which permeabilizes cells. By using the data obtained, corrected ΔΨ values were calculated (Fig. 3B) (38). At a pHout of 1, ΔΨ was −26 mV. ΔΨ increased to −64 mV at a pHout of 3 (Fig. 3B), remained approximately constant at pHout values between 3 and 5, and increased further to −140 mV at a pHout of 7. In the absence of urea, ΔΨ was close to zero at pHout values between 1 and 5. At pHout values of >5 ΔΨ increased steeply to values similar to those observed in the presence of urea (Fig. 3B).
FIG. 3.
ΔΨ of H. pylori determined by the distribution of the lipophilic cation [14C]TPP+ across the cytoplasmic membrane after incubation for 8 min in citrate-phosphate buffer at the appropriate pHout. (A) Apparent values of ΔΨ; (B) values after correction for binding as described by Zaritsky et al. (38). Open circles, cells in the absence of urea; solid circles, cells in the presence of 20 mM urea; gray circles, cells in the presence of 2% n-butanol.
The data obtained with the permeant anion S14CN− confirmed that in the presence of urea H. pylori does not invert its ΔΨ to an internally positive value. S14CN− uptake by the cells was not diminished by n-butanol (Table 1), indicating that this uptake reflects binding rather than accumulation inside the cytoplasm. Moreover, addition of 10 mM KSCN, which uncouples cells of A. acidocaldarius (19), an observation which we confirmed, did not have any effect on the pHin of H. pylori at a pHout of 2 (results not shown). Taken together, these data indicate that down to a pHout of 1 H. pylori maintains an internally negative ΔΨ, presumably because of the electrogenic extrusion of NH4+ cations from the cytoplasm (see below).
TABLE 1.
Apparent ΔΨ and ratio of total 14C in the cells and to total 14C in the medium (ATin/ATout) for H. pylori determined by using S14CN− as a potentiometric probea
| pHout | Compound(s) added to medium | ATin/ATout | Apparent ΔΨ (mV) |
|---|---|---|---|
| 1 | None | 42 | 100 |
| Urea + butanol | 47 | 103 | |
| 1.2 | Urea | 41 | 99 |
| 2.1 | None | 53 ± 23 | 106 ± 11 |
| Urea + butanol | 42 ± 27 | 100 ± 16 | |
| 2.4 | Urea | 36 ± 2 | 96 ± 2 |
| 3 | None | 38 ± 21 | 97 ± 14 |
| Urea + butanol | 61 ± 55 | 111 ± 21 | |
| 3.3 | Urea | 39 ± 1 | 98 ± 1 |
| 4 | None | 44 ± 7 | 101 ± 4 |
| Urea + butanol | 33 ± 15 | 93 ± 12 | |
| 4.8 | Urea | 46 ± 21 | 102 ± 12 |
Cells were incubated for 8 min in citrate-phosphate buffer in the presence of urea at different pHout values. Apparent ΔΨ values and the ratio of radioactivity in the cells to radioactivity in the medium (ATin/ATout) were calculated from the distribution of S14CN− across the cell membrane, assuming that all S14CN− that accumulated was present in the cytoplasm. Urea (20 mM) or 20 mM urea and 2% n-butanol were added as indicated.
PMF.
PMF can be calculated from ΔpH and ΔΨ with the equation: PMF = ΔΨ − 61.5(ΔpH) (in millivolts at 37°C) (20). By using the data for ΔpH and ΔΨ corrected for probe binding (Fig. 1B and 3B, respectively), cells suspended in the presence of urea had negative PMF values (−254 to −181 mV) at pHout values of 1.2 to 3.3. These values dropped to −96 and −62 mV at pHout values of 4.8 and 5.8, respectively, and increased to −143 mV at a pHout of 7.2. In the absence of urea the PMF was also low at pHout values of 4.8 and 5.8 and increased to about −130 mV at pHout values of ≥6.3, conditions under which H. pylori grows (Table 2).
Urease.
