Abstract
Tetragenococcus halophila D10 catalyzes the decarboxylation of l-aspartate with nearly stoichiometric release of l-alanine and CO2. This trait is encoded on a 25-kb plasmid, pD1. We found in this plasmid a putative asp operon consisting of two genes, which we designated aspD and aspT, encoding an l-aspartate-β-decarboxylase (AspD) and an aspartate-alanine antiporter (AspT), respectively, and determined the nucleotide sequences. The sequence analysis revealed that the genes of the asp operon in pD1 were in the following order: promoter → aspD → aspT. The deduced amino acid sequence of AspD showed similarity to the sequences of two known l-aspartate-β-decarboxylases from Pseudomonas dacunhae and Alcaligenes faecalis. Hydropathy analyses suggested that the aspT gene product encodes a hydrophobic protein with multiple membrane-spanning regions. The operon was subcloned into the Escherichia coli expression vector pTrc99A, and the two genes were cotranscribed in the resulting plasmid, pTrcAsp. Expression of the asp operon in E. coli coincided with appearance of the capacity to catalyze the decarboxylation of aspartate to alanine. Histidine-tagged AspD (AspDHis) was also expressed in E. coli and purified from cell extracts. The purified AspDHis clearly exhibited activity of l-aspartate-β-decarboxylase. Recombinant AspT was solubilized from E. coli membranes and reconstituted in proteoliposomes. The reconstituted AspT catalyzed self-exchange of aspartate and electrogenic heterologous exchange of aspartate with alanine. Thus, the asp operon confers a proton motive metabolic cycle consisting of the electrogenic aspartate-alanine antiporter and the aspartate decarboxylase, which keeps intracellular levels of alanine, the countersubstrate for aspartate, high.
The gram-positive lactic acid bacterium Tetragenococcus halophila is used to ferment soy sauce, which contains large amounts of amino acids, including l-aspartate (aspartate), sugars, such as hexoses and pentoses, and sodium chloride (ca. 17%) (27). Some strains of T. halophila catalyze decarboxylation of aspartate with nearly stoichiometric release of l-alanine (alanine) and CO2 (11, 27). Based on analogy to our previous work on Lactobacillus subspecies M3 (1), aspartate decarboxylation is thought to be advantageous for tetragenococcal cells because aspartate consumption concomitant with release of alanine generates rather than consumes metabolic energy and regulates the intracellular pH. The net charge movement during the exchange of aspartate with alanine results in a membrane potential of physiological polarity. Furthermore, decarboxylation reactions consume scalar protons and thus generate a pH gradient of physiological polarity. The combined activities of the precursor-product exchange and decarboxylation result in a proton motive force (PMF) that is sufficiently high to drive ATP synthesis via FoF1 ATPase. Such metabolic systems are proposed as proton motive metabolic cycles, and the prototype model is found in the oxalate-formate exchange system of Oxalobacter formigenes (2, 4, 16). Over the last 10 years, a number of presumed proton motive metabolic decarboxylation cycles have been identified from several bacteria, including glutamate to γ-aminobutyrate (6, 10, 24), malate to lactate (20), citrate to lactate (17, 18), and histidine to histamine (19).
Some aspartate-decarboxylating strains (AspD+) of T. halophila become decarboxylation-defective strains (AspD−) after treatment with curing agents, such as ethidium bromide or acridine orange (11). In one such AspD+ strain, T. halophila D10, we previously found a 25-kb plasmid encoding the aspartate decarboxylation trait (11). In the present study, we report the cloning, sequencing, and expression of the asp operon consisting of two genes, which we designated aspD and aspT, encoding an aspartate β-decarboxylase (AspD) and an aspartate-alanine antiporter (AspT), respectively. In addition, we used proteoliposomes to demonstrate the electrogenic character of the aspartate-alanine exchange catalyzed by AspT. This is the first discovery of a proton motive metabolic cycle encoded on a plasmid and the first report of the aspT gene coding for the aspartate-alanine antiporter.
MATERIALS AND METHODS
Cells and plasmids.
T. halophila D10 (11) was used as the source of plasmid pD1 encoding the asp operon, which consists of the aspD and aspT genes. Escherichia coli strain XL1 blue (Kanr) (Stratagene) was used for cloning experiments with the vector pBluescript II KS(−) (Stratagene). Strain XL1 blue harboring pMS421 (Specr LacIq) was called strain XL3 (2) and was used for expression of the asp operon with pTrc99A (Amersham-Pharmacia Biotech).
Growth of cells and plasmid preparation.
T. halophila D10 from which plasmid pD1 was to be extracted was grown for 72 h in lactobacillus MRS broth (Difco) with 5% (wt/vol) NaCl at 30°C. Plasmids were prepared by the method of Anderson and McKay (5), except that the lysis broth was supplemented with 5% NaCl because T. halophila requires NaCl for optimal growth. The plasmid samples were electrophoresed on horizontal 0.7% agarose gels. Plasmids were electrophoretically fractionated from the gels and purified by standard DNA recombinant techniques. E. coli cells were grown aerobically or anaerobically at 30 or 37°C in Luria broth with antibiotics as required (100 μg of ampicillin per ml, 50 μg of kanamycin per ml, and 50 μg of spectinomycin per ml).
Subcloning of asp operon from pD1.
