Abstract
Background: Simvastatin has been shown to increase bone growth when applied topically to murine bone; however, it caused considerable soft tissue inflammation at high doses (2.2 mg), making future clinical use problematic. This study evaluated the effect of lower simvastatin doses and cyclooxygenase synthase (COX) inhibitors on tissue inflammation and bone growth in rats, and gene expression in mice.
Methods: Adult female rats were untreated or treated with a single dose of 0.1, 0.5, 1.0, 1.5, or 2.2 mg simvastatin in methylcellulose gel in a polylactic acid membrane (SIM) on the lateral aspect of the mandible. The contralateral mandible side was implanted with methylcellulose gel/polylactic acid membrane alone (GEL), and 5 rats in each dose pairing were evaluated histomorphometrically after 3, 7 and 24 days. Subsequent rats were similarly treated with 0.5 mg simvastatin (optimal dose) and daily intraperitoneal injections of COX-2 inhibitor (NS-398; 1 mg/kg × 7days; n = 16), general COX inhibitor (indomethacin; 1 mg/kg × 7days; n = 16), or no inhibitor (n = 10) and evaluated histomorphometrically after 7 or 24 days by analysis of variance (ANOVA). Gene arrays also were used to evaluate osteogenic gene expression from 0.5 mg simvastatin in murine calvaria (n = 12).
Results: There was a 45% increase in bone area with 0.5 mg simvastatin vs. gel control (P<0.001; similar to 2.2 mg dose), and clinical swelling was reduced compared to the high simvastatin dose (P<0.05). The 0.1 mg simvastatin dose failed to stimulate significant bone growth. Both NS-398 and indomethacin reduced inflammation and bone growth. Simvastatin significantly upregulated procollagen, fibronectin and matrix metalloproteinase-13 genes.
Conclusions: Reducing simvastatin dose from 2.2 mg to 0.5 mg reduced inflammation to a more clinically-acceptable level without sacrificing bone-growth potential, but COX-associated inflammation appears to be necessary for in vivo bone growth.
Keywords: mandible, histology, rats, mice, osteogenic genes
Introduction
Inflammatory diseases occurring adjacent to bone, such as periodontitis, lead to bone resorption creating bony defects which may cause tooth loss. Methods to repair these defects typically use implantation of bone particles to create space and stimulate host bone formation through the graft.1 Topical application of biological growth factors may augment bone growth, including use of bone morphogenetic protein-2 (BMP-2).2 However, protein growth factors are expensive, may degrade rapidly at the treatment site, and could potentially elicit immune responses.3
If pharmacological compounds can upregulate the necessary autogenous growth factors to stimulate bone growth, this approach may prove to be a cost-effective way to correct bone defects. Topically-applied simvastatin,* a cholesterol-lowering drug, has been shown to stimulate bone growth over mouse calvaria in vivo without using a bone-graft scaffold, presumably through stimulating BMP-2.3,4 A single simvastatin dose (2.2 mg) in methylcellose gel under a polylactic acid (PLA) membrane caused substantial bone growth in this model, but considerable soft-tissue inflammation also was noted at the site of activity.5 The inflammatory response would make future clinical application problematic, and raises questions about the role of inflammation in simvastatin-induced bone growth.
The hypothesis of this study was that inflammation could be reduced to a level more appropriate for future clinical use, either by reducing simvastatin dose or using anti-inflammatory drugs, while preserving bone-growth potential. The experimental protocol used an adult rat bilateral mandible model to: 1) evaluate the topically-applied simvastatin dose on histologic bone growth and inflammation in vivo; and 2) evaluate the effects of anti-inflammatory cyclooxogenase inhibitors, including selective COX-2 inhibitor NS-398 and non-selective COX inhibitor indomethacin, on this topical simvastatin-induced bone growth and inflammation. Potential autogenous mediators of topical simvastatin-induced bone growth/inflammation were preliminarily explored using gene arrays in a murine calvarial model.
Materials and Methods
Rat Bilateral Mandible Model
All animal procedures were approved by the Institutional Animal Care & Use Committee at the University of Nebraska. A rat bilateral mandible model was used consisting of a randomly-selected active drug side where the simvastatin (SIM) dose was incorporated into a methylcellulose gel, placed into a polylactic acid (PLA) dome, then inserted supraperiosteally over the facial aspect of the mandible (Fig. 1). The contralateral control side received the same treatment, but the gel had no simvastatin (GEL). Another control used the gel alone on one side and an untreated (UN) opposite side, as a means for testing the systemic effects of the simvastatin gel when compared to GEL in SIM-treated animals.
Figure 1.