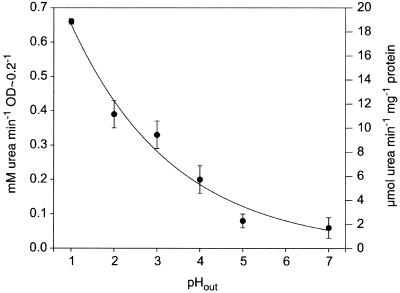
The urease activity of intact cells was measured after acid shock by determining the decrease in the urea concentration in the medium, which was 20 mM at the moment of the acid shock. When the pHout increased from 1 to 7, the urease activity of a cell suspension at an OD578 of ∼0.2 decreased approximately exponentially from 0.66 mM urea/min at pH 1 to 0.08 mM urea/min at pH 7 (Fig. 4). Urease activity was also measured in extracts of H. pylori cells which had been exposed to pHout values of 1 to 7 for 30 min in the presence of the concentrations of urea indicated (Table 3). Control experiments showed that these concentrations were saturating and not inhibitory. Irrespective of the preincubation conditions, the urease activities in H. pylori cell extracts were similar, and the average value was 57 ± 7.5 μmol min−1 mg of protein−1 (Table 3). These data indicate that the increase in cell urease activity at a low pHout was due to activation of an enzyme-transporter function rather than to an increase in gene expression during incubation of the cells at low pH.
FIG. 4.
Urease activity of H. pylori in APM at different pHout values in the presence of 20 mM urea. Urease activity was measured by determination of urea in the medium as described by Rahmatullah and Boyde (23). OD, optical density.
TABLE 3.
Urease activities of H. pylori cell extracts after 30 min of acid shock in the presence of ureaa
| Preincubation conditions | Urease activity (μmol of urea min−1 mg of protein−1) |
|---|---|
| pH 1 + 50 mM urea | 57.5 |
| pH 2 + 20 mM urea | 50.8 ± 9.3 |
| pH 3 + 20 mM urea | 51.4 ± 11.8 |
| pH 4 + 20 mM urea | 68.3 ± 6.5 |
| pH 5 + 20 mM urea | 49 ± 11 |
| pH 7 | 66.4 ± 5.5 |
Urease activity was measured by determining ammonia release with cell extracts in 100 mM sodium phosphate buffer (pH 7.4) supplemented with 10 mM urea using the phenol-hypochlorite assay (35).
For comparative analysis, in vivo urease activity was also calculated per milligram of cell protein (Fig. 4). The protein content of a cell extract from 1 ml of a cell suspension (OD578, ∼0.2) was 36 ± 9 μg (n = 6). According to this calculation the maximal urease activity in vivo (i.e., at a pHout of 1) was about 18 U/mg of cell protein, only one-third of the in vitro activity in cell extracts (∼57 U/mg of cell protein) (Fig. 4 and Table 3).
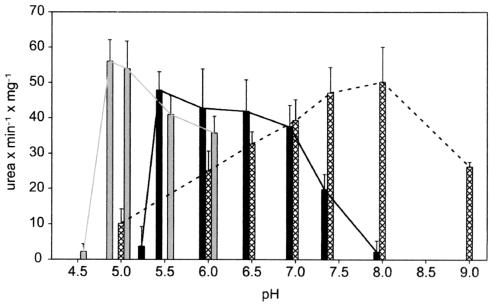
The pH optimum of urease from H. pylori cell extract was determined in different buffer systems (Fig. 5). In sodium phosphate buffers urease activity was maximal at pH 8, which is close to the value reported for sodium citrate/phosphate buffers (pH 7.4) (28). However, in sodium-MES buffers urease activity exhibited a broad pH maximum between pH 5.5 and 7, and in sodium citrate buffer the pH optimum was even lower (pH 4.8 to 5). We concluded that H. pylori urease activity strongly depends on the buffer system used and has an acidic pH optimum in the absence of phosphate.
FIG. 5.
pH optima of H. pylori urease from cell extracts in different buffer systems. Gray bars, 100 mM citrate-NaOH; solid bars, 100 mM MES-NaOH; cross-hatched bars, 100 mM sodium phosphate. Urease activity was determined by the phenol-hypochlorite assay (35).