All basic molecular biology procedures were carried out as described by Sambrook et al. (23). SalI digestion of plasmid pDI gave three fragments of DNA (12.2, 10.8, and 2.2 kb). The 10.8-kb SalI fragment was ligated to pBluescript II KS(−), giving pAspS, and a nested deletion series was prepared for sequencing. After identification of the genes encoding AspD and AspT, the DNA sequence in this region was confirmed by second-strand sequencing using plasmids selected from the same deletion series. The 12.2- and 2.2-kb SalI fragments were also ligated to pBluescript II KS(−), and the nucleotide sequences of both strands were determined with a nested deletion series and/or primer walking.
A new NcoI site was introduced at the initiation codon of the aspD gene in the asp operon on pAspS, producing pAsp(Nco). Subsequently, pAspS(Nco) was digested with NcoI plus SalI to give a 4.3-kb fragment containing the complete asp operon. This 4.3-kb fragment was ligated into the NcoI-SalI site of pTrc99A to generate pTrcAsp, in which expression of the asp operon was regulated by the trc promoter. The asp operon was translated from the ATG codon in the NcoI site. As a final step, pTrcAsp was placed in E. coli XL3 for functional tests.
To generate histidine-tagged AspD (AspDHis), a histidine tag (6× histidine) and a new XhoI site were introduced at the C-terminal end of aspD in pTrcAsp by using a QuikChange site-directed mutagenesis kit (Stratagene) with oligonucleotides AspD6His+ (5′ C TAT GAT AAA TTC CAA CAA AAA TAA CAC CAC CAC CAC CAC CAC CCA TGG AAT TCG AGC TCG AGA CCC GGG GAT CCT CTA GAG TCG ACC; the underlining indicates the new XhoI site) and AspD6His− (5′ GGT CGA CTC TAG AGG ATC CCC GGG TCT CGA GCT CGA ATT CCA TGG GTG GTG GTG GTG GTG GTG TTA TTT TTG TTG GAA TTT ATC ATA G). Then the mutated plasmid was digested with XhoI to excise a fragment of aspT and ligated to generate the resulting plasmid, pTrcAspDHis.
Sequencing.
Double-stranded DNA was sequenced with an ABI Prism BigDye terminator cycle sequencing reaction kit (PE Applied Biosystems) by automated DNA sequencing using an ABI Prism 377 DNA sequencer (PE Applied Biosystems). A nested deletion series was sequenced by using universal primers for pBluescript II KS(−); as primers for sequencing the asp operon opposite strand, we designed appropriate complementary synthetic oligonucleotides.
RNA isolation and primer extension.
Cells of T. halophila D10 grown for 72 h in lactobacillus MRS broth with 5% (wt/vol) NaCl and 10 mM l-aspartate at 30°C were harvested and washed once in 50 mM Tris-Cl (pH 7.5), and RNA was isolated with Sepasol-RNA I Super kits (Nacalai Tesque, Kyoto, Japan) used according to the manufacturer's instructions. Primer extension analysis was performed by using oligonucleotides AspDL48 (5′-TCC AAG AAA AGG GCA GCA ACT) and AspDL229 (5′-GCC TTC TTT TTC AAC ATA GCC). The primers end labeled with IRD800 infrared dye were purchased from Aloka (Tokyo, Japan). Each primer (10 pmol) was annealed to 15 μg of total RNA, and primer extension reactions were performed with Ready-To-Go RT-PCR beads (Amersham Pharmacia Biotech) as recommended by the manufacturer's protocol. The cDNAs, which were synthesized along with sequence ladders generated with the same primers, were electrophoresed on an 8% polyacrylamide gel with 7 M urea by using a Li-Cor automated DNA sequencer 4200G (Aloka). The sequencing reaction for the ladders was performed with Thermo Sequenase cycle sequencing kits (Amersham Pharmacia Biotech).
Expression of asp operon in E. coli.
An overnight preculture of E. coli XL3 carrying pTrcAsp or pTrc99A was diluted 100-fold in fresh Luria broth containing 10 mM aspartate and 2 mM pyridoxal 5′-phosphate (PLP). The cell suspension was incubated statically for 48 h at 30°C. At 12 h prior to cell harvest, 200 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added to the cultures. IPTG-induced cells and uninduced control cells were washed with and resuspended in 50 mM potassium morpholineethanesulfonic acid (MES) buffer (pH 6). The aspartate decarboxylation reaction was started by adding 10 mM aspartate to the cell suspension (80 mg [dry weight] of cells/ml) and incubating the reaction mixture at 30°C for 20 min. Concentrations of aspartate and alanine in the reaction mixtures were measured with a Hitachi L8500 amino acid analyzer.
Assay of AspD activity.
IPTG-induced E. coli cells harboring pTrcAspDHis or pTrc99A were disrupted in lysis buffer (50 mM sodium phosphate buffer [pH 8], 300 mM NaCl, 10 mM imidazole) by sonication or high-pressure lysis as described below, and cell extracts were prepared by centrifugation (15,000 × g) for 10 min. Aspartate decarboxylation was monitored by the appearance of the product alanine as follows. The reaction mixture contained 200 mM sodium acetate buffer (pH 5.3), 5 mM pyruvate, 0.1 mM PLP, and 0.003% (wt/vol) hydroxypropyl cellulose (Wako, Tokyo, Japan). After incubation of cell extracts in the mixture, the reaction was terminated by boiling for 5 min. The solution was then clarified by centrifugation (15,000 × g) for 5 min, and the concentration of l-alanine in the supernatant was determined by using alanine dehydrogenase and the method described previously (28). Aspartate decarboxylase activities were calculated by determining the rate of alanine appearance.