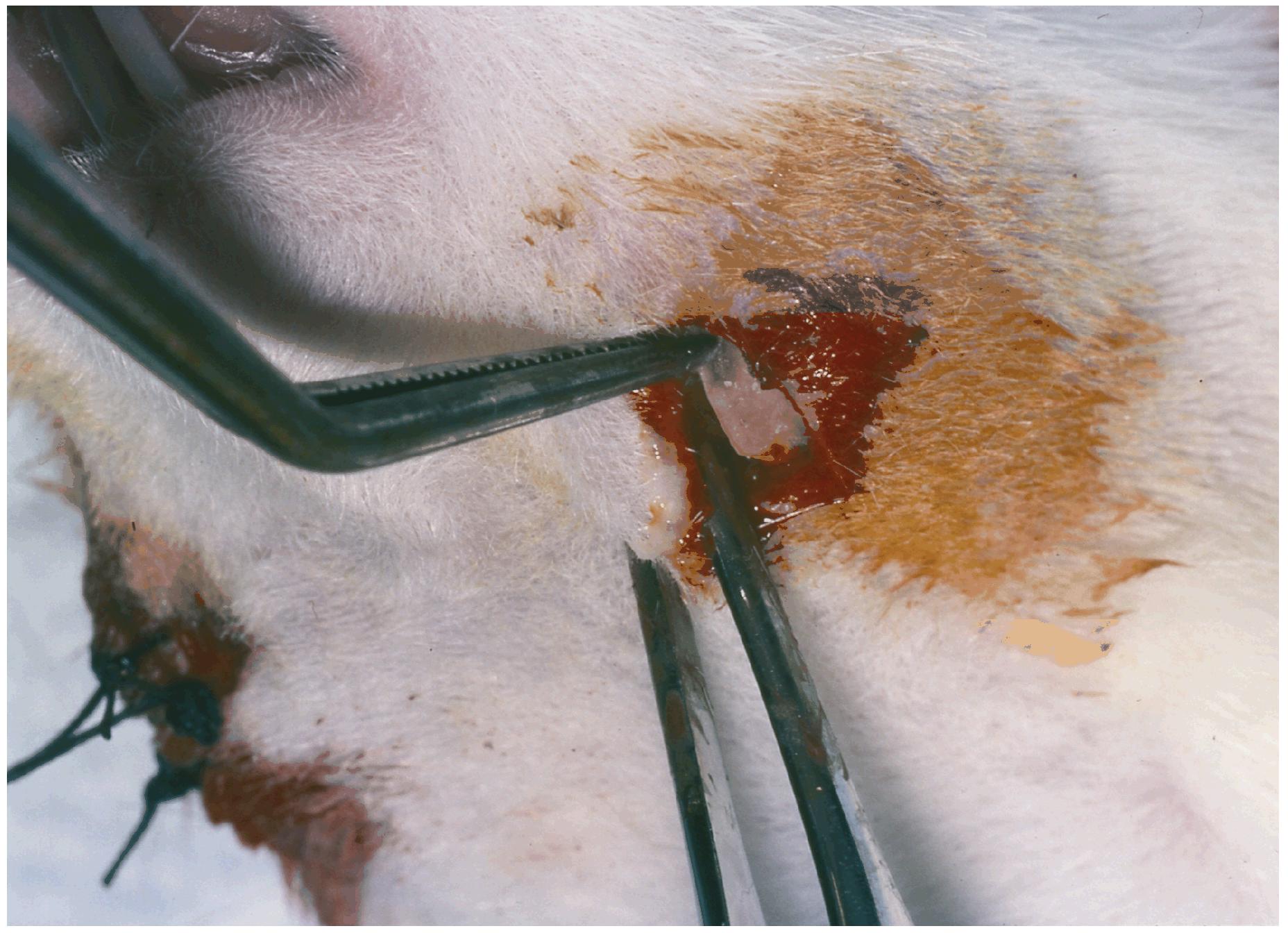
PLA dome membrane carrying simvastatin in methycellulose gel or gel alone is inserted into tunnel with the open side of the membrane positioned towards the lateral aspect of the mandible. The opposite side shows incisions closed with silk interrupted sutures.
Local Delivery System
Concave, impermeable, biocompatible PLA membranes and methycellulose gel were prepared as previously described.5 Simvastatin doses were added to the gel to achieve a final concentration of 0.1, 0.5, 1.0, 1.5 or 2.2 mg SIM/30 μl gel. Thirty μl of the simvastatin in gel (SIM) or gel alone (GEL) were added to the chamber of each dome prior to implantation into the animal.
Animal Procedures
Mature female Sprague-Dawley rats† were used, five animals for each of the 6 experimental groups with bilateral topical placement of: 0.1 mg SIM/GEL; 0.5 mg SIM/GEL; 1.0 mg SIM/GEL; 1.5 mg SIM/GEL; 2.2 mg SIM/GEL; UN/GEL); and 3 healing periods (days 3,7 and 24) for a total of 90 rats. The high dose would be the equivalent of approximately 7.3 mg/kg if given systemically. The skin around the mandible was shaved and swabbed with disinfectant.
Immediately before surgery, animals were sedated with an intramuscular injection of two parts ketamine (100 mg/ml) and one part xylazine (20 mg/ml)‡ at a dosage of 0.2 ml/100 g, and additional sedation was given as necessary. An incision was made inferior to the angle of the mandible extending to the mandibular bone. Then a tunnel was created slightly wider than the dome diameter (8mm) by sharp dissection supraperiosteally. The dome membrane loaded with gel was positioned open-side toward the jaw, and the tunnel entry closed with 4-0 silk sutures. After healing times (3, 7 and 24 days) were attained, the animals were killed by CO2 asphyxiation. All rats were weighed at baseline and autopsy. Before dissection of the mandible, the size of swelling around the dome was noted using a clinical swelling index: 0 = no swelling; 1 = < 1 cm; 2 = 1 - < 1.5 cm; 3 = 1.5 - < 2.0 cm; 4 = ≥ 2.0 cm. The mandibles then were removed, and the skin dissected away leaving bone, soft tissue, muscles, and membrane domes intact.
COX Inhibitor Administration
For systemic NS-398, a 1.0 mg/kg dose was delivered by intraperitoneal (IP) injections at baseline and daily for 7 days in 16 rats. This was carried out in animals that received 0.5 mg of SIM on one side and GEL on the contralateral side. Half of the animals were euthanized each after 7 and 24 days. Similarly, indomethacin was administered IP at baseline and for 7 days at a 1.0 mg/kg dose, also in 16 animals that had received 0.5 mg SIM on one side and GEL on the contralateral side. Half were killed each after 7 and 24 days. Both doses were chosen based on literature demonstrating reduction of inflammation in rats.6-8 Ten additional rats were treated as above, except COX inhibitor was not added.
Sample Processing
Specimens were placed into 10% buffered formalin solution for at least 24 hours immediately after collection, decalcified in 5% formic acid§ for one week at 4°C, and cut (with razor blade) in cross-section at the angle of the mandible to include the mandibular bone, overlying facial soft tissues, and the membrane dome. Subsequent sections, therefore, included both sides of the jaw at the same location relative to the membrane domes (Fig. 2). The specimens were dehydrated in alcohol and embedded in paraffin. Five μm thick cross-sections were cut at 200 μm intervals (3 areas to represent an average response), coded for animal number and keyed for left and right sides, and stained with hematoxylin-eosin.
Figure 2.

Photomicrographs (hematoxylin and eosin) of rat bilateral mandible specimens; left show the left side of the rat mandible, right show the right side of the same mandible. All animals were euthanized after 7 days. Bar lengths represent 1 mm and inserts are 20-fold magnifications of the main panels. The white arrows indicate bone surface facing domes, the open arrows indicate the inflammatory infiltrate around the membrane, and black arrows locate the remnants of the membrane. Inserted images toward the midline illustrate the periosteal surface of the mandible taken from the region indicated by the white arrow. Inserted images toward the periphery illustrate the inflammatory infiltrate taken from the region indicated by the open arrows. Note that the shapes of the jaws change in each animal, but are consistent bilaterally in the same animal. The mandible side in (A) had received 0.5 mg of simvastatin and the opposite side (B) received gel control. The white arrow on (A) and midline insert show new bone growth beneath periosteum and osteoblast lining cells on the inferior and lateral aspect, while the right side shows less new bone growth and primarily flat-lining cells in that area. The open arrow in (A) shows a wide area of inflammatory infiltrate (lymphocytes and neutrophils; peripheral insert) and infiltrate away from the bone in (B). The mandible side in (C) received 0.5 mg of simvastatin and gel control in (D), but the animal also received daily doses of 1.0 mg/kg NS-398 intraperitoneally for seven days post-implant. The white arrows and midline inserts show minimal new bone growth or osteoblastic cells on either side of the mandible. Open arrows point to a more contained inflammatory infiltrate. The rat mandible side in (E) received 0.5 mg of simvastatin and gel control in (F). The animal also received 1.0 mg/kg indomethacin intraperitoneally daily for 7 days. The white arrows and midline inserts indicate minimal new bone growth or osteoblastic cells on either side of the mandible. The open arrows show that inflammation is minimal on the right and appears well contained on the left.
Histomorphometric Analysis
All sections were measured by two masked evaluators at 40X and 400X for total bone area (Tt. Ar), periosteal bone surface length (Ps. Pm), and osteoblast, flat lining cell, and osteoclast surfaces (Ob.Pm, Fl.Pm,Oc.Pm). Cell surfaces were defined as in previous studies:9-11 osteoblast surface when covered by plump cells with a single large, round to ovoid, lightly stained nucleus; flat cell surface when covered by cells with a single long, flat, darkly stained nucleus; and osteoclast surface when cells were large with pleomorphic cell body, multinucleated with prominent nucleoli, and located in Howship's lacunae or resorption pits with irregular surfaces. A surface covered by mononuclear cells that were not readily identifiable as osteoblasts or flat cells was considered a transitional surface and classified either as osteoblast surface or flat cell surface based on how closely the cells resembled either cell type. All evaluated surfaces were on the facial and inferior surfaces of the inferior 2 mm of the mandible (Fig. 2). Area of inflammatory infiltrate was measured as mm2 of grids containing infiltrate evaluated at 40X. Morphometric analysis of inflammatory cell types was accomplished by grid intersection point counting at 400X and the percent of each inflammatory cell type (plasma cell, lymphocyte, neutrophil, macrophage/ monocyte) and tissue/cell type (muscle, fibroblast, fibrous connective tissue, vessels) were counted in two areas (adjacent to lateral bone surface and adjacent to the dome). The distance between the dome and bone was measured.
Mouse Gene Analysis
Twelve Swiss ICR mice were used for the gene array experiment to evaluate simvastatin regulation of a panel of genes associated with osteogenesis. Fifty μl PLA domes were fabricated. A 0.5 mg simvastatin/50 μl methylcellulose gel dose was placed into 6 domes. In 6 other domes 50 μl of methylcellulose gel alone was placed. The mice were anesthetized with two parts ketamine (100 mg/ml) and one part xylazine (20 mg/ml)‡ at a dose of 0.1-0.15 ml/100 g per animal, and additional sedation was given as necessary. The simvastatin or gel alone domes were placed with the open side adjacent to the mouse calvarial periosteum. The wounds were sutured closed with 4-0 silk sutures. Three mice containing simvastatin and 3 mice containing gel alone were killed at day 3. The remaining mice were killed at day 14.
The calvaria of each mouse was surgically extracted and was snap-frozen in liquid nitrogen immediately after euthanasia, transferred to a mortar and pestle and pulverized. RNA extraction, purification and concentration followed the protocol of commercially-available kits.” Samples analyzed with ultraviolet spectrophotometry revealed all samples with concentrations of > 3 μg/10 μl (260 nm) and 260/280 nm quality values of 1.7-2.0.
Three μg of RNA from each sample were used to run separate gene arrays# for each animal according to manufacturer's instructions. Twelve osteogenic gene arrays total were performed (6 arrays for day 3 and 6 arrays for day 14 animals). Each array included DNA of 96 genes involved in the regulation of osteogenic differentiation and 4 housekeeping genes in tetra-spots on a single membrane. Genes included growth factors and associated molecules, matrix and associated proteins, and cell adhesion molecules. After sample RNA was expanded with reverse transcriptase polymerase chain reaction, biotinylated cDNA probes were created, hybridized to the membrane, and developed with alkaline phosphatase-streptavidin and chemiluminescent substrate.
The membranes were properly oriented on radiographic film in a dark room and the film was exposed for 1 or 5 minutes. The developed radiographic films were scanned into the computer and inverted. The images were converted to a Text Image File (TIF) and using the ScanAlyze program (developed by Dr. Michael Eisen, Stanford University), the TIF was imported into the program and oriented on a grid. The software analyzed the intensities of the spots on the gene arrays and created a spreadsheet with the intensities. Finally, each gene intensity was divided by the housekeeping gene β-actin intensity on the same film.
Statistical Analysis
All histologic and clinical inflammation measurements were compared among treatment groups and time periods by repeated measures analysis of variance (ANOVA). Dome location and weight change also were evaluated by ANOVA. Pearson correlation coefficients and ANOVA were used to compare inflammation levels (area of infiltrate and percent inflammatory cell type) with bone formation parameters using the 7 day specimens. For gene arrays, the differences between groups (SIM vs. GEL) and time (day 3 vs. day 14) were compared with parametric t tests and nonparametric Kruskal – Wallis tests.
Results
Simvastatin Dose Response
Correlation coefficients showed that simvastatin dose did not affect the weight change of the animal during the postoperative healing period (data not shown). However, rats lost significant weight, 15.6 ± 1.8 grams (5%) 3 days after surgery, which was reduced to 6.6 ± 1.1 and 7.7 ± 1.6 grams net loss 7 and 24 days after baseline, respectively.
The effect of simvastatin dose on general measures of inflammation (clinical swelling and amount of inflammatory infiltrate) is summarized in Figures 3 and 4. All graph values represent least squares means and estimated standard errors based on ANOVA. Each dose of simvastatin more than 0.1 mg caused a significantly higher level of swelling than its contralateral gel control or the untreated jaws. The untreated side also showed less swelling than gel alone. However, gel alone controls in animals treated with contralateral simvastatin did not have more swelling compared to gel controls in rats not treated with simvastatin (UN), suggesting minimal systemic effect of the simvastatin implants (Fig. 3). The area of inflammatory infiltrate also was higher around simvastatin doses greater than 0.1 mg compared to gel controls (Fig. 4). Both swelling and inflammatory infiltrate peaked at day 7. In addition, swelling decreased significantly (P < 0.05) around every simvastatin dose lower than 2.2 mg, but infiltrate area remained consistent around all doses of simvastatin except 0.1 mg.
Figure 3.
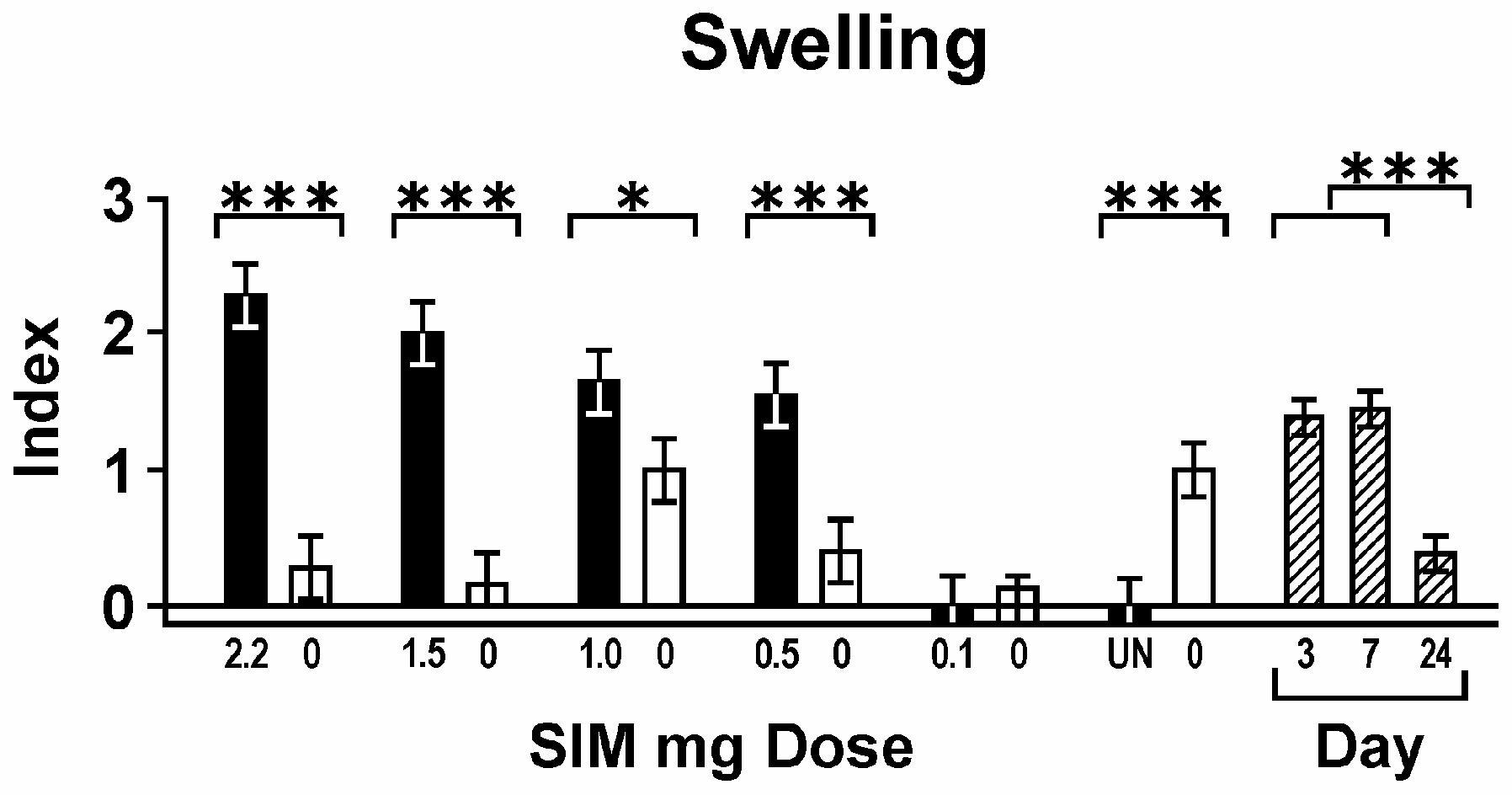
Swelling; clinical swelling index (mean ± standard error) associated with decreasing doses of simvastatin in mg (black bars; means across all days) and contralateral gel controls (0 mg, adjacent white bars). UN = untreated mandibular sides. Day post-implant are diagonal striped bars (means across all doses). For Figures 3-10, brackets indicate significant differences among groups or days at P < 0.05(*), P < 0.01(**) and P < 0.001(***).
Figure 4.
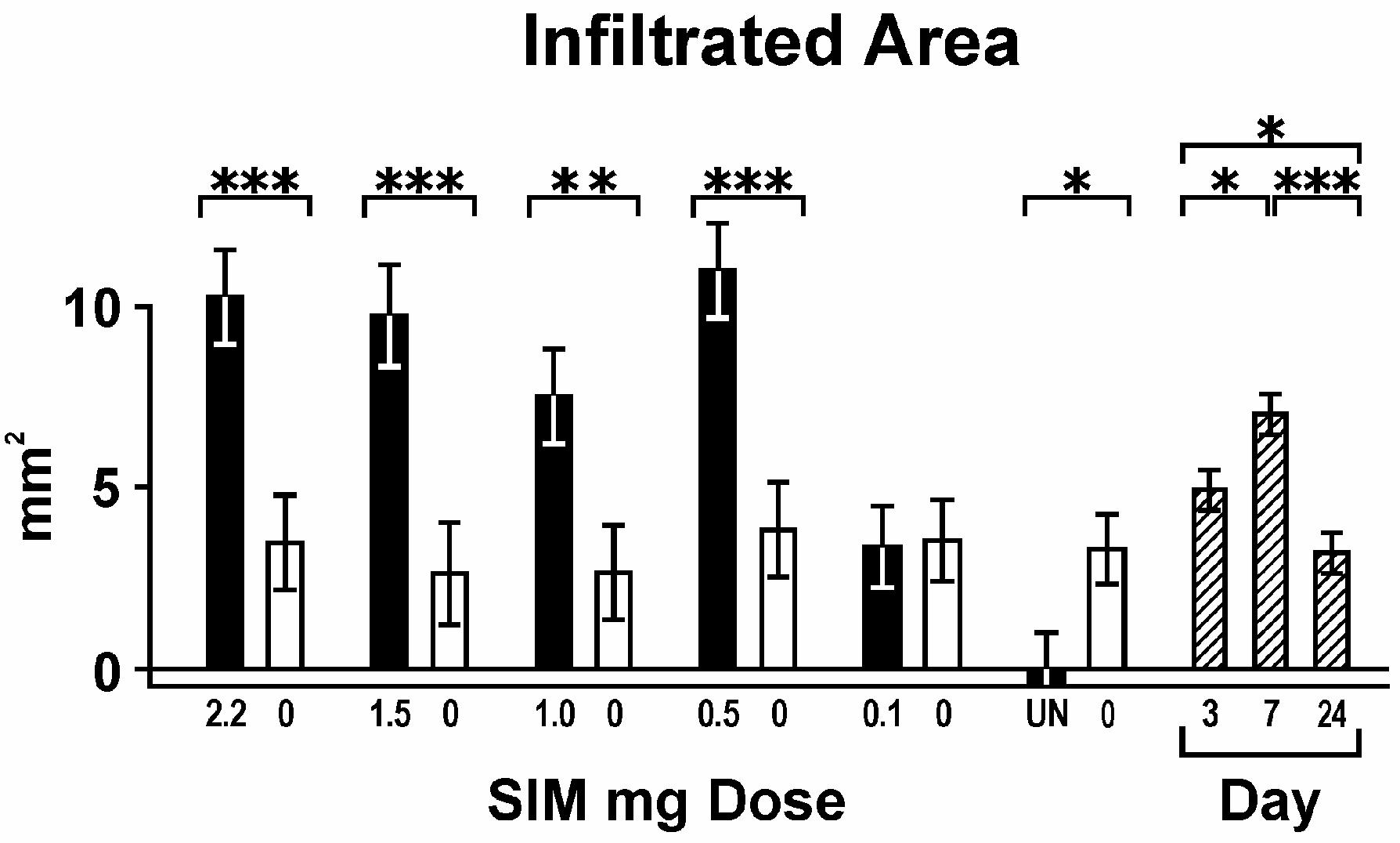
Infiltrated area; associated with simvastatin dose (mg; means across all days), contralateral gel control (0 mg); and days post-implant (means across all doses).
The increase in mandibular area induced by simvastatin dose as a percentage of its contralateral gel control (measure of bone growth) is graphically displayed in Fig. 5. Each simvastatin dose, except 1.0 and 0.1 mg, showed significantly more area than its gel control and the untreated hemi-mandibles. The bone surface lined with osteoblasts also was significantly higher on the SIM side at doses greater than 0.1 mg, but not different among simvastatin doses (data not shown). Osteoblast numbers peaked at day 7 (26.0 ± 2.6% bone surface; P <0.001). Almost no osteoclasts were noted on the bone periphery during the early time periods following simvastatin stimulation (day 3: 0.01 ± 0.5%; day 7: 0.2 ± 0.5%), but increased osteoclasts were seen after 24 days (3.1 ± 0.5%, P <0.001). None of the inflammatory or bone growth measurements were affected by how close the dome was located relative to bone.
Figure 5.

Mandibular area; cross-sectional area of the inferior 2 mm of the mandible associated with simvastatin dose (SIM mg) expressed as percent of its contralateral gel control (GEL). Asterisks above bars represent significant differences between SIM and GEL according to P values described in Fig. 3.
Since the histologic space on the lateral aspect of the rat mandible was primarily muscle, infiltrating cell types during healing were easily detectable. Adjacent to the lateral bone surface, no cell type was significantly altered by local simvastatin relative to its gel control. Fibroblasts were the predominant cell type next to bone, peaking at day 7 (Fig. 6). The density of collagen-like tissue and blood vessels followed the same pattern as the fibroblast numbers.
Figure 6.
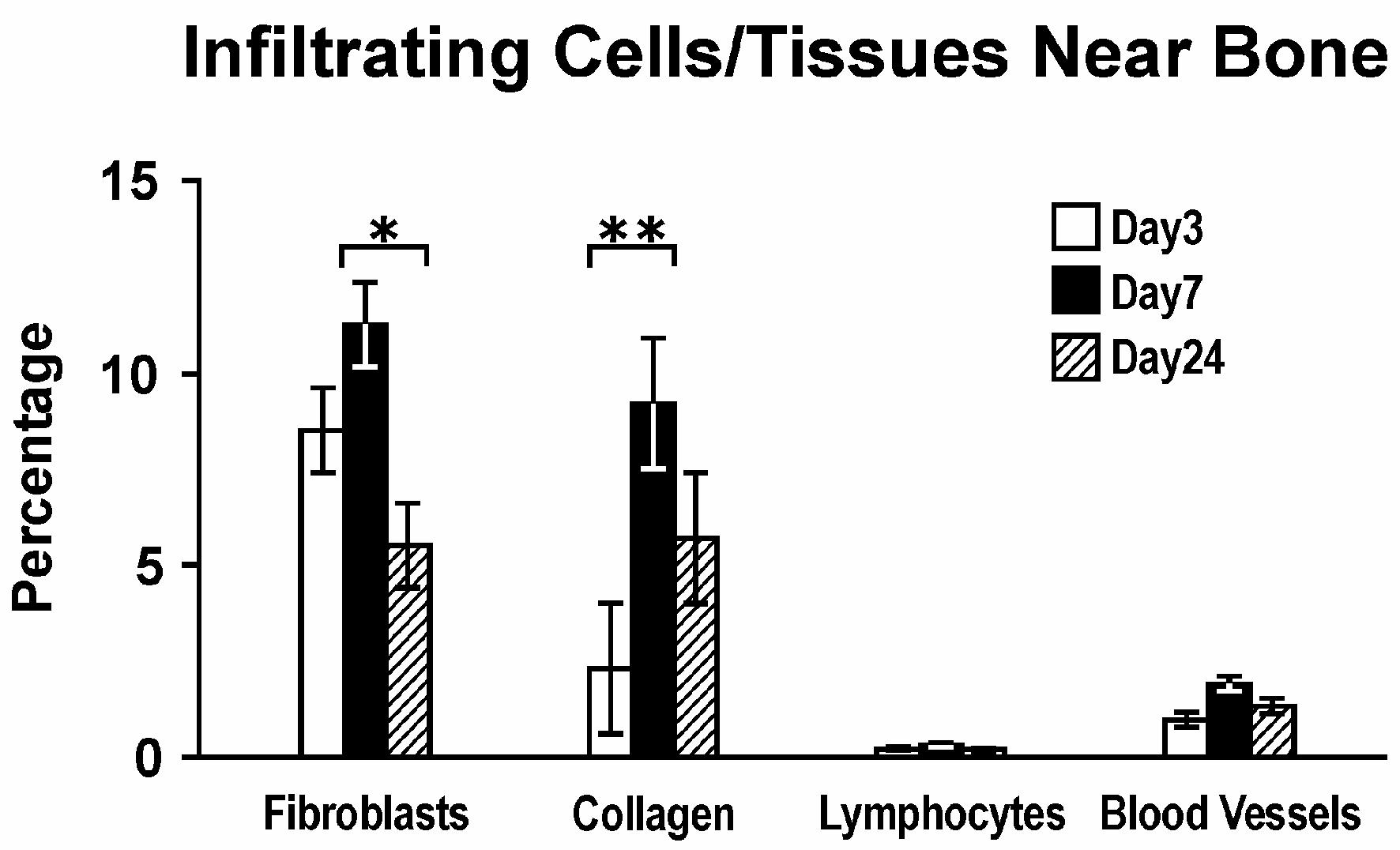
Infiltrating cells/tissues near bone; percentage of cells or tissues displacing muscle tissue adjacent to bone facing PLA/simvastatin domes across all doses relative to days post-implant.
Adjacent to the dome membrane, the primary inflammatory cell type was lymphocytes. Infiltrating cell types near the dome membrane generally were not statistically different between SIM vs. GEL groups (data not shown) except for increased neutrophils at all SIM doses greater than 0.1 mg, peaking at day 3 (10.1 ± 1.3%; P < 0.01).
Inhibitory Effects of NS-398 and Indomethacin
The effect of systemic COX-2 inhibitor (NS-398) and general COX inhibitor (indomethacin) is summarized in Figures 7 and 8. Clinical swelling at days 7 and 24 remained unchanged by the COX inhibitors when 0.5 mg simvastatin was used locally. However, the area of infiltrate was significantly reduced by both NS-398 and indomethacin. Swelling and infiltrate area decreased significantly between days 7 and 24, as was the case with the simvastatin dose experiments (Figs. 3, 4).
Figure 7.

Swelling; clinical swelling index associated with 0.5 mg simvastatin and contralateral gel controls (0 mg) across all days given with NS-398 and indomethacin or without (control) systemic COX inhibitors, and days post-implant across all conditions.
Figure 8.

Infiltrated area; associated with 0.5 mg simvastatin and contralateral gel control (0 mg) across all days given with or without systemic COX inhibitors, and days post-implant across all conditions.
Mandibular area was significantly different between 0.5 mg SIM and GEL sides in control rats, but not in rats treated with either NS-398 or indomethacin (Fig. 9). When these measurements were compared with 0.5 mg SIM/GEL control rats, indomethacin significantly reduced the mandibular area (bone growth). A trend toward reduced mandibular area also was seen with NS-398. The osteoblast surface percentage was significantly greater (and flat-lining cell surfaces significantly less) on the simvastatin side in control animals, but no differences between SIM and GEL were noted in the NS-398- or indomethacin-treated rats (Fig. 10). NS-398 significantly reduced osteoblast percentages from control, while indomethacin nearly eliminated osteoblasts on both SIM and GEL sides. Most of the osteoblasts were seen at day 7, while the few osteoclasts seen predominated at day 24 (0.8 ± 0.2%). The primary cell type displacing muscle near the bone was fibroblasts, with concomitant collagen (Fig. 11). NS-398 significantly reduced these cells/tissue, while indomethacin nearly eliminated them. Indomethacin also severely reduced blood vessels near bone. Around the dome membrane, more neutrophils were seen on the SIM than GEL side for SIM/control and SIM/NS-398, but not for SIM/indomethacin (data not shown).
Figure 9.

Mandibular area; cross-sectional area of the inferior 2 mm of the mandible associated with 0.5 mg SIM dose expressed as percent of its contralateral control (GEL), given with or without systemic COX inhibitor. † = P < 0.1; asterisks above bar represent significant difference between SIM and GEL according to P values described in Fig. 3.
Figure 10.
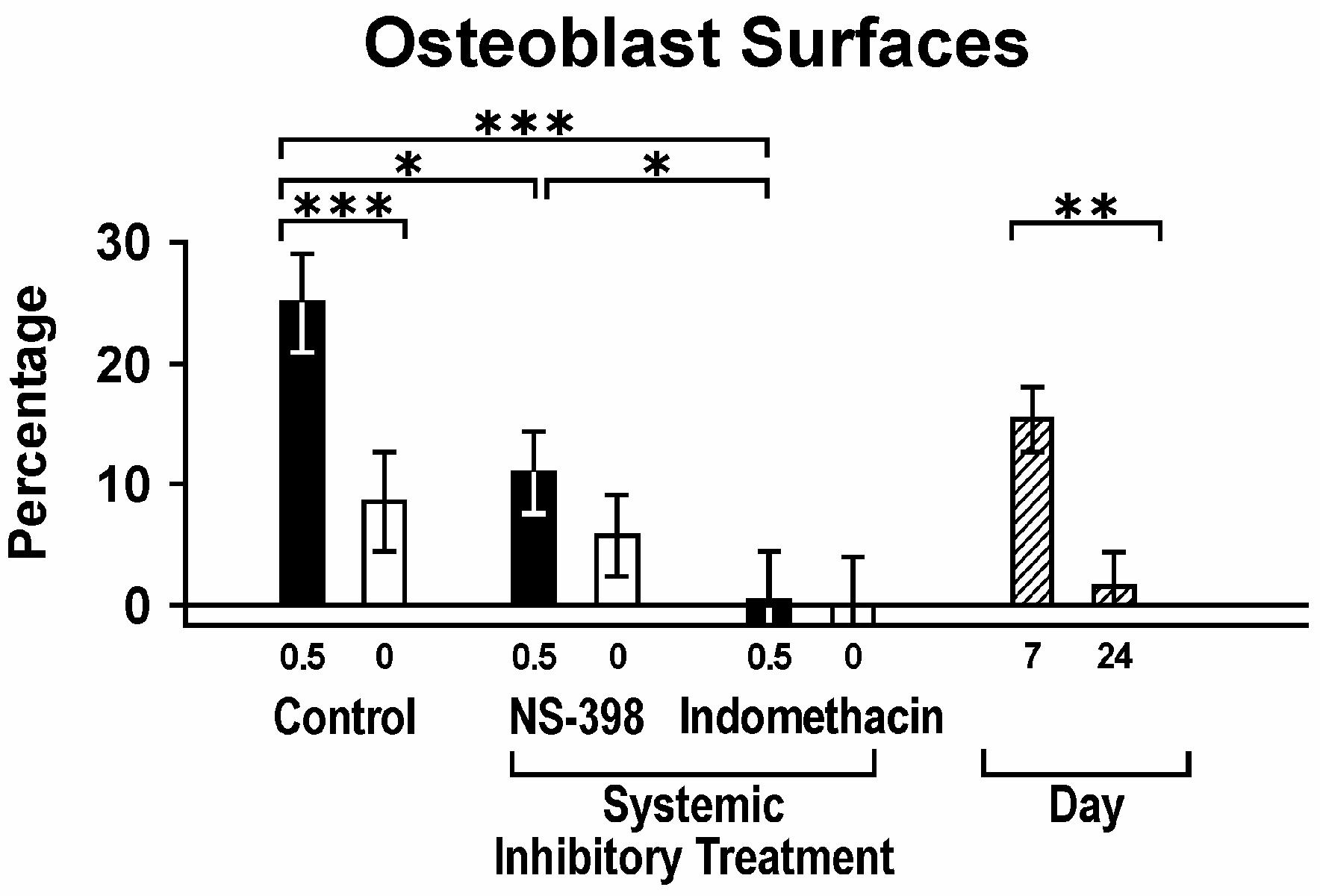
Osteoblast surfaces; percent bone surface facing PLA domes lined with osteoblast-like cells relative to 0.5 mg SIM and contralateral gel control (0 mg) across all days, with or without systemic COX inhibitor; and days post-implant across all conditions.
Figure 11.

Infiltrating cells/tissue near bone; percentage of cells or tissues displacing muscle tissue adjacent to bone facing PLA/0.5 mg SIM domes across all days with or without systemic COX inhibitor. Asterisks above bars represent significant differences between SIM and GEL. Both NS-398 and indomethacin significantly reduced fibroblasts and collagen (P < 0.01), and indomethacin reduced blood vessel density (P < 0.05) (brackets not shown).
In order to further examine the association between cells/tissues near bone and bone growth, the density of cells/tissue was correlated with the appearance of osteoblasts. Measurements which had significant positive correlation coefficients with osteoblast surface were: infiltrate area (r = 0.68, P < 0.0001), percent lymphocytes (r = 0.46, P = 0.0019), blood vessels (r = 0.67, P < 0.0001), fibroblasts (r = 0.77, P < 0.0001) and collagen (r = 0.70, P < 0.0001).
Simvastatin-Induced Gene Upregulation
The results from the gene array analysis demonstrated that 71 of 96 osteogenic genes were significantly upregulated (P ≤ 0.05) between day 3 and day 14, while one gene (epidermal growth factor) was downregulated. Eight specific genes were significantly upregulated in the simvastatin animals vs. the control animals after 14 days, using both parametric t tests and nonparametric Kruskal-Wallis tests (procollagen Ia1, IIIa1, Va1, VIa2, XIa1, XIIa1,fibronectin, and matrix metalloproteinase [MMP-13]; Fig. 12). In all cases, all simvastatin animals had higher gene intensity/β-actin intensity data points than all gel controls at the 14- day measurement. Two genes (procollagen type 6a2 and MMP-13) had average simvastatin-induced gene/β-actin intensities > 2-fold higher than the gel controls. The BMP-2 gene induction in mouse calvaria by simvastatin was minimal.
Figure 12.

Osteogenic gene expression; significant differences in murine calvaria treated with 0.5 mg simvastatin in methylcellulose gel/PLA (x) vs. methylcellulose gel alone (•). Horizontal lines represent mean values at days 3 and 14.
Discussion
This study has confirmed that local simvastatin upregulates bone growth. Using the rat bilateral mandible model allowed each simvastatin dose to be compared directly with gel alone in the same animal, and at the same location in the mandible relative to the dome placement. This was confirmed by the finding that dome placement was relatively constant from side-to-side and placement location did not affect inflammation or bone growth. Supraperiosteal placement was used because pilot studies showed that simvastatin stimulation over periosteum in contact with bone was necessary for optimal bone growth (data not shown). The animal procedures also caused minimal long-term (days 7 and 24) weight loss.
Simvastatin has been shown to induce inflammation around sites of local application.5 The current study demonstrated that by lowering the 2.2 mg simvastatin dose, signs of clinical inflammation can be reduced, yet significant bone growth can be retained at 0.5 mg simvastatin. The uncharacteristic drop in mandibular area noted with the 1.0 mg simvastatin dose (Fig. 5) was associated with an unusually high number of specimens where the dome could not be located (and less bone growth was seen) on the simvastatin side of the 7 and 24 day histologic sections (5/10), when bone growth should be noted. This suggests that domes were lost or moved away from the bone surface during healing. Except for 0.1 mg simvastatin, swelling remained significantly higher at each simvastatin dose than its gel control, and area of infiltrate remained high at all simvastatin doses except 0.1 mg (Figs. 3 and 4). Clearly, the 0.1 mg simvastatin dose demonstrated minimal inflammation (swelling, infiltrate area), but little bone growth (mandibular area or osteoblast surface) over its contralateral gel control. Based on these findings, 0.5 mg simvastatin appears to be the optimal dose for single local application in a GEL/PLA matrix. This study did not analyze the quality of the bone formed. It has been shown that the daily administration of simvastatin perorally significantly increased the compressive strength of the fifth lumbar vertebral body in rats,12 but the effects of local simvastatin on bone strength await future evaluation.
Products of the mevalonate pathway, besides synthesizing cholesterol, are also important for the prenylation of some kinds of small GTPases.13 Since such prenylation is of importance for both the activation of osteoclasts and the inhibition of the synthesis of BMP-2, statin's inhibition of small GTPases may increase bone mass systemically.14 The promotive effects of statins for the differentiation of osteoblasts also has been reported.15
Based on the fact that simvastatin may induce apoptosis of osteoclasts and hinder bone resorption processes,16 our results were consistent with this mechanism, showing very few osteoclasts or resorption lacunae. These processes are indicative of a modeling mechanism where bone formation is initiated without prior resorption on the same surface.9,17 Simvastatin may have stimulated intact periosteum to create an osteoblastic surface adjacent to the mandibles in the rats with a resultant increase in bone formation (Fig. 2). In fact, when the periosteum was disrupted using a critical-size defect in rat calvaria, minimal bone growth was noted from the periphery into the defect (data not shown). Osteoclast numbers increased after 24 days, suggesting a remodeling process may have begun later on the newly formed bone surfaces.
The use of a selective COX-2 inhibitor, NS-398, did not appear to reduce swelling, but infiltrate area was significantly reduced on the SIM side with both NS-398 and indomethacin (Fig. 8). In addition, the bone-growth properties of simvastatin were reduced on the SIM side with indomethacin, and the mandibular area adjacent to the 0.5 mg dose was not different from gel control in the same animal (Fig. 9). The percentage of osteoblast surface on the SIM side also was reduced by NS-398, and osteoblasts were nearly eliminated by indomethacin (Fig. 10). This parallels other studies where non-selective COX inhibitors, such as indomethacin, have been shown to delay bone formation in rats.18 COX inhibitors also reduce heterotopic bone formation associated with hip surgery. 19 The results of the current study, demonstrating that the COX-inhibition of inflammation also reduced the desired bone-formative properties of simvastatin, is suggestive that some degree of COX activity may be necessary in the simvastatin-induced bone-modeling process. COX-2 knockout mice have shown decreased intramembranous bone formation induced by both inflammation and fibroblast growth factor, which was rescued by prostaglandin (PG) E2 in stromal cell culture.20 The in vitro effects of indomethacin and nitric oxide-donating NSAIDs [5,5-dimethyl-3-(3-fluorophenyl)-4-(4 methylsulphonal) phenyl-2(5H)-furanone;DFU] on human osteoblasts have been shown to cause a reduction in osteoblast numbers.21 Blocking COX and subsequent PGE2 could thereby reduce the number of osteoblasts on the bone surface and associated bone synthesis. These results also support concerns about the possible adverse effects of using NSAIDs during postoperative care of bone-regeneration procedures.
According to the results of the gene array analysis, simvastatin may stimulate bone by more than one chemical pathway. Simvastatin has been shown to enhance mineralized nodule formation in culture, whereas coincubation with mevalonate, geranylgeranyl pyrophosphate, specific phosphatidylinositide-3 kinase inhibitor (LY294002), or vascular endothelial growth factor (VEGF) receptor 2 inhibitor (SU1498) abrogated statin-induced mineralization.22 Thus, statins appear to stimulate VEGF expression in vitro in osteoblasts via reduced protein prenylation and the phosphatidylinositide-3 kinase pathway, promoting osteoblastic differentiation. Studies also have demonstrated that simvastatin induces BMP-2 in vitro.3,4 Therefore, it was surprising that the in vivo gene array analysis showed minimal BMP-2 expression with simvastatin implants after either 3 or 14 days. Similarly, VEGF factors A, B and C were not stimulated by the simvastatin implants. It is possible that sampling times missed the period where BMP-2 or VEGF gene expression occurred. Osteoblast numbers in the rats in the current study peaked at day 7, making day 3 a logical time to test gene activation. Previous studies have shown that bone-growth factors were elevated 2 and 3 weeks following implantation in sheep of poly (lactide-co-glycolide) and insulin-like growth factor, respectively.23 Seventy-one of 96 osteogenic genes evaluated in the gene array were upregulated between day 3 and 14 (only one was down-regulated), suggesting that later times were more likely to show gene activation. However, if gene activation occurred at 3 weeks, it would be at the same time where maximum bone growth had already occurred in mice.5
Procollagen genes type I, III, V, VI, XI and XII, which were upregulated in the simvastatin group (Fig. 12), are found to be involved in events leading up to osteogenesis. Fibroblasts and collagen also were the predominant cell type and tissue infiltrating near the bone surface in the current rat mandible model, and were highly correlated with the percent of osteoblast surface. Collagen extracellular matrix has a role in the cellular signaling pathway and is fundamental to the developmental expression of the osteoblast phenotype and formation of the mineralized matrix. Collagen affects the growth and differentiation of fetal rat calvarial osteoblasts in vitro.24 PGE2 induces collagen systhesis in osteoblasts in vitro and bone formation in vivo via the osteoblast EP4 receptor.25,26 Since NS-398 and indomethacin reduced fibroblast-like cells, collagen and bone-lining osteoblasts