DISCUSSION
In the presence of urea, cells of H. pylori survive for several hours at pH 1 when the pHin is kept at a value close to neutral, indicating homeostasis of pHin (30). This process is crucial for the cells, since at low pHin values cytoplasmic enzymes become inactive, as experiments with protonophores at low pHout values with both acidophiles (19) and H. pylori (24, 30) have shown. Urease has been proposed to play the following role in this homeostasis of pHin (29, 30, 37). In the cytoplasm NH3, a product of the urease reaction, binds protons leaking in from the acidic medium. This process occurs because all prokaryotes, including acidophiles, possess a cytoplasmic membrane with some permeability for protons (33, 34). The NH4+ produced is then removed from the cytoplasm via a hypothetical NH4+ efflux system. The present study was carried out to obtain more information about this mechanism. To do this, cells were suspended in the presence or absence of urea in citrate-phosphate buffers at a wide range of pH values. It has been shown previously that at a pHout of 2 cells do not lose viability rapidly in this type of buffer (30). At pHout values of <4, pH homeostasis of H. pylori depended on urease activity (Fig. 1), which increased exponentially with the inverse of pHout (Fig. 4). This observation is consistent with the fact that H. pylori relies on the availability of urea for survival at pHout values of <4 (17). A urea-dependent rise in cellular pH has previously been observed for H. pylori suspended in buffer at pH 3 to 5 and was attributed to changes in the periplasmic pH (18). Several points argue against the notion that the changes in cellular pHin observed in the present study reflect changes in the periplasmic pH: (i) the pHin values reported here for energized and nonenergized H. pylori cells are very similar to those of a gram-positive organism and several archaeal acidophiles (2, 19, 26, 33) which do not possess a periplasmic space; (ii) protons are expected to move rapidly through the outer membrane porins of gram-negative bacteria, thereby restricting the possibility of building up a large pH gradient across this membrane; (iii) if the H. pylori outer membrane had limited permeability, the probes used for measuring the ΔΨ and ΔpH of H. pylori (Fig. 1, 2, and 3) (14, 18) were not expected to reach the cytoplasmic membrane, contrary to what was observed; and (iv) the anionic form of [14C]salicylic acid is expected to move rapidly across the H. pylori outer membrane, making this probe unsuitable for detection of a ΔpH across this membrane. Since this probe did, however, detect a urea-dependent and protonophore-sensitive ΔpH, we concluded that it detects pH differences across the cytoplasmic membrane and that we measured pHin rather than the periplasmic pH of H. pylori in this study and previously (30).
The exact pHin in H. pylori during survival at pH 1 is not known. It is between 5.8, the apparent value determined in growth medium (30), and 4.9, the value corrected for probe binding reported here (Fig. 1B). The latter value is close to pH 5.0, the value for the pH optimum of urease activity in cell extracts assayed in sodium citrate buffer (Fig. 5), and it is higher than pH 4.6, the value reported for the extremely acidophilic thermophilic archaeon Picrophilus oshimae (33). This suggests that urease is active under conditions under which it is most needed by the cells (i.e., at a pHout of 1) and that this effect contributes to the survival of the organism under extremely acidic conditions. However, the pH optimum of urease activity was strongly dependent on the type of buffer used for the assay (Fig. 5), and at present it is not known how the ionic composition of the H. pylori cytoplasm affects the enzyme activity and pH optimum of its urease.
Recently, Ha et al. (8) have reported that in pH 3 buffer H. pylori urease is inactive. However, in nonbuffered, nonstirred solutions these authors observed some residual activity of the enzyme at pH 3 (8). This observation was taken to support the altruistic autolysis hypothesis for the survival of H. pylori at low pH (10). According to this hypothesis, some of the H. pylori cells lyse, thereby releasing their urease into the medium, where it binds to the surface of other, still viable H. pylori cells (8, 10). At a low medium pH and in the presence of urea this surface-bound urease is thought to create a cloud of ammonia around the cells, which protects the cells by binding protons, thereby increasing the local pH just outside the cells to a more neutral value (8, 10). We criticize this concept on several grounds. First, the data of Ha et al. do not support the conclusions of these authors. In their nonbuffered solutions, urease activity was completely nonproportional to enzyme concentration, reflecting ill-defined conditions. Moreover, from their data it can be calculated that at the highest protein concentration the turnover rate of the urease at pH 3 was only about 2.5% of the maximal turnover rate at neutral pH, raising doubt concerning the contention that at low pH the urease activity of the enzyme sticking to the surface of nonlysed cells is sufficient for creating and maintaining the cloud of neutralizing ammonia around the cells (8). Second, survival of H. pylori at pH 1 has been observed (30). At pH 1 to 1.3 the stomach contents are well buffered, since it takes about 99 to 29 mM OH− ions to raise the pH to 3. Hence, any results specifically obtained in nonbuffered solutions at pH 3, such as those reported by Ha et al. (8), are irrelevant for the survival of H. pylori at pH 1. Finally, we contend that the altruistic autolysis hypothesis does not apply to the low-pH conditions under which we observed survival of H. pylori cells. This argument is based on the observation that addition of a protonophore to cells in either buffer or growth medium at pH 1 to 4 eliminates all urease activity as pHin reaches the same value as pHout (24, 30), suggesting that at these low pH values there is no external urease that is still active. Nevertheless, cells do not lose viability at pHout values of 1 to 4 before the protonophore is added, suggesting that the presence of surface-bound urease is not essential for survival of H. pylori at low pH.