Purification of AspDHis.
Crude cell extract (684 μg of protein) containing AspDHis was loaded on a Qiagen Ni2+-nitrilotriacetic acid (NTA) column, washed, and eluted according to the manufacturer's instructions.
Electrophoresis and Western blotting.
Samples of fractions obtained during purification of AspDHis were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 12% acrylamide, as described previously (13), and protein content was evaluated by staining with Coomassie brilliant blue. The position of AspDHis was verified by Western blotting of a duplicate gel in which proteins were transferred to polyvinylidene difluoride membranes (Millipore, Tokyo, Japan) and exposed to anti-6His mouse monoclonal antibodies (Covance Inc., Princeton, N.J.). Binding of the primary antibody was visualized by using anti-mouse immunoglobulin G (heavy and light chains) horse polyclonal antibody (Vector Laboratories Inc., Burlingame, Calif.) and an AP detection kit (Bio-Rad) according to the manufacturer's instructions.
Preparation of membrane vesicles, solubilization, and reconstitution of AspT.
IPTG-induced cells and uninduced control cells (5 mg of protein each) were harvested by centrifugation and washed with 100 mM potassium phosphate (pH 7); membrane vesicles were prepared by high-pressure lysis in the presence of 100 mM potassium phosphate (pH 7), as previously described (3), and the vesicles were stored at −70°C as concentrated stock preparations (10 to 20 mg of protein per ml).
Membrane vesicles (1 to 2 mg of protein) were solubilized (3) by using 1.25% (wt/vol) octylglucoside in the presence of 0.4% (wt/vol) acetone-ether-washed E. coli phospholipid, 100 mM potassium phosphate (pH 7), 4 mM magnesium sulfate, 1 mM dithiothreitol, 0.75 mM phenylmethylsulfonyl fluoride, and 20% glycerol. Control extracts were prepared in the same way but without added protein.
Reconstitution was in a final volume of 1 ml by using 400 μl of detergent extract (or control lipid extract), 130 μl of bath-sonicated liposomes (5.9 mg of E. coli phospholipid), and 18 μl of 15% octylglucoside, with the balance comprised of 1 mM dithiothreitol and 100 mM phosphate (pH 7) as either the potassium or N-methylglucamine (NMG) salt. After incubation for 20 min on ice, proteoliposomes (or control liposomes) were formed at 23°C by rapid injection into 20 ml of a loading buffer containing 100 mM potassium NMG phosphate (pH 7) and 1 mM dithiothreitol along with 100 mM aspartate (potassium or NMG salt, as specified below). After an additional 20 min, the substrate-loaded proteoliposomes (or liposomes) were recovered by centrifugation and washing (3), with resuspension in 100 mM potassium sulfate or 100 mM NMG sulfate plus 100 mM potassium phosphate or 100 mM NMG phosphate (pH 7) and 1 mM dithiothreitol (resuspension buffers). The final resuspension volume was usually 300 μl, which resulted in protein and lipid concentrations of approximately 50 to 250 μg/ml and 13 mg/ml, respectively (3). When proteoliposomes (liposomes) were loaded with alanine, the same procedure was used, except that the buffer for loading, washing, and resuspension contained 200 mM alanine instead of aspartate and the resuspension volume for proteoliposomes was 20 μl.
Assays of transport.
To assay for [3H]aspartate transport by aspartate-loaded particles, proteoliposomes were diluted 20-fold from the concentrated stock preparations into an appropriate volume of assay buffer (resuspension buffer lacking dithiothreitol) along with other required materials. After 1 to 3 min of preincubation at 23°C, 3H-labeled substrate was added to a normal concentration of 100 μM, and at different times 50- to 100-μl aliquots were removed for membrane filtration (0.22-μm pore-size GSTF Millipore filters), followed by two washes with 5 ml of assay buffer (3). For transport assays of alanine-loaded proteoliposomes, proteoliposomes were diluted 100-fold with the assay buffer containing 200 μM [3H]aspartate with or without 1 μM valinomycin. In this way, it was possible to generate a membrane potential whose polarity was either interior positive (potassium outside, NMG inside) or interior negative (NMG outside, potassium inside). As an additional basis for comparison, proteoliposomes were loaded with NMG-alanine and tested by using the NMG-based assay buffer.
Protein estimation.
Protein content was measured by using a modification of the method of Schaffner and Weissman (25), as described elsewhere (3).
Chemicals.
l-[2,3-3H]Aspartic acid (1.07 GBq/mmol) was purchased from Amersham-Pharmacia Biotech. Octyl-β-d-glucoside was obtained from Dojin (Kumamoto, Japan). E. coli phospholipid was provided by Avanti Polar Lipids, Inc. (Alabaster, Ala.) (3).
Nucleotide sequence accession number.
The nucleotide sequence of the asp operon has been deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases under accession number AB072729.
RESULTS
Cloning of the asp operon.
