Abstract
1. The area and circumference of surface fibres of sartorius muscles were measured from photomicrographs of frozen sections of whole muscles, and compared with the values obtained assuming a circular cross-section. The latter assumption gave an over-estimate of the mean area of 28%, but only a 2% over-estimate of the circumference. In isolated, single fibres, the assumption gave over-estimates of 25 and 6%, of area and circumference respectively.
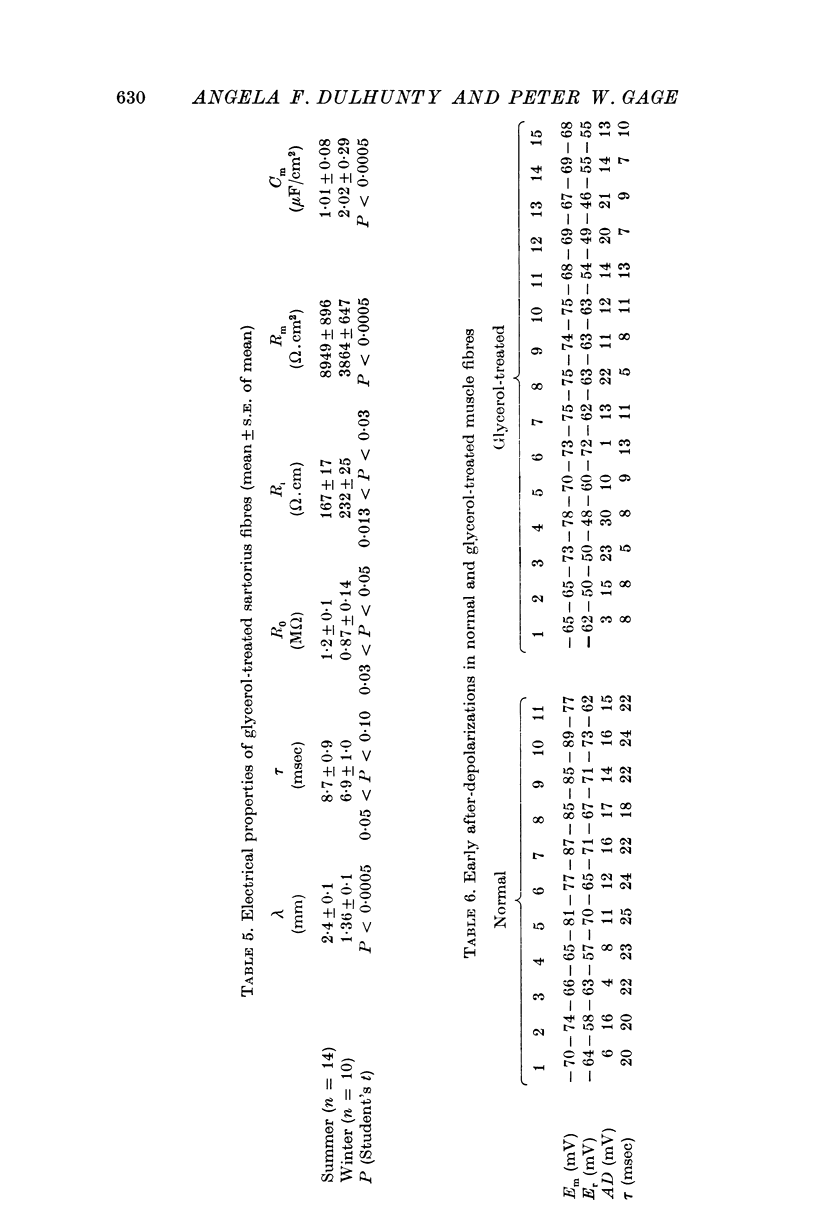
2. The passive electrical properties of fibres were different in summer and winter. The mean internal resistivity, membrane resistance and membrane capacitance were 147 Ω.cm, 7·6 kΩ.cm2 and 4 μF/cm2 in summer, and 194 Ω.cm, 3·9 kΩ.cm2 and 6·7 μF/cm2 in winter, in fibres of comparable diameters in situ. In single fibres in summer, the mean values were 120 Ω.cm, 8·6 kΩ.cm2 and 3·6 μF/cm2.
