Abstract
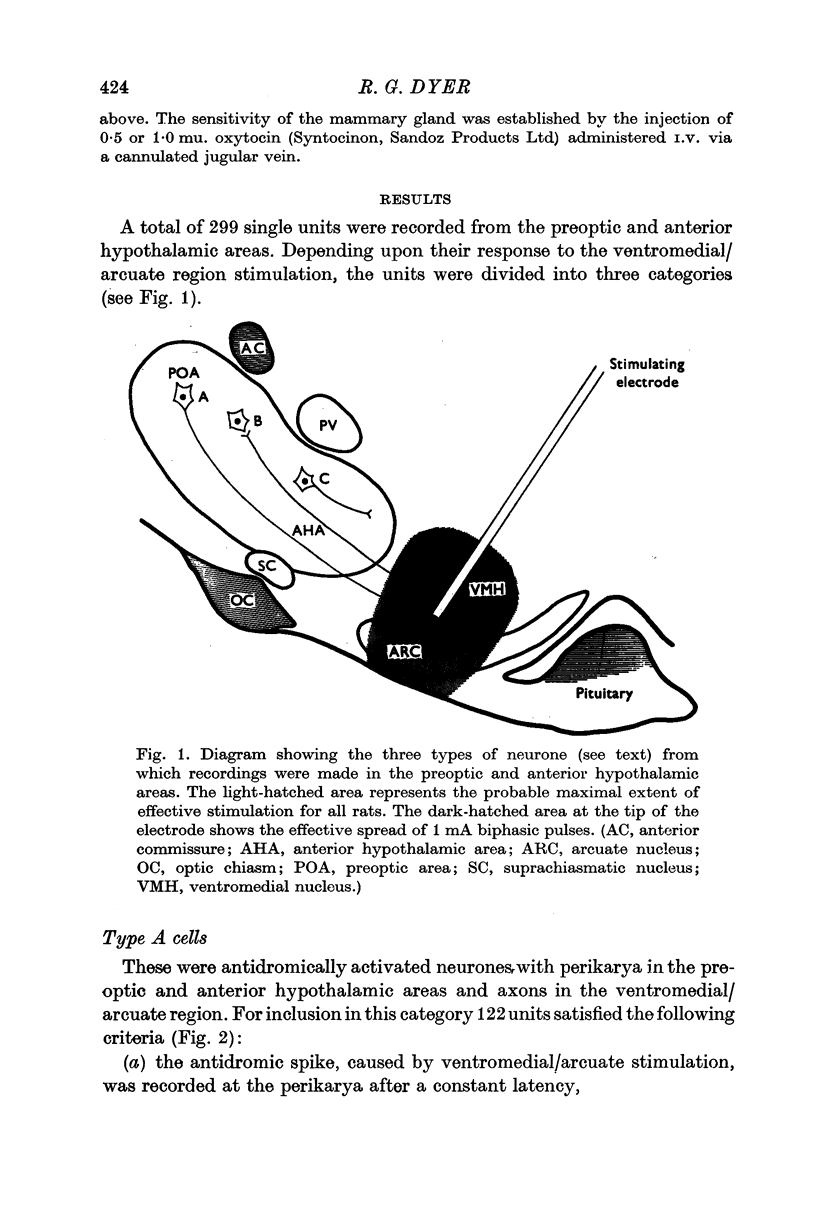
1. Extracellular action potentials were recorded from 299 single units in the preoptic and anterior hypothalamic areas of fifty-two female rats anaesthetized with urethane. The units were categorized by their response to single biphasic pulses (ca. 1 mA; 1 msec duration) applied to the ventromedial/arcuate region of the hypothalamus.
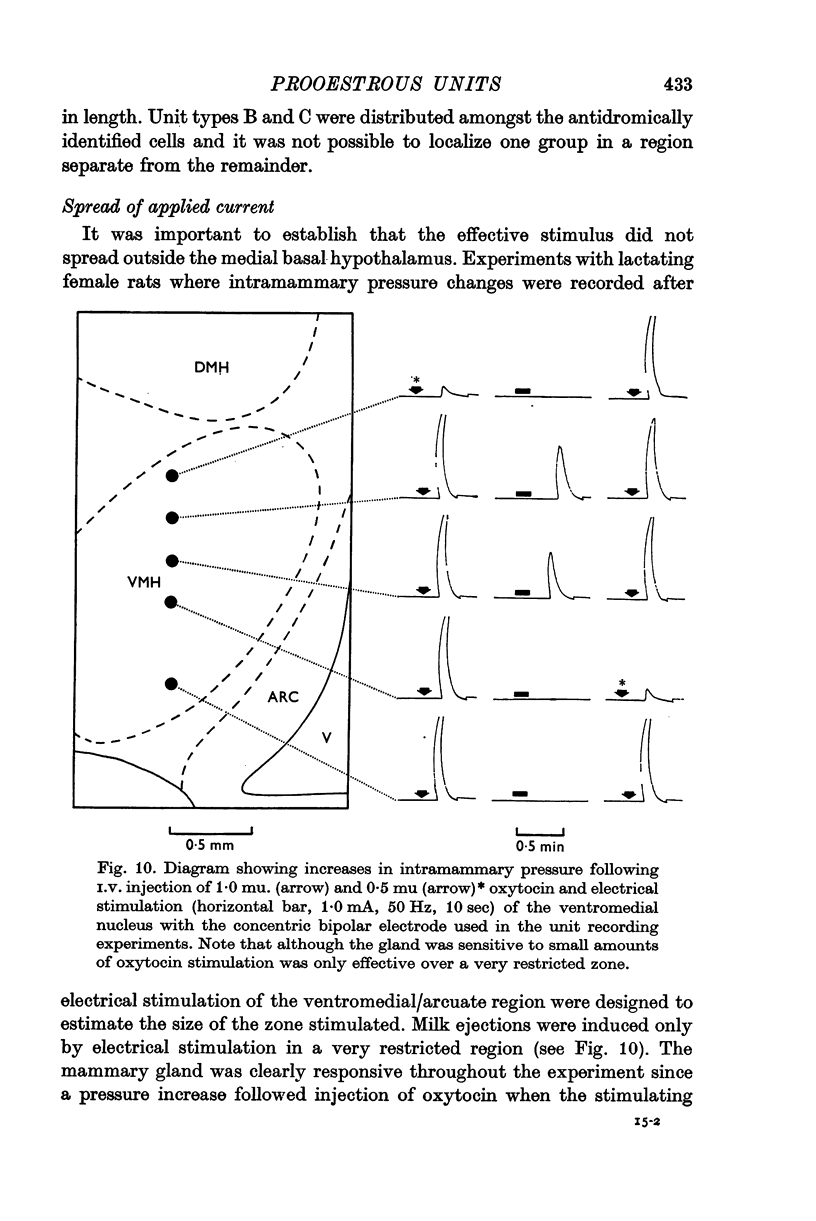
2. Experiments with five lactating rats demonstrated that the effective zone of stimulation was confined within the ventromedial/arcuate region. This observation was supported by further evidence obtained during unit recording sessions.