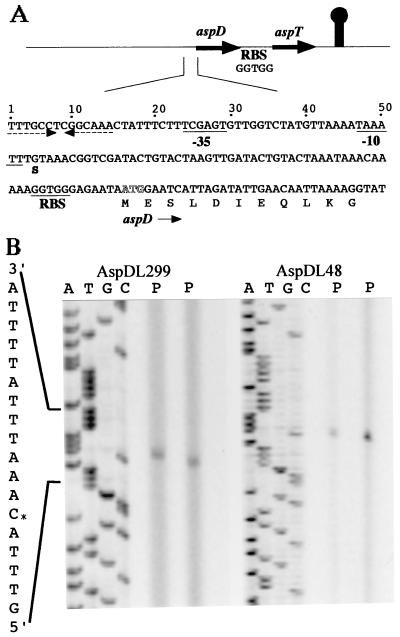
We found the aspartate metabolic genes in plasmid pD1 (ca. 25 kb) and determined the nucleotide sequences of these genes. Figure 1 shows the gene organization of the asp operon in the plasmid and the nucleotide sequence of its putative promoter region. We identified a gene cluster consisting of two genes, designated aspD and aspT (Fig. 1A); aspD and aspT code for an l-aspartate-β-decarboxylase (EC 4.1.1.12) and an aspartate-alanine antiporter, respectively. These two genes were oriented in the same direction, and there was no potential open reading frame immediately upstream of the 5′ end of aspD or downstream of the 3′ end of aspT in this direction; the minimum size for a putative open reading frame used to analyze the sequence was 40 amino acids. The asp gene cluster seems to form an operon (Fig. 1A). Figure 1A also shows the nucleotide sequence upstream of the 5′ end of the aspD gene. A potential core sequence of the ribosome binding site (RBS) for tetragenococcal genes is GGTGG, which is also observed in the xyl operon of T. halophila (26); an RBS preceded the ATG start codon of the aspD gene, and another RBS was between aspD and aspT.
FIG. 1.
Determination of the aspDT mRNA 5′ end and gene organization of the asp operon of T. halophila and its putative 5′ flanking region. (A) At the top, the genes are indicated by arrows (not to scale). The directions of transcription are also indicated by the arrows. The noncoding region following the aspT gene contained a sequence with a potential structure in the mRNA that was similar to the rho-independent transcription termination signal of E. coli (indicated by a mushroom-like symbol). The two open reading frames each contained an ATG codon and were preceded by possible RBSs at distances of 7 to 9 nucleotides. The assigned initiation codons are putative. The termination codons were TAA for both aspD and aspT. The intergenic noncoding region in the asp cluster was 25 bp between aspD and aspT. At the bottom, the nucleotide sequence of the 5′ flanking region for aspD is shown. ATG in white letters indicates the location of the start codon for aspD. The dashed arrows indicate a palindrome. The transcription initiation site is labeled S, and putative −35 and −10 regions deduced from the transcript analysis described below are underlined. An RBS is also underlined. (B) Primer extension analysis was carried out with oligonucleotides AspDL229 and AspDL48 and RNA from aspartate-grown cells of T. halophila D10. Lanes P, primer extension aliquot (duplicate); lanes C, G, T, and A, sequence ladder generated with AspDL229 or AspDL48. The +1 site is marked with an asterisk.
The transcription initiation site for the asp operon was determined by primer extension analysis. Primers AspDL48 and AspD229L were complementary to the 48 and 229 nucleotides, respectively, downstream from the translation initiation site, and cDNA products from the two primers independently assigned the same transcription initiation site located 62 nucleotides upstream from the translation initiation site (Fig. 1B). Probable sequences of the so-called −35 and −10 (or Pribnow) boxes (9) were found (Fig. 1A). A potential transcription termination hairpin (ΔG = −10 kcal/mol) is located 73 bp downstream from the translation end of aspT.
Primary amino acid sequences of the asp gene products.
We searched current protein databases with the BLAST network service and the United States patent database. The aspD gene product (AspD) was predicted to be composed of 532 amino acids and to have a molecular mass of 60,073 Da. On the basis of the database searches, known aspartate β-decarboxylase genes have been isolated only from Alcaligenes faecalis (7) and Pseudomonas dacunhae (22). Although the products of these two genes exhibited significant relatedness to each other (93% identity, 96% similarity), the tetragenococcal aspD gene product (AspD) was less related to them (37 and 35% identity, respectively; 59 and 58% similarity, respectively). Figure 2 shows an alignment of the amino acid sequences of the three aspartate β-decarboxylases. PLP attachment motifs (7) were conserved in all three aspartate β-decarboxylases.
FIG. 2.
Similarity of the amino acid sequence of T. halophila AspD (AspD/Th) (DDBJ/EMBL/GenBank accession no. AB072729) to the amino acid sequences of P. dacunhae aspartate-β-decarboxylase (AspD/Pd) (22) and A. faecalis aspartate-β-decarboxylase (AsdA/Af) (DDBJ/EMBL/GenBank accession no. AF168368). Asterisks indicate identical amino acid residues. The box and triangles indicate predicted pyridoxal phosphate attachment sites and substrate binding sites, respectively. The numbers for each line are the numbers of amino acid residues starting from the amino terminus. Dashes indicate gaps.
Analysis of the deduced AspT amino acid sequence revealed a novel hydrophobic protein consisting of 543 amino acid residues having a predicted mass of 57,212 Da (data not shown). No proteins with significant relatedness to AspT were found in the protein databases examined. Analyses of AspT hydropathy by using the methods of the TMpred (12) and PHDhtm (21) programs predicted the presence of 10 to 12 hydrophobic segments, each of sufficient length to constitute a transmembrane helix (data not shown).
Expression of asp operon in E. coli.