At pH 4 to 7, the pHin of H. pylori cells was slightly lower in the presence of urea than in the absence of urea (Fig. 1B). Moreover, in the presence of urea the cells did not develop as negative a ΔΨ as other prokaryotes develop in this pH range (Fig. 3B) and therefore had a low PMF (Table 2). At present, the reason for this result is not clear. It may be an artifact of the methods used. In the case of ΔpH, salicylate may not have been completely trapped within the cells due to the formation of a pH gradient ranging from the cytoplasm to the external medium rather than a steep pH change at the cytoplasmic membrane under these conditions. However, it is noteworthy that this effect has nothing to do with neutralization of the microenvironment or the periplasm, particularly at an extremely low pH, since proton and ammonia diffusion through the outer membrane is expected to be some orders of magnitude greater than the production of ammonia by urease activity. In addition, it is also important to mention that H. pylori contains several drug efflux systems (1, 32), and it may very well be that some of the probes used in the present study for measurement of ΔpH and ΔΨ are exported from the cells by these systems.
A remarkable result of the present study was that unlike acidophiles (2, 33), at pHout values of <3 H. pylori did not reverse the sign of its ΔΨ from internally negative to internally positive (Fig. 3 and Table 1). This conclusion disagrees with that of previous work, in which an internally positive ΔΨ was postulated for H. pylori at pH 3 (14). We agree with Matin et al. that at a low pH the cells take up the lipophilic anion SCN−. However, SCN− uptake was not diminished by a protonophore or by permeabilization of the cells with n-butanol (Table 1), indicating that it reflects binding to the cells rather than accumulation in the cytoplasm. The question that then arises is, by which mechanism is the internally negative ΔΨ generated. It depends on the presence of urea in the medium. Moreover, addition of urea to the cells causes immediate hyperpolarization (internally negative) of the cells at low pHout (28) (Fig. 2). We propose that this hyperpolarization is caused by the electrogenic efflux of NH4+ cations by a hypothetical transporter. However, further work, including identification of such a transport system, is needed to show whether this proposal is correct.
Finally, our finding that even at pH 1 the urease activity of intact cells was only one-third of the urease activity in cell extracts prepared from the equivalent number of cells (Fig. 4 and Table 3) has important implications for the mechanism(s) by which urease activity is regulated. It shows that besides the well-documented pH-dependent activation of the urea carrier UreI (24, 27, 36) and the possible direct activation of urease activity by low pHin (this study), other still unknown factors may regulate urease activity in H. pylori cells.
Acknowledgments
We thank L. Herrmann and K. Melchers (Byk Gulden, Konstanz, Germany) for helpful discussions.
This work was supported by the Fonds der Chemischen Industrie.