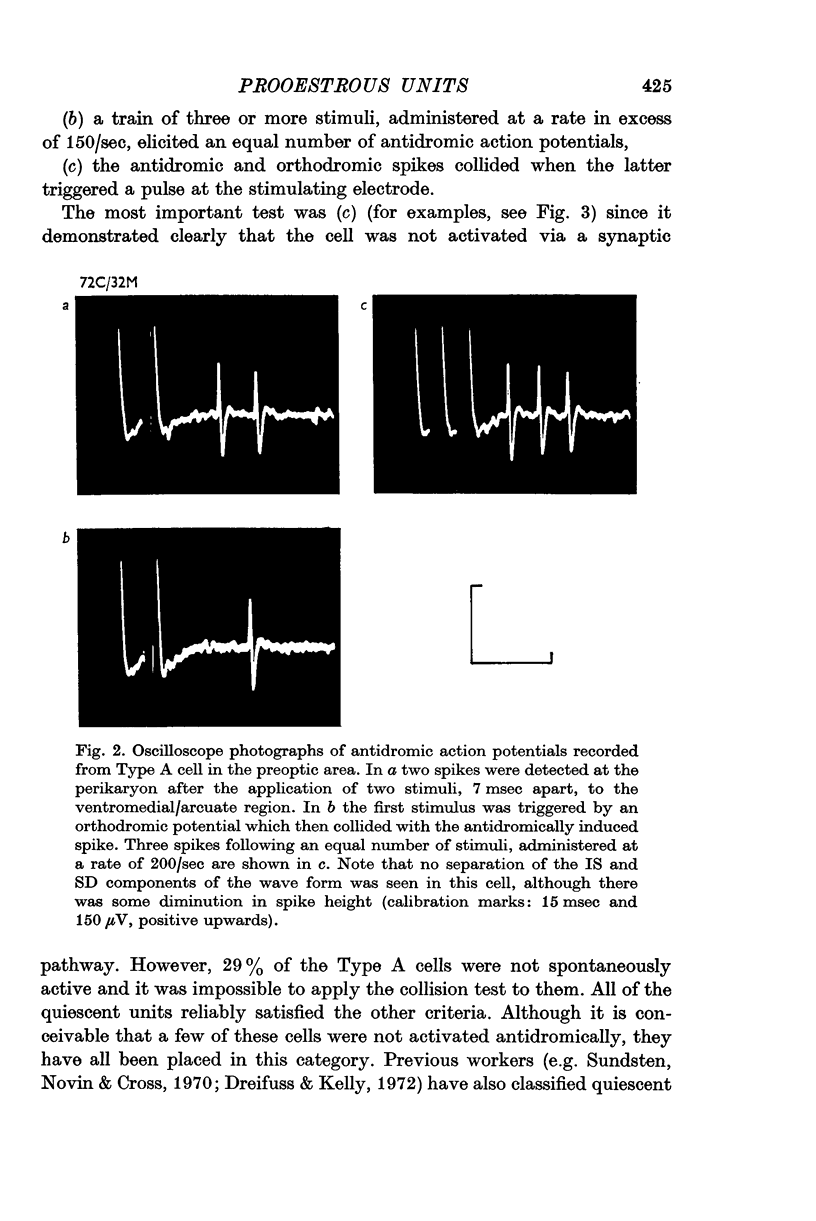
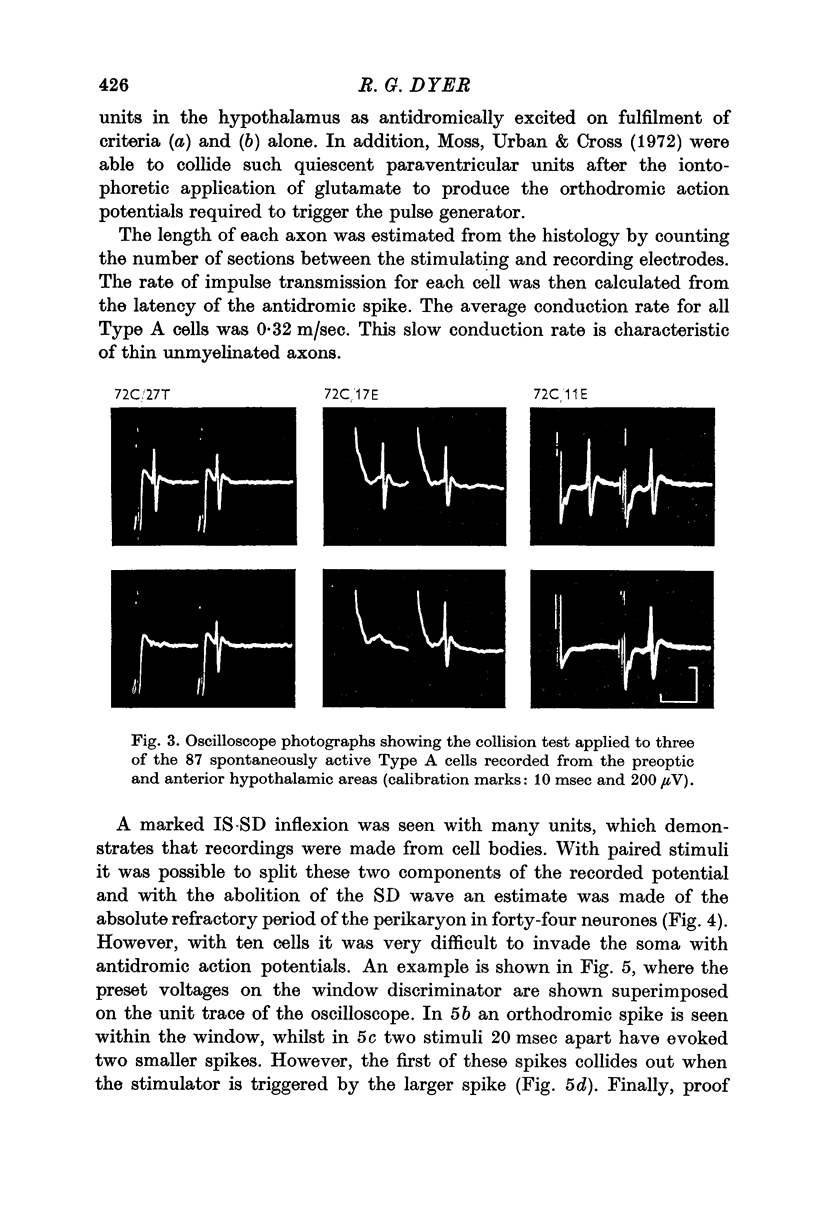
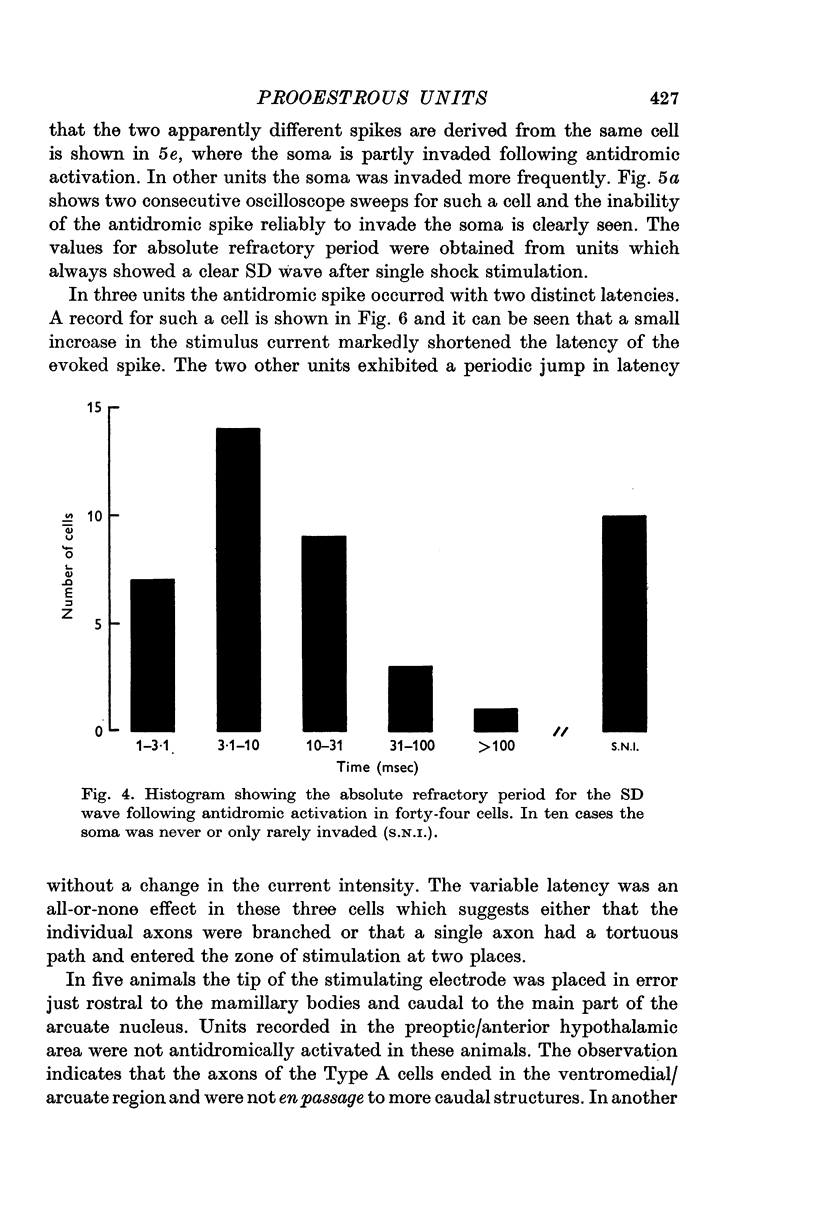
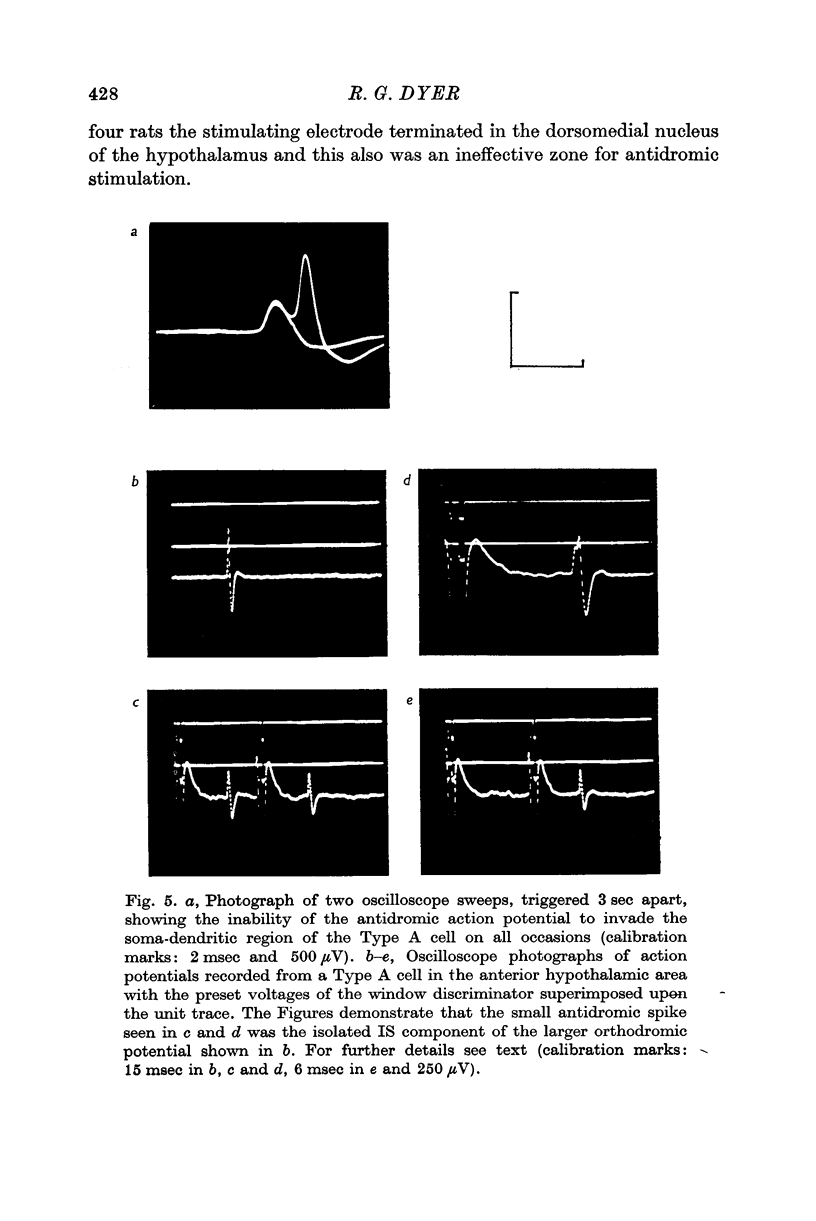
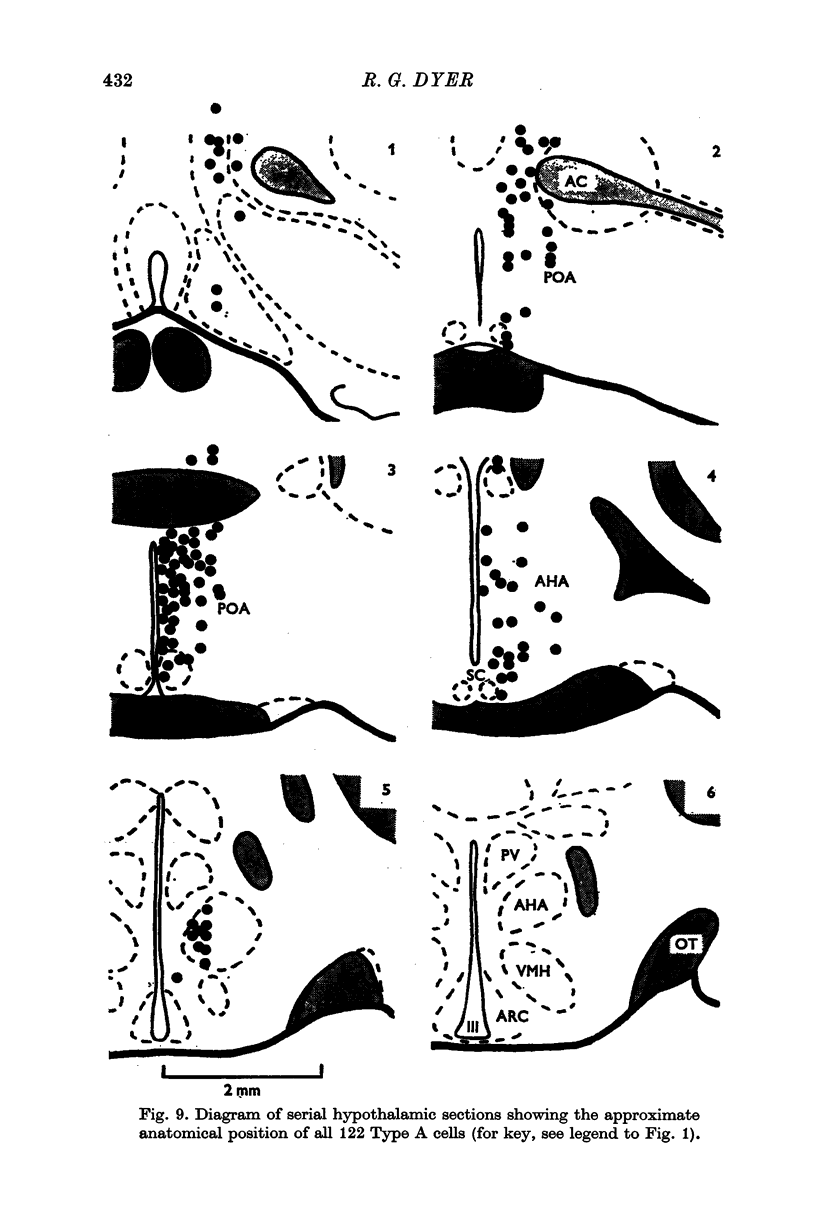
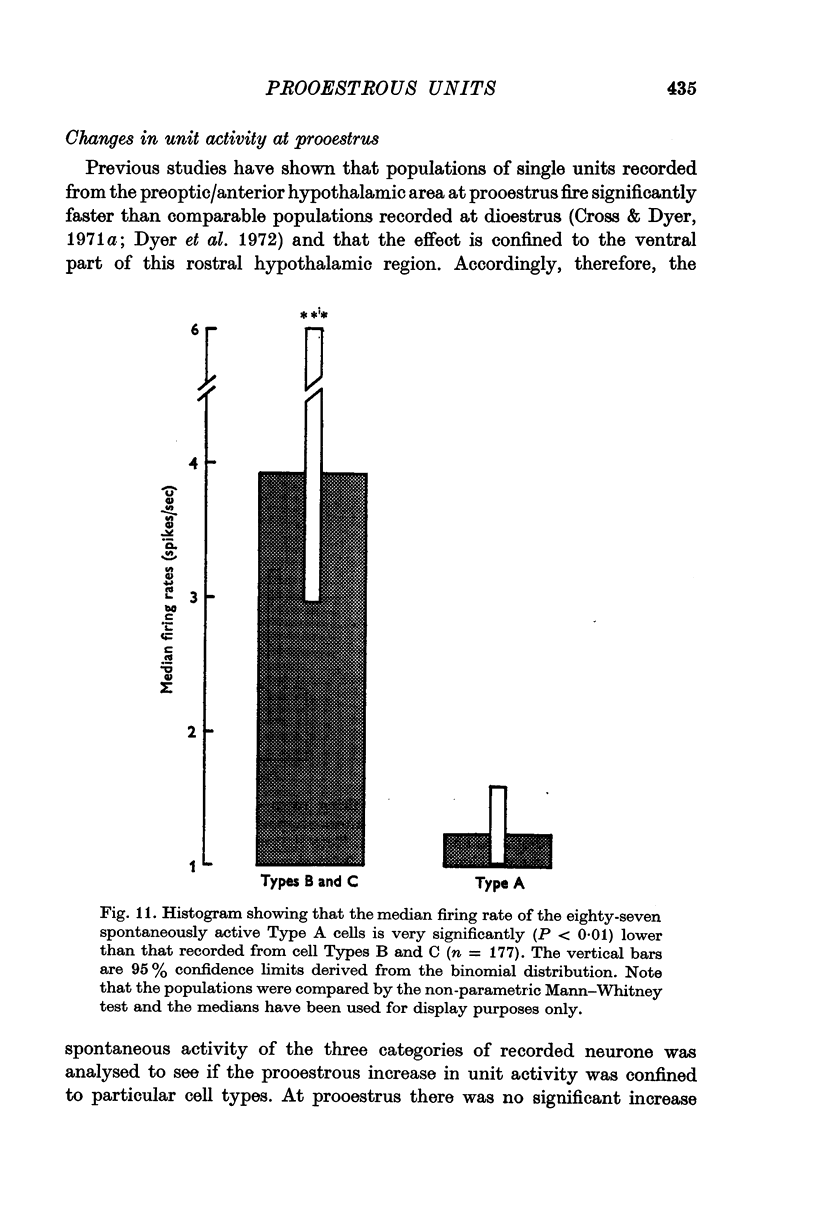
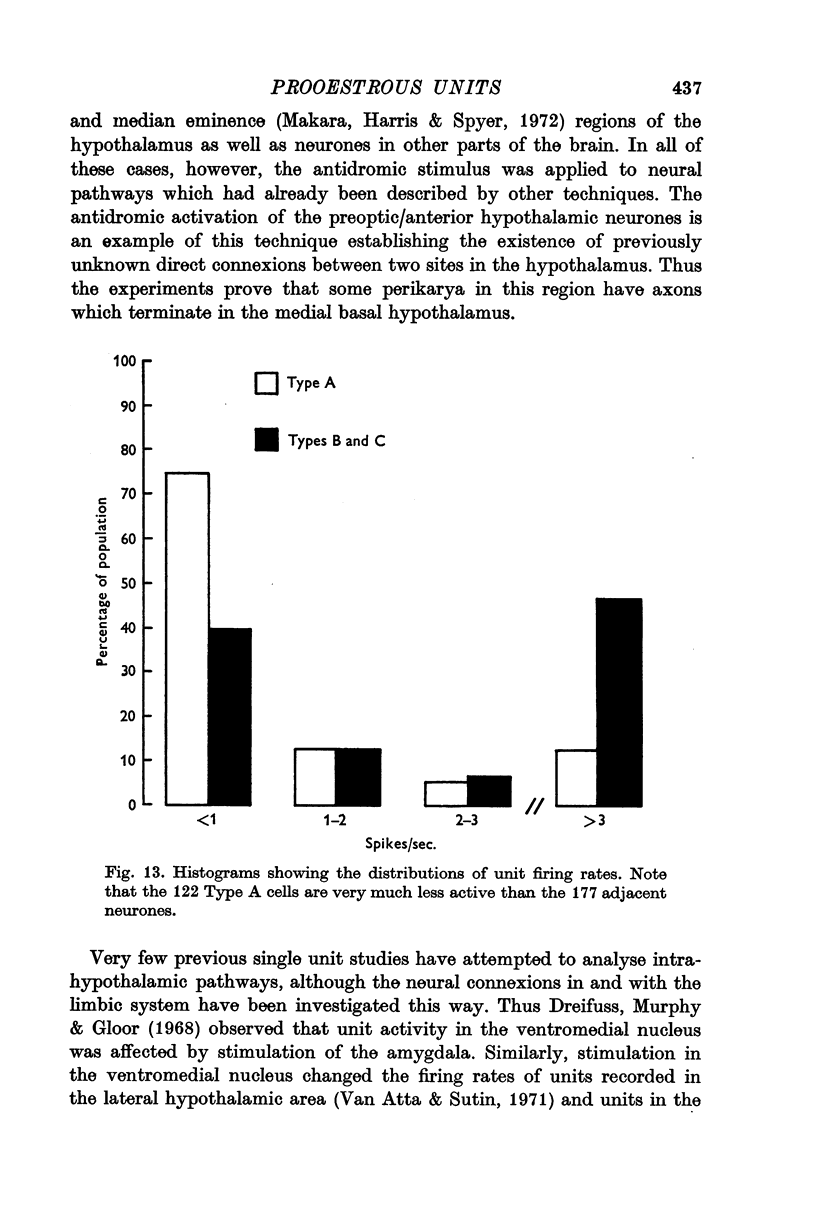
3. Antidromic action potentials were recorded from 122 (41%) of the neurones monitored in preoptic and anterior hypothalamic areas. These Type A cells were characterized by their very slow discharge rate (median for spontaneously active units 1·2 spikes/sec) when contrasted with adjacent cells (median 3·9 spikes/sec). Orthodromic action potentials were not observed in thirty-six Type A cells. The antidromically identified neurones had an average conduction rate of 0·32 m/sec. The absolute refractory period of the soma, computed by separation of the IS and SD components of the antidromic action potentials recorded from forty-four neurones, ranged from < 3·0 to > 100 msec. With ten units it was not possible to obtain a soma dendritic (SD) wave by antidromic activation even though the initial segment (IS) wave was seen clearly and the orthodromic potentials consisted of both IS and SD waves.