To determine whether the genes tentatively identified as the asp operon specify the aspartate decarboxylase AspD and the AspT transport protein, we constructed a vector (pTrcAsp) in which aspD and aspT were cotranscribed in the authentic order (Fig. 1) under control of the trc promoter. Expression of the asp operon and assays of its function were then carried out in E. coli XL3. This strain also harbored middle-copy compatible plasmid pMS421 encoding the gene for LacIq, which strongly repressed expression of the asp operon in the absence of IPTG. This enabled pTrcAsp to be propagated without the selective pressures that might accompany leaky protein expression. The results shown in Fig. 3 document that the cloned asp operon encoded the proteins catalyzing aspartate decarboxylation in washed cells of E. coli. IPTG-induced cells with pTrcAsp or pTrc99A were harvested and washed as described above. The washed cell suspensions were incubated with 10 mM aspartate. Cells harboring pTrcAsp metabolized aspartate with nearly stoichiometric release of alanine. By contrast, neither aspartate metabolism nor alanine production was observed in E. coli cells harboring the pTrc99A vector. Aspartate decarboxylase activities were detected intracellularly but not extracellularly in E. coli cells containing pTrcAsp (data not shown). These results suggest that gene products of the asp operon are able to catalyze inward transport of aspartate, intracellular decarboxylation of aspartate, and subsequent outward excretion of alanine.
FIG. 3.
Aspartate decarboxylation by E. coli cells harboring pTrcAsp (asp operon). The asp operon in pTrcAsp was expressed by induction with 200 μM IPTG for 12 h at 30°C. Cells were harvested, resuspended in 50 mM potassium MES buffer (pH 6) containing 10 mM aspartate, and incubated for 20 min at 30°C. The concentrations of aspartate (open symbols) and alanine (solid symbols) in the reaction mixtures were monitored (□ and ♦, pTrcAsp; ○ and ▴, pTrc99A). The vector without inserts was pTrc99A. The error bars indicate standard errors.
aspD encodes l-aspartate β-decarboxylase.
We also expressed AspDHis in E. coli XL3 and purified the tagged protein from crude cell extracts with an Ni2+-NTA column (Fig. 4). The specific activity of AspDHis in the crude cell extract was 0.093 mkat/kg of protein (Fig. 4A, lane 2), and the purified enzyme had a specific activity of 0.54 mkat/kg of protein (Fig. 4A, lane 4). AspD activities were not detectable in crude extracts from cells with pTrc99A (data not shown). The molecular mass of AspD (AspDHis) predicted from observation of the SDS-PAGE gel was almost identical to that calculated from the deduced amino acid sequence of AspD.
FIG. 4.
Purification of AspDHis. (A) SDS-PAGE profiles obtained during purification of AspDHis. Lanes 2 and 3, 114.0 μg of protein of the crude extract and the immediate flowthrough from an Ni2+-NTA column, respectively; lane 4, wash fluid taken just prior to elution of AspDHis by 50 mM sodium phosphate buffer (pH 8) containing 300 mM NaCl and 250 mM imidazole; lane 5, 2.4 μg of purified AspDHis. An arrow indicates the position of AspDHis. (B) Western blot of the profiles described for panel A. The contents of a duplicate SDS-PAGE gel were transferred to a polyvinylidene difluoride membrane and probed with antiserum against the histidine tag.
aspT encodes the aspartate-alanine exchange protein.
To further investigate whether the aspartate decarboxylation shown in Fig. 3 is organized as a proton motive metabolic cycle (16) involving antiport of the precursor (aspartate) and the product (alanine), we employed reconstituted membrane proteins to probe for the presence of a carrier which could mediate the predicted antiport reaction.
We initially focused on studying aspartate-loaded proteoliposomes, with the expectation that AspT would catalyze an aspartate self-exchange. Proteoliposomes prepared from membranes of E. coli harboring pTrcAsp were loaded with 100 mM potassium aspartate and 0.1 M potassium phosphate buffer (pH 7) and suspended in an NMG-based medium (0.1 M NMG sulfate, 0.1 M NMG phosphate; pH 7) to which 100 μM external [3H]aspartate was added. [3H]aspartate was loaded into proteoliposomes by aspartate self-exchange. In the experiment whose results are shown in Fig. 5A, the steady-state incorporation of [3H]aspartate was approximately 200 nmol/mg of protein. Moreover, the incorporated material was readily expelled after a later addition of excess unlabeled aspartate or alanine, as if [3H]aspartate had been taken up by an exchange reaction and alanine had been the countersubstrate for aspartate. Since accumulated [3H]aspartate was released by either aspartate or alanine (Fig. 5A), it seems feasible that both compounds served as substrates. As expected, a membrane potential (outside positive) generated by addition of valinomycin had no effect on the release of [3H]aspartate induced by unlabeled aspartate (Fig. 5A), because this release was attributable to the electroneutral property of aspartate self-exchange (Fig. 5A). On the other hand, the steady-state level of aspartate self-exchange observed in the control proteoliposomes prepared from E. coli cells with the pTrc99A vector was less than 15% of the level observed in the proteoliposomes from E. coli cells with pTrcAsp, and the incorporated [3H]aspartate in the control proteoliposomes (pTrc99A) was released after a later addition of unlabeled aspartate but was not expelled after a subsequent addition of unlabeled alanine even in the presence or absence of valinomycin (Fig. 5A). The results for aspartate transport observed with the control proteoliposomes suggested that E. coli cells had weak background activities of aspartate self-exchange in the assay conditions used but did not have activities of heterologous exchange of aspartate with alanine. Hence, the major activities of aspartate transport observed in the proteoliposomes from E. coli with pTrcAsp are attributable to the function of expressed AspT proteins.
FIG. 5.