REFERENCES
- 1.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 2.Bakker, E. P. 1990. The role of alkali-cation transport in energy coupling of neutrophilic and acidophilic bacteria. FEMS Microbiol. Rev. 75:319-334. [Google Scholar]
- 3.Bakker, E. P., and W. E. Mangerich. 1981. Interconversion of components of the bacterial proton motive force by electrogenic potassium transport. J. Bacteriol. 147:820-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakker, E. P., H. Rottenberg, and S. R. Caplan. 1976. An estimation of the light-induced electrochemical potential difference of protons across the membrane of Halobacterium halobium. Biochim. Biophys. Acta 440:557-572. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Cabrini, G., and A. S. Verkman. 1986. Potential-sensitive response mechanism of diS-C3-(5) in biological membranes. J. Membr. Biol. 92:171-182. [DOI] [PubMed] [Google Scholar]
- 7.Dawson, R. M. C., D. C. Elliot, W. H. Elliot, and K. M. Jones. 1986. Data for biochemical research. Oxford Science Publications, Clavendon Press, Oxford, United Kingdom.
- 8.Ha, N. C., S. T. Oh, J. Y. Sung, K. A. Cha, M. H. Lee, and B. H. Oh. 2001. Supramolecular assembly and acid resistance of Helicobacter pylori urease. Nat. Struct. Biol. 8:505-509. [DOI] [PubMed] [Google Scholar]
- 9.Hazell, S. L. 1990. Urease and catalase as virulence factors of Helicobacter pylori, p. 3-14. In H. Menge (ed.), Helicobacter pylori. Springer-Verlag, Berlin, Germany.
- 10.Krishnamurthy, P., M. Parlow, J. B. Zitzer, N. B. Vakil, H. L. T. Mobley, M. Levy, S. H. Phadnis, and B. E. Dunn. 1998. Helicobacter pylori containing only cytoplasmic urease is susceptible to acid. Infect. Immun. 66:5060-5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lolkema, J. S., K. J. Hellingwerf, and W. N. Konings. 1982. The effect of “probe binding”on the quantitative determination of the proton-motive force in bacteria. Biochim. Biophys. Acta 681:85-94. [Google Scholar]
- 12.Marshall, B. J., L. J. Barrett, C. Prakash, R. W. McCallum, and R. L. Guerrant. 1990. Urea protects Helicobacter (Campylobacter) pylori from the bactericidal effect of acid. Gastroenterology 99:697-702. [DOI] [PubMed] [Google Scholar]
- 13.Matin, A. 1999. pH homeostasis in acidophiles. Novartis Found. Symp. 221:152-163. [DOI] [PubMed] [Google Scholar]
- 14.Matin, A., E. Zychlinsky, M. Keyhan, and G. Sachs. 1996. Capacity of Helicobacter pylori to generate ionic gradients at low pH is similar to that of bacteria which grow under strongly acidic conditions. Infect. Immun. 64:1434-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matzke, J., B. Schwermann, and E. P. Bakker. 1997. Acidostable and acidophilic proteins: the example of the alpha-amylase from Alicyclobacillus acidocaldarius. Comp. Biochem. Physiol. A Comp. Physiol. 118:475-479. [DOI] [PubMed] [Google Scholar]
- 16.McGowan, C. C., T. L. Cover, and M. J. Blaser. 1994. The proton pump inhibitor omeprazole inhibits acid survival of Helicobacter pylori by a urease-independent mechanism. Gastroenterology 107:1573-1578. [DOI] [PubMed] [Google Scholar]
- 17.McGowan, C. C., T. L. Cover, and M. J. Blaser. 1996. Helicobacter pylori and gastric acid: biological and therapeutic implications. Gastroenterology 110:926-938. [DOI] [PubMed] [Google Scholar]
- 18.Meyer-Rosberg, K., D. R. Scott, D. Rex, K. Melchers, and G. Sachs. 1996. The effect of environmental pH on the proton motive force of Helicobacter pylori. Gastroenterology 111:886-900. [DOI] [PubMed] [Google Scholar]
- 19.Michels, M., and E. P. Bakker. 1985. Generation of a large, protonophore-sensitive proton motive force and pH difference in the acidophilic bacteria Thermoplasma acidophilum and Bacillus acidocaldarius. J. Bacteriol. 161:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell, P. 1966. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol. Rev. 41:445-502. [DOI] [PubMed] [Google Scholar]