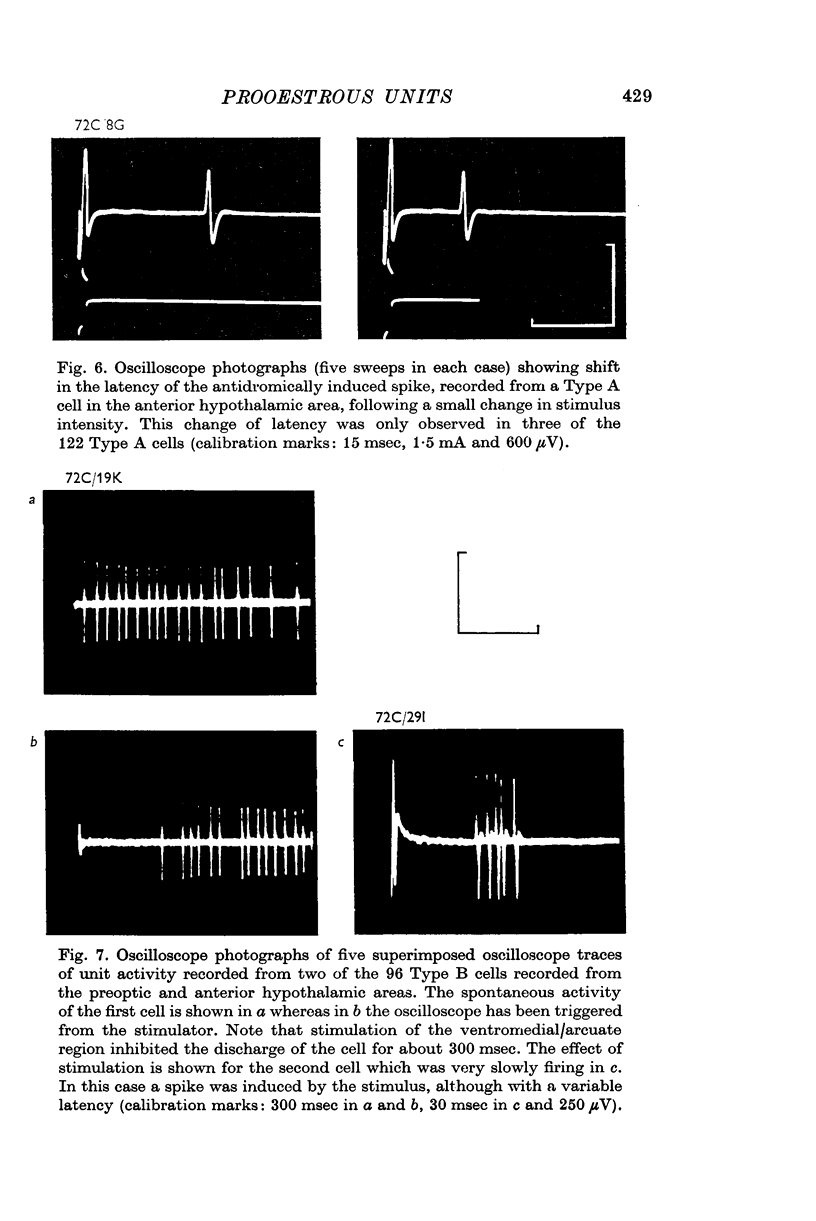
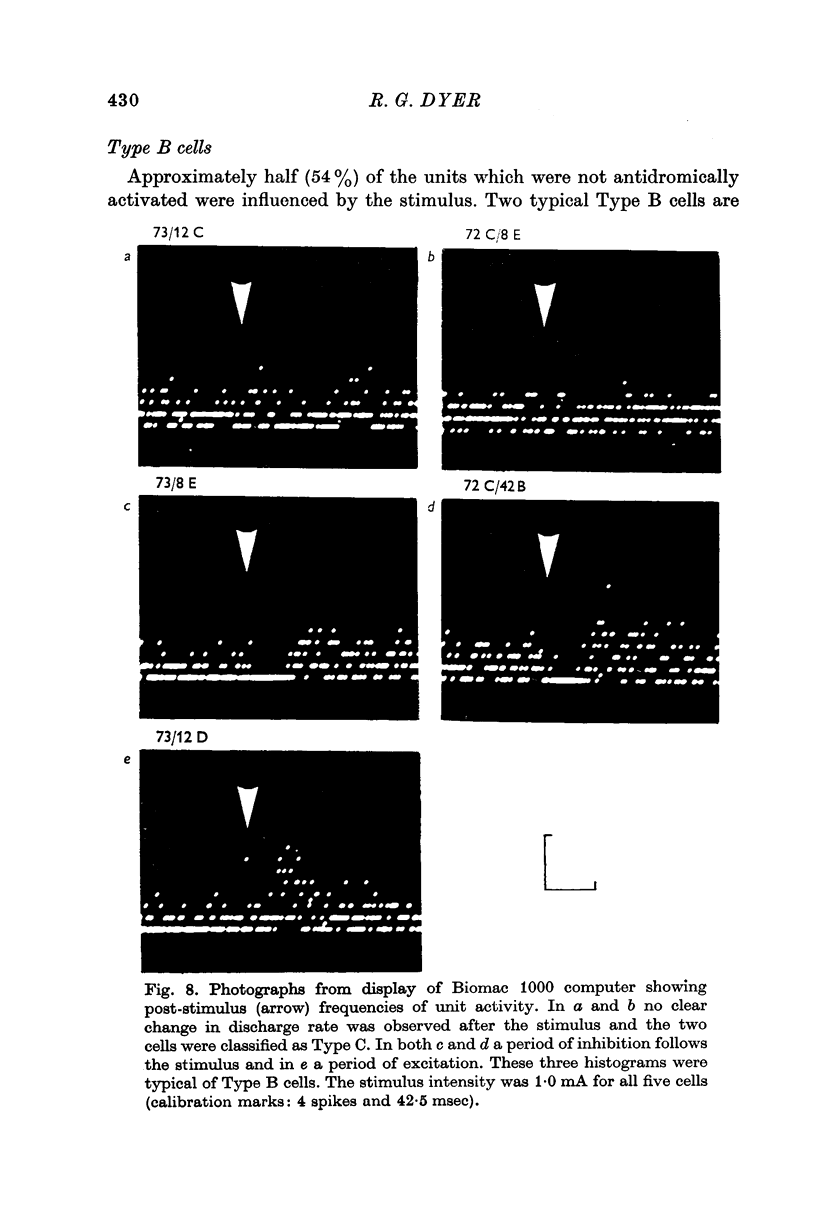
4. Type B cells (32% of population) were excited and/or inhibited by the ventromedial/arcuate stimulation but were not antidromically activated. This post-stimulatory change in discharge rate lasted for up to 400 msec.