AspT expressed in E. coli catalyzes electroneutral aspartate self-exchange and electrogenic exchange of aspartate with alanine. (A) A detergent extract of IPTG-induced cells carrying pTrcAsp or pTrc99A was used to prepare proteoliposomes (or liposomes). Proteoliposomes loaded with 100 mM potassium aspartate and 100 mM potassium phosphate (pH 7) were placed in an NMG-based medium (0.1 M NMG sulfate, 0.1 M NMG phosphate; pH 7) at a concentration of 10 to 25 μg of protein/ml along with 100 μM [3H]aspartate and either 1 μM valinomycin (open symbols) or ethanol (solid symbols). After 8 min (arrow), aliquots from each tube received 10 mM NMG-aspartate (▵ and ▴, pTrcAsp; ⋄, pTrc99A), 10 mM NMG-alanine (○ and •, pTrcAsp; □, pTrc99A), or the equivalent volume of assay buffer (▹). Samples were taken and used for filtration and washing at the times indicated. Control liposomes (▿) were also examined without the addition of unlabeled substrates. Error bars are omitted in the case of pTrc99A (⋄ and □) and control liposomes (▿) for clarity. (B) Proteoliposomes (or liposomes) were loaded with 200 mM alanine plus 100 mM phosphate (pH 7) as either the NMG salt (•, □, and ▪, pTrcAsp; ▵, pTrc99A) or the potassium salt (○, pTrcAsp) and diluted 100-fold into assay media containing 200 μM [3H]aspartate along with 100 mM sulfate plus 100 mM phosphate as the NMG salt (○, □, and ▪, pTrcAsp) or the potassium salt (•, pTrcAsp); with one exception (□) (−Val), 1 μM valinomycin (+Val) was also present. Samples were taken and used for filtration and washing at the times indicated. The presence of external potassium (Kout) or internal potassium (Kin) is indicated. The control proteoliposomes (No K[+Val] or No K[−Val]), whose behavior was largely unaffected by valinomycin, showed aspartate transport virtually identical to that found for the potassium-loaded proteoliposomes not exposed to valinomycin. Liposomes (⊕) without protein loaded with the NMG-based buffer containing 200 mM alanine were also diluted into the potassium-based buffer with 200 μM [3H]aspartate plus 1 μM valinomycin. Error bars are omitted for Kin[+Val] (○), pTrc99A (▵), and control liposomes (⊕) for clarity.
Because the conversion of aspartate to alanine by E. coli with pTrcAsp was very nearly stoichiometric (Fig. 3), we expected that the heterologous aspartate-alanine exchange would be a one-for-one antiport. We also anticipated that the reaction would be an electrogenic exchange, since at physiological pH aspartate is monovalent (pK1[COOH] = 2.09, pK2[NH3+] = 9.82, pK3[COOH] = 3.86), while alanine is neutral (pK1[COOH]) = 2.34, pK2[NH3+]) = 9.69). This view was initially suggested by the results shown in Fig. 5A (proteoliposomes from E. coli with pTrcAsp). However, the release of accumulated [3H]aspartate after addition of alanine (Fig. 5A) was observed to be slower than that after addition of aspartate in the absence of valinomycin (Fig. 5A). The slow excretion of [3H]aspartate after addition of alanine is attributable to the outside-negative membrane potential formed by aspartate excretion coupled with alanine uptake; the resulting increase in polarization of the membrane (outside negative) would have retarded the heterologous exchange itself. When an outside-positive membrane potential was imposed across the proteoliposome membrane by an outward potassium diffusion potential in the presence of valinomycin, [3H]aspartate release induced by alanine (heterologous exchange) (Fig. 5A) was accelerated to the same extent as the release induced by aspartate (homologous exchange) (Fig. 5A). Hence, we concluded that the aspartate self-exchange and aspartate-alanine heterologous exchange reactions were catalyzed by AspT expressed with pTrcAsp (Fig. 5A).
The electrogenic nature of the aspartate-alanine exchange catalyzed by AspT was further supported by the results of experiments shown in Fig. 5B. A detergent extract from IPTG-induced cells with pTrcAsp was also used to prepare proteoliposomes loaded with the potassium or NMG salt of alanine to monitor the exchange of aspartate with alanine (Fig. 5B). The proteoliposomes were diluted into media containing NMG phosphate or potassium phosphate, so that addition of valinomycin negatively or positively polarized the membranes. Imposition of an internally positive electrical potential strongly stimulated aspartate transport, whereas imposition of an internally negative potential significantly inhibited the transport reaction. In such experiments (Fig. 5B), control proteoliposomes were prepared and assayed by using NMG as the internal and external cations (Fig. 5B). These controls, whose behavior was largely unaffected by valinomycin, showed aspartate transport virtually identical to that found for the potassium- or NMG-loaded proteoliposomes not exposed to valinomycin. On the other hand, proteoliposomes prepared from membranes of E. coli harboring the pTrc99A vector were also loaded with alanine and examined; however, the proteoliposomes of pTrc99A scarcely showed aspartate transport activity even in the presence of an inside-positive membrane potential (Fig. 5B). Therefore, the activities of aspartate-alanine exchange observed in the proteoliposomes from E. coli with pTrcAsp are attributable to the function of AspT. The pattern of responses indicates that the aspartate-alanine exchange is electrogenic, with a negative charge moving in parallel with aspartate.