3. In glycerol-treated fibres the mean specific membrane capacitance was 1·0 μF/cm2 in summer and 2·0 μF/cm2 in winter. The internal resistivity and specific membrane resistance were 167 Ω.cm and 8·9 kΩ.cm2 in summer, and 232 Ω.cm and 3·9 kΩ.cm2 in winter.
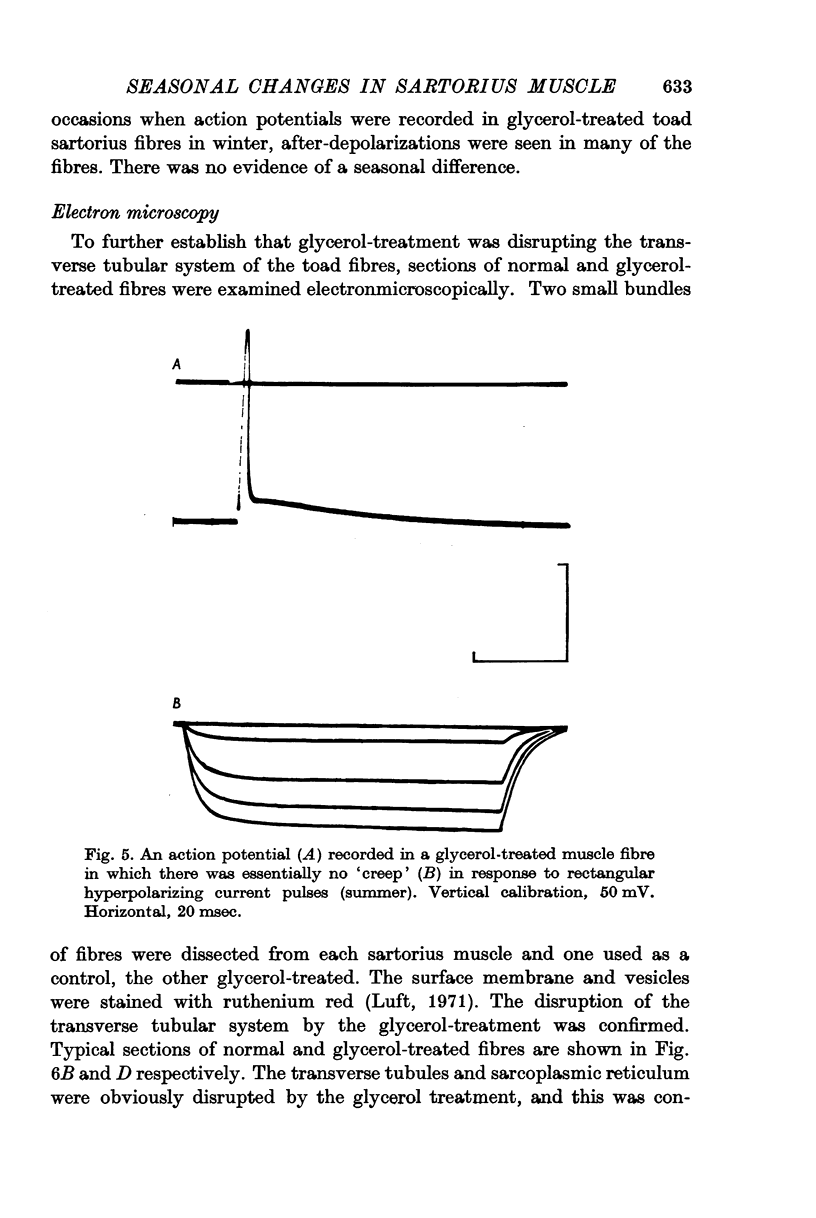
4. Early after-depolarizations were recorded in glycerol-treated fibres which had a low membrane capacitance, did not twitch and showed little `creep'. Electron micrographs of glycerol-treated fibres showed disruption of the transverse tubular system and sarcoplasmic reticulum.
5. After exposure of muscles to 400 mM urea or acetamide for 1 hr, muscle fibres did not twitch and had a reduced membrane capacitance in Ringer solution.