5. Type C cells (27% of population) showed no change in spontaneous activity after stimulation of the ventromedial arcuate area. These neurones had discharge rates similar to Type B cells.
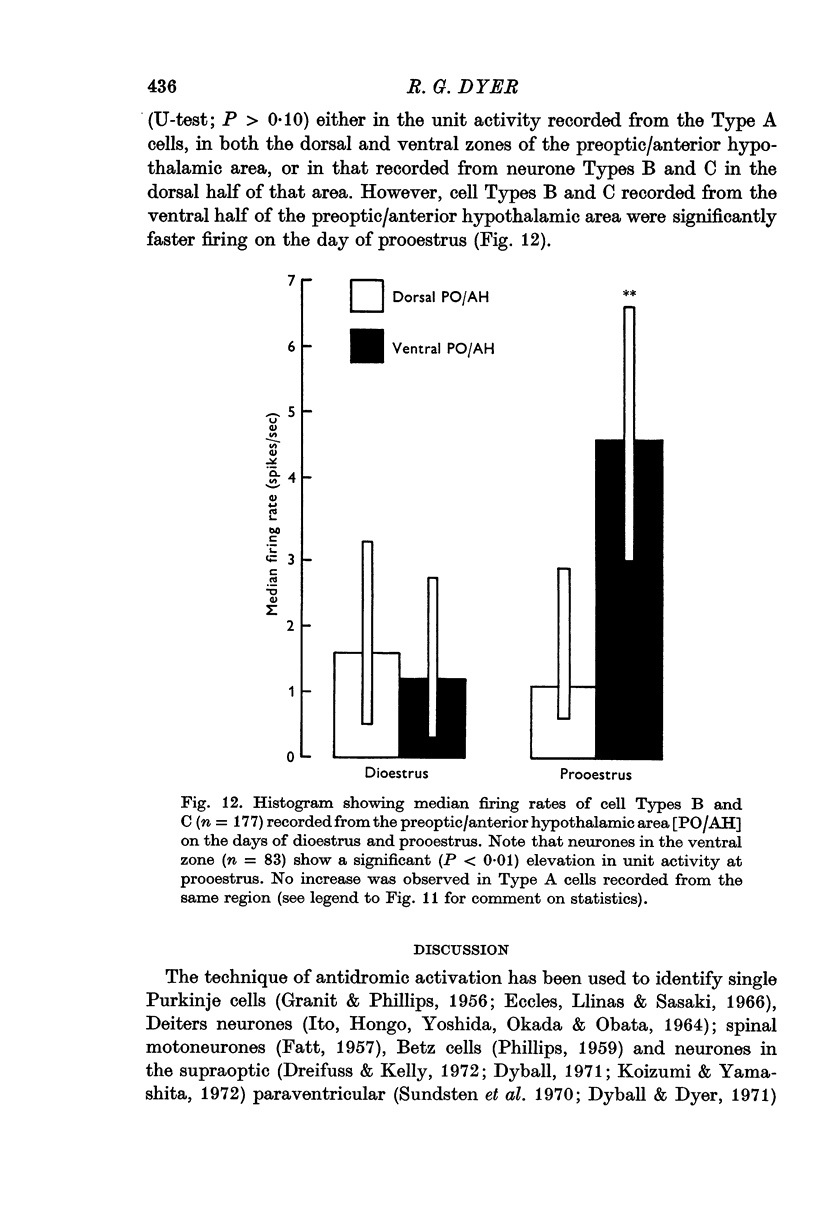
6. The previously reported prooestrous increase in firing rate, recorded from neurones in the ventral part of the preoptic/anterior hypothalamic area was restricted to cell Types B and C.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake C. A., Sawyer C. H. Ovulation blocking actions of urethane in the rat. Endocrinology. 1972 Jul;91(1):87–94. doi: 10.1210/endo-91-1-87. [DOI] [PubMed] [Google Scholar]
- Cramer O. M., Barraclough C. A. Effect of electrical stimulation of the preoptic area on plasma LH concentrations in proestrous rats. Endocrinology. 1971 May;88(5):1175–1183. doi: 10.1210/endo-88-5-1175. [DOI] [PubMed] [Google Scholar]
- Crighton D. B., Schneider H. P., McCann S. M. Localization of LH-releasing factor in the hypothalamus and neurohypophysis as determined by an in vitro method. Endocrinology. 1970 Aug;87(2):323–329. doi: 10.1210/endo-87-2-323. [DOI] [PubMed] [Google Scholar]
- Cross B. A., Dyer R. G. Ovarian modulation of unit activity in the anterior hypothalamus of the cyclic rat. J Physiol. 1972 Apr;222(1):25P–25P. [PubMed] [Google Scholar]
- Cross B. A., Dyer R. G. Unit activity in rat diencephalic islands--the effect of anaesthetics. J Physiol. 1971 Jan;212(2):467–481. doi: 10.1113/jphysiol.1971.sp009336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross B. A., Silver I. A. Electrophysiological studies on the hypothalamus. Br Med Bull. 1966 Sep;22(3):254–260. doi: 10.1093/oxfordjournals.bmb.a070483. [DOI] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S. Recurrent inhibition of antidromically identified rat supraoptic neurones. J Physiol. 1972 Jan;220(1):87–103. doi: 10.1113/jphysiol.1972.sp009696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Murphy J. T., Gloor P. Contrasting effects of two identified amygdaloid efferent pathways on single hypothalamic neurons. J Neurophysiol. 1968 Mar;31(2):237–248. doi: 10.1152/jn.1968.31.2.237. [DOI] [PubMed] [Google Scholar]
- Dyball R. E., Dyer R. G. Plasma oxytocin concentration and paraventricular neurone activity in rats with diencephalic islands and intact brains. J Physiol. 1971 Jul;216(1):227–235. doi: 10.1113/jphysiol.1971.sp009520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball R. E., Koizumi K. Electrical activity in the supraoptic and paraventricular nuclei associated with neurohypophysial hormone release. J Physiol. 1969 May;201(3):711–722. doi: 10.1113/jphysiol.1969.sp008783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball R. E. Oxytocin and ADH secretion in relation to electrical activity in antidromically identified supraoptic and paraventricular units. J Physiol. 1971 Apr;214(2):245–256. doi: 10.1113/jphysiol.1971.sp009430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer R. G., Beechey P. A window discriminator, with visual display, suitable for unit recording. Electroencephalogr Clin Neurophysiol. 1971 Dec;31(6):621–624. doi: 10.1016/0013-4694(71)90080-0. [DOI] [PubMed] [Google Scholar]
- Dyer R. G., Cross B. A. Antidromic identification of units in the preoptic and anterior hypothalamic areas projecting directly to the ventromedial and arcuate nuclei. Brain Res. 1972 Aug 11;43(1):254–258. doi: 10.1016/0006-8993(72)90291-0. [DOI] [PubMed] [Google Scholar]