DISCUSSION
In the present work, we demonstrated the presence of asp operon-encoded proteins, which catalyze decarboxylation of aspartate with nearly stoichiometric release of alanine, in plasmid pD1 found in the halophilic lactic acid bacterium T. halophila strain D10. Analysis of the nucleotide sequence of plasmid pD1 showed that the asp operon consisted of two genes, aspD and aspT, presumably encoding an aspartate decarboxylase (AspD) and an aspartate-alanine antiporter (AspT) (Fig. 1A). These predictions based on the sequence analysis were supported by the nearly stoichiometric conversion of aspartate to alanine observed in E. coli cells in which the asp operon was expressed (Fig. 3) and by the finding that purified AspDHis indicated the activity of l-aspartate-β-decarboxylase (Fig. 4). Evidence of the electrogenic character of recombinant AspT, as determined by using a proteoliposome reconstitution system (Fig. 5), further supported the idea that the gene products of the asp operon, AspD and AspT, coordinate a proton motive metabolic cycle (16).
Since the intergenic region between aspD and aspT did not seem to form any secondary structure, the probable gene organization of the asp operon is promoter → aspD → aspT, and the two genes are likely to be cotranscribed by the predicted promoter upstream of the 5′ end of aspD.
At this time, only two aspartate β-decarboxylases, one originating from P. dacunhae (22) and one originating from A. faecalis (7), can be found in the United States patent and protein databases, respectively. It is noteworthy that these two decarboxylases have been used industrially for enzymatic or fermentative production of l-alanine or d-aspartate. The deduced amino acid sequence of tetragenococcal AspD is only moderately related to those of aspartate β-decarboxylases from P. dacunhae and A. faecalis (37 to 38% identity); however, the relatedness between the two decarboxylases from P. dacunhae and A. faecalis is significant (93% identity). Therefore, tetragenococcal AspD seems to be evolutionarily distant from the two other decarboxylases, although the PLP attachment sites are well conserved in the three decarboxylases (Fig. 2). The activities of AspD were localized in cytoplasmic fractions prepared from both the authentic T. halophila D10 cells and E. coli cells in which the tetragenococcal asp operon was expressed with pTrcAsp (data not shown).
Analysis of the AspT amino acid sequence revealed a hydrophobic membrane protein (data not shown), and the biochemical features of AspT transport demonstrated by using proteoliposomes (Fig. 5) are sufficient to classify AspT as a conventional secondary transport protein, in particular, an electrogenic antiporter similar to OxlT from O. formigenes (2, 4, 14, 15, 16). However, there is no apparent sequence homology between AspT and known membrane carriers, including PMF-generating antiporters (2, 17, 24), suggesting that the structures of PMF-generating antiporters are divergent. We previously found an electrogenic aspartate-alanine antiporter in Lactobacillus subspecies M3 by employing a proteoliposome reconstitution system (1); however, the predicted gene encoding AspT in Lactobacillus subspecies M3 has not yet been cloned. Consequently, at this time we are not able to compare the deduced structures of AspTs from T. halophila and Lactobacillus subspecies M3. Cloning of the lactobacillus aspT gene is also in progress. Based on the features of the tetragenococcal asp operon products described above, we expect that the cytoplasmic aspartate decarboxylase (AspD) and the electrogenic aspartate-alanine antiporter (AspT) compose a proton motive metabolic cycle similar to the oxalate-formate exchange system observed in O. formigenes (4) and to the aspartate-alanine exchange system observed in Lactobacillus subspecies M3 (1).
T. halophila naturally grows from a neutral pH (pH 7) toward an acidic pH (pH 4 to 5) due to lactic acid production in the presence of a large amount of sodium chloride (17%, wt/vol) (11, 27). The presence of a large amount of extracellular cations (H+, Na+) might force tetragenococcal cells to pump out the cations. The proton motive metabolic cycle encoded in the asp operon may contribute directly to the extrusion of protons from cells or indirectly to sodium extrusion (Na+/H+ exchange) coupled with a PMF generated from the cycle, because T. halophila is an anaerobe and is thought to lack oxidative proton pumps. Similar bacterial proton motive metabolic cycles coupled with glutamate decarboxylation have recently been recognized as cycles that are involved in acid tolerance (6, 8, 10, 24).
To our knowledge, this is the first report of plasmid-encoded genes involving a proton motive metabolic cycle and the first report of an aspT gene coding for an aspartate-alanine antiporter. The entire sequence of plasmid pD1 contains transposon-like sequences and probable genes encoding machinery required for conjugal transfer (Abe and Higuchi, unpublished data), showing the possibility of mobility of the asp operon not only among strains but also among genomes and plasmids. Studies of the origin of the mobile asp operon would also be interesting from an evolutionary point of view.
Acknowledgments
We thank Kinzi Uchida for advice and Kuniko Shiraishi for excellent technical assistance throughout this research.