Full text
PDF




















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Potassium conductance changes in skeletal muscle and the potassium concentration in the transverse tubules. J Physiol. 1972 Aug;225(1):33–56. doi: 10.1113/jphysiol.1972.sp009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C. Volume and twitch tension changes in single muscle fibers in hypertonic solutions. J Gen Physiol. 1968 Nov;52(5):793–809. doi: 10.1085/jgp.52.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. S., Gage P. W. Ionic conductances of the surface and transverse tubular membranes of frog sartorius fibers. J Gen Physiol. 1969 Mar;53(3):279–297. doi: 10.1085/jgp.53.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faichney G. J., Davies H. L., Scott T. W., Cook L. J. The incorporation of linoleic acid into the tissues of growing steers offered a dietary supplement of formaldehyde-treated casein-safflower oil. Aust J Biol Sci. 1972 Feb;25(1):205–212. [PubMed] [Google Scholar]
- Freeman S. E., Satchell D. G., Chang C. S., Gay W. S. The effect of high K+ solutions and fatty acid substrates on metabolic pathways in toad heart. Comp Biochem Physiol. 1968 Jul;26(1):31–44. doi: 10.1016/0010-406x(68)90310-1. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Action potentials, afterpotentials, and excitation-contraction coupling in frog sartorius fibers without transverse tubules. J Gen Physiol. 1969 Mar;53(3):298–310. doi: 10.1085/jgp.53.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Capacitance of the surface and transverse tubular membrane of frog sartorius muscle fibers. J Gen Physiol. 1969 Mar;53(3):265–278. doi: 10.1085/jgp.53.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. Tension development in highly stretched vertebrate muscle fibres. J Physiol. 1966 May;184(1):143–169. doi: 10.1113/jphysiol.1966.sp007908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., PADSHA S. M. Effect of nitrate and other anions on the membrane resistance of frog skeletal muscle. J Physiol. 1959 Apr 23;146(1):117–132. doi: 10.1113/jphysiol.1959.sp006182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E. G. Potassium exchange and afterpotentials in frog sartorius muscles treated with glycerol. J Gen Physiol. 1970 Dec;56(6):692–715. doi: 10.1085/jgp.56.6.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nakajima S. The effect of diameter on the electrical constants of frog skeletal muscle fibres. J Physiol. 1972 Feb;221(1):105–120. doi: 10.1113/jphysiol.1972.sp009742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox R. B., Heslop-Harrison J. Pollen-wall proteins: localization and enzymic activity. J Cell Sci. 1970 Jan;6(1):1–27. doi: 10.1242/jcs.6.1.1a. [DOI] [PubMed] [Google Scholar]
- Moore L. E. Anion permeability of frog skeletal muscle. J Gen Physiol. 1969 Jul;54(1):33–52. doi: 10.1085/jgp.54.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Hodgkin A. L. Effect of diameter on the electrical constants of frog skeletal muscle fibres. Nature. 1970 Sep 5;227(5262):1053–1055. doi: 10.1038/2271053a0. [DOI] [PubMed] [Google Scholar]
- PUGSLEY I. D. MICROELECTRODE MEASUREMENTS OF MEMBRANE RESISTANCE AND CAPACITANCE IN THE SARTORIUS MUSCLE OF THE TOAD (BUFO MARINUS). Aust J Exp Biol Med Sci. 1963 Dec;41:615–628. doi: 10.1038/icb.1963.51. [DOI] [PubMed] [Google Scholar]
- Schneider M. F. Linear electrical properties of the transverse tubules and surface membrane of skeletal muscle fibers. J Gen Physiol. 1970 Nov;56(5):640–671. doi: 10.1085/jgp.56.5.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan P. C., Howell J. N., Eisenberg R. S. The capacitance of skeletal muscle fibers in solutions of low ionic strength. J Gen Physiol. 1972 Mar;59(3):347–359. doi: 10.1085/jgp.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachar J., Zacharova D., Adrian R. H. Observations on "detubulated" muscle fibres. Nat New Biol. 1972 Oct 4;239(92):153–155. doi: 10.1038/newbio239153a0. [DOI] [PubMed] [Google Scholar]