- Dyer R. G., Pritchett C. J., Cross B. A. Unit activity in the diencephalon of female rats during the oestrous cycle. J Endocrinol. 1972 Apr;53(1):151–160. doi: 10.1677/joe.0.0530151. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The action of antidromic impulses on the cerebellar Purkinje cells. J Physiol. 1966 Jan;182(2):316–345. doi: 10.1113/jphysiol.1966.sp007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P. Electric potentials occurring around a neurone during its antidromic activation. J Neurophysiol. 1957 Jan;20(1):27–60. doi: 10.1152/jn.1957.20.1.27. [DOI] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G. Excitatory and inhibitory processes acting upon individual Purkinje cells of the cerebellum in cats. J Physiol. 1956 Sep 27;133(3):520–547. doi: 10.1113/jphysiol.1956.sp005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. V., Johnson J. H., Goldman B. D., Whitmoyer D. I., Sawyer C. H. Effects of electrochemical stimulation of the ventral hippocampus on hypothalamic electrical activity and pituitary gonadotropin secretion in female rats. Endocrinology. 1971 Sep;89(3):704–713. doi: 10.1210/endo-89-3-704. [DOI] [PubMed] [Google Scholar]
- Halász B., Gorski R. A. Gonadotrophic hormone secretion in female rats after partial or total interruption of neural afferents to the medial basal hypothalamus. Endocrinology. 1967 Apr;80(4):608–622. doi: 10.1210/endo-80-4-608. [DOI] [PubMed] [Google Scholar]
- ITO M., HONGO T., YOSHIDA M., OKADA Y., OBATA K. ANTIDROMIC AND TRANS-SYNAPTIC ACTIVATION OF DEITERS' NEURONES INDUCED FROM THE SPINAL CORD. Jpn J Physiol. 1964 Dec 15;14:638–658. doi: 10.2170/jjphysiol.14.638. [DOI] [PubMed] [Google Scholar]
- Kalra S. P., Ajika K., Krulich L., Fawcett C. P., Quijada M., McCann S. M. Effects of hypothalamic and preoptic electrochemical stimulation on gonadotropin and prolactin release in proestrous rats. Endocrinology. 1971 May;88(5):1150–1158. doi: 10.1210/endo-88-5-1150. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Kelly W. A. The influence of urethane on ovulation in the rat. Endocrinology. 1972 Jun;90(6):1594–1599. doi: 10.1210/endo-90-6-1594. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W. Unit activity in the hypothalamus, septum and preoptic area of the rat: characteristics of spontaneous activity and the effect of oestrogen. J Endocrinol. 1967 Feb;37(2):177–189. doi: 10.1677/joe.0.0370177. [DOI] [PubMed] [Google Scholar]
- Makara G. B., Harris M. C., Spyer K. M. Identification and distribution of tuberoinfundibular neurones. Brain Res. 1972 May 26;40(2):283–290. doi: 10.1016/0006-8993(72)90134-5. [DOI] [PubMed] [Google Scholar]
- Mok A. C., Mogenson G. J. Effect of electrical stimulation of the septum and the lateral preoptic area on unit activity of the lateral habenular nucleus in the rat. Brain Res. 1972 Aug 25;43(2):361–372. doi: 10.1016/0006-8993(72)90393-9. [DOI] [PubMed] [Google Scholar]
- Moss R. L., Law O. T. The estrous cycle: its influence on single unit activity in the forebrain. Brain Res. 1971 Jul 23;30(2):435–438. doi: 10.1016/0006-8993(71)90097-7. [DOI] [PubMed] [Google Scholar]
- Moss R. L., Urban I., Cross B. A. Microelectrophoresis of cholinergic and aminergic drugs on paraventricular neurons. Am J Physiol. 1972 Aug;223(2):310–318. doi: 10.1152/ajplegacy.1972.223.2.310. [DOI] [PubMed] [Google Scholar]
- PHILLIPS C. G. Actions of antidromic pyramidal volleys on single Betz cells in the cat. Q J Exp Physiol Cogn Med Sci. 1959 Jan;44(1):1–25. doi: 10.1113/expphysiol.1959.sp001364. [DOI] [PubMed] [Google Scholar]
- Sundsten J. W., Novin D., Cross B. A. Identification and distribution of paraventricular units excited by stimulation of the neural lobe of the hypophysis. Exp Neurol. 1970 Feb;26(2):316–329. doi: 10.1016/0014-4886(70)90129-9. [DOI] [PubMed] [Google Scholar]
- Terasawa E., Sawyer C. H. Changes in electrical activity in the rat hypothalamus related to electrochemical stimulation of adenohypophyseal function. Endocrinology. 1969 Jul;85(1):143–149. doi: 10.1210/endo-85-1-143. [DOI] [PubMed] [Google Scholar]
- Van Atta L., Sutin J. The response of single lateral hypothalamic neurons to ventromedial nucleus and limbic stimulation. Physiol Behav. 1971 May;6(5):523–536. doi: 10.1016/0031-9384(71)90200-9. [DOI] [PubMed] [Google Scholar]