REFERENCES
- 1.Abe, K., H. Hayashi, and P. C. Maloney. 1996. Exchange of aspartate and alanine. J. Biol. Chem. 271:3079-3084. [DOI] [PubMed] [Google Scholar]
- 2.Abe, K., Z. S. Ruan, and P. C. Maloney. 1996. Cloning, sequencing, and expression in Escherichia coli of OxlT, the oxalate:formate exchange protein of Oxalobacter formigenes. J. Biol. Chem. 271:6789-6793. [DOI] [PubMed] [Google Scholar]
- 3.Ambudkar, S. V., and P. C. Maloney. 1986. Bacterial anion exchange, use of osmolyte during solubilization and reconstitution of phosphate-linked antiport from Streptococcus lactis. J. Biol. Chem. 261:10079-10086. [PubMed] [Google Scholar]
- 4.Anantharam, V., M. J. Allison, and P. C. Maloney. 1989. Oxalate:formate exchange. The basis for energy coupling in Oxalobacter. J. Biol. Chem. 264:7244-7250. [PubMed] [Google Scholar]
- 5.Anderson, D. G., and L. L. McKay. 1983. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl. Environ. Micorobiol. 46:549-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castanie-Cornet, M. P., and J. W. Foster. 2001. Escherichia coli acid resistance: cAMP receptor protein and a 20 bp cis-acting sequence control pH and stationary phase expression of the gadA and gadBC glutamate decarboxylase genes. Microbiology 147:709-715. [DOI] [PubMed] [Google Scholar]
- 7.Chen, C. C., T. L. Chou, and C. Y. Lee. 2000. Cloning, expression and characterization of l-aspartate β-decarboxylase gene from Alcaligenes faecalis CCRC 11585. J. Ind. Microbiol. Biotechnol. 25:132-140. [Google Scholar]
- 8.Cotter, P. D., C. G. Gahan, and C. Hill. 2001. A glutamate decarboxylase system protects Listeria monocytogenes in gastric fluid. Mol. Microbiol. 40:465-475. [DOI] [PubMed] [Google Scholar]
- 9.Dahl, M. K., J. Degenkolb, and W. Hillen. 1994. Transcription of the xyl operon is controlled in Bacillus subtilis by tandem overlapping operators spaced by four base-pairs. J. Mol. Biol. 243:413-424. [DOI] [PubMed] [Google Scholar]
- 10.Higuchi, T., H. Hayashi, and K. Abe. 1997. Exchange of glutamate and γ-aminobutyrate in a Lactobacillus strain. J. Bacteriol. 179:3362-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higuchi, T., K. Uchida, and K. Abe. 1998. Aspartate decarboxylation encoded on the plasmid in the soy sauce lactic acid bacterium, Tetragenococcus halophila D10. Biosci. Biotechnol. Biochem. 62:1601-1603. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann, K., and W. Stoffel. 1993. TM-base-A database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler 374:166. [Google Scholar]
- 13.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 14.Maloney, P. C. 1995. Bacterial transporters. Curr. Opin. Cell Biol. 6:571-582. [DOI] [PubMed] [Google Scholar]
- 15.Maloney, P. C., and T. H. Wilson. 1996. Ion-coupled transport and transporters, p. 1130-1148. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. D. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. ASM Press, Washington, D.C.
- 16.Maloney, P. C., R. Y. Yan, and K. Abe. 1994. Bacterial anion exchange: reductionist and integrative approaches to membrane biology. J. Exp. Biol. 196:471-482. [DOI] [PubMed] [Google Scholar]
- 17.Marty-Teysset, C., C. Posthuma, J. S. Lolkema, P. Schmitt, C. Divies, and W. N. Konings. 1995. Membrane potential generating transport of citrate and malate catalysed by CitP of Leuconostoc mesenteroides. J. Biol. Chem. 270:25370-25376. [DOI] [PubMed] [Google Scholar]
- 18.Marty-Teysset, C., C. Posthuma, J. S. Lolkema, P. Schmitt, C. Divies, and W. N. Konings. 1996. Proton motive force generation by citrolactic fermentation in Leuconostoc mesenteroides. J. Bacteriol. 178:2178-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molenaar, D., J. S. Bosscher, B. TenBrink, A. J. M. Driessen, and W. N. Konings. 1993. Generation of a proton motive force by histidine decarboxylation and electrogenic histidine/histamine antiport in Lactobacillus buchneri. J. Bacteriol. 175:2864-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poolman, B., D. Molenaar, E. J. Smid, T. Ubbink, T. Abee, P. P. Renault, and W. N. Konings. 1991. Malolactic fermentation: electrogenic malate uptake and malate/lactate antiport generate metabolic energy. J. Bacteriol. 173:6030-6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rost, B., P. Fariselli, and R. Casadio. 1996. Topology prediction for helical transmembrane proteins at 86% accuracy. Protein Sci. 7:1704-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rozzell, J. D. May 1991. Method and compositions for the production of l-alanine and derivatives thereof. U.S. Patent 5,019,509.
- 23.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 24.Sanders, J. W., K. Leenhouts, J. Burghoorn, J. R. Brands, G. Venema, and J. Kok. 1998. A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol. Microbiol. 27:299-310. [DOI] [PubMed] [Google Scholar]
- 25.Schaffner, W., and C. Weissman. 1973. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal. Biochem. 56:502-514. [DOI] [PubMed] [Google Scholar]
- 26.Takeda, Y., K. Takase, I. Yamato, and K. Abe. 1998. Sequencing and characterization of the xyl operon of a gram-positive bacterium, Tetragenococcus halophila. Appl. Environ. Micorobiol. 64:2513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uchida, K. 1988. Trends in preparation and uses of fermented and acid-hydrolyzed soy sauce, p. 78-83. In Proceedings of the World Congress on Vegetable Protein Utilization in Human Foods and Animal Feedstuffs. Kraft, Inc.
- 28.Williamson, D. H. 1985. l-Alanine, p. 341-344. In H. U. Bergmeyer (ed.), Methods of enzymatic analysis, 3rd ed., vol. VIII. VCH Publishers, Deerfield Beach, Fla. [Google Scholar]