- 21.Morgan, D. R., R. Freedman, C. E. Depew, and W. G. Kraft. 1987. Growth of Campylobacter pylori in liquid media. J. Clin. Microbiol. 25:2123-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padan, E., D. Zilberstein, and S. Schuldiner. 1981. pH homeostasis in bacteria. Biochim. Biophys. Acta 650:151-166. [DOI] [PubMed] [Google Scholar]
- 23.Rahmatullah, M., and T. R. Boyde. 1980. Improvements in the determination of urea using diacetyl monoxime; methods with and without deproteinisation. Clin. Chim. Acta 107:3-9. [DOI] [PubMed] [Google Scholar]
- 24.Rektorschek, M., A. Buhmann, D. Weeks, D. Schwan, K. W. Bensch, S. Eskandari, D. Scott, G. Sachs, and K. Melchers. 2000. Acid resistance of Helicobacter pylori depends on the UreI membrane protein and an inner membrane proton barrier. Mol. Microbiol. 36:141-152. [DOI] [PubMed] [Google Scholar]
- 25.Rottenberg, H. 1979. The measurement of membrane potential and delta pH in cells, organelles, and vesicles. Methods Enzymol. 55:547-569. [DOI] [PubMed] [Google Scholar]
- 26.Schäfer, G., M. Engelhard, and V. Müller. 1999. Bioenergetics of the Archaea. Microbiol. Mol. Biol. Rev. 63:570-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott, D. R., E. A. Marcus, D. L. Weeks, A. Lee, K. Melchers, and G. Sachs. 2000. Expression of the Helicobacter pylori ureI gene is required for acidic pH activation of cytoplasmic urease. Infect. Immun. 68:470-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott, D. R., D. Weeks, C. Hong, S. Postius, K. Melchers, and G. Sachs. 1998. The role of internal urease in acid resistance of Helicobacter pylori. Gastroenterology 114:58-70. [DOI] [PubMed] [Google Scholar]
- 29.Stingl, K., K. Altendorf, and E. P. Bakker. 2002. Acid survival of Helicobacter pylori: how does urease activity trigger cytoplasmic pH homeostasis? Trends Microbiol. 10:70-74. [DOI] [PubMed] [Google Scholar]
- 30.Stingl, K., E. M. Uhlemann, G. Deckers-Hebestreit, R. Schmid, E. P. Bakker, and K. Altendorf. 2001. Prolonged survival and cytoplasmic pH homeostasis of Helicobacter pylori at pH 1. Infect. Immun. 69:1178-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todd, M. J., and R. P. Hausinger. 1989. Competitive inhibitors of Klebsiella aerogenes urease. Mechanisms of interaction with the nickel active site. J. Biol. Chem. 264:15835-15842. [PubMed] [Google Scholar]
- 32.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 33.van de Vossenberg, J. L. C. M., A. J. Driessen, W. Zillig, and W. N. Konings. 1998. Bioenergetics and cytoplasmic membrane stability of the extremely acidophilic, thermophilic archaeon Picrophilus oshimae. Extremophiles 2:67-74. [DOI] [PubMed] [Google Scholar]
- 34.van de Vossenberg, J. L. C. M., T. Ubbink-Kok, M. G. Elferink, A. J. Driessen, and W. N. Konings. 1995. Ion permeability of the cytoplasmic membrane limits the maximum growth temperature of bacteria and archaea. Mol. Microbiol. 18:925-932. [DOI] [PubMed] [Google Scholar]
- 35.Weatherburn, M. W. 1967. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 39:971-974. [Google Scholar]
- 36.Weeks, D. L., S. Eskandari, D. R. Scott, and G. Sachs. 2000. A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science 287:482-485. [DOI] [PubMed] [Google Scholar]
- 37.Young, G. M., D. Amid, and V. L. Miller. 1996. A bifunctional urease enhances survival of pathogenic Yersinia enterocolitica and Morganella morganii at low pH. J. Bacteriol. 178:6487-6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaritsky, A., M. Kihara, and R. M. Macnab. 1981. Measurement of membrane potential in Bacillus subtilis: a comparison of lipophilic cations, rubidium ion, and a cyanine dye as probes. J. Membr. Biol. 63:215-231. [DOI] [PubMed] [Google Scholar]
- 39.Zychlinsky, E., and A. Matin. 1983. Cytoplasmic pH homeostasis in an acidophilic bacterium, Thiobacillus acidophilus. J. Bacteriol. 156:1352-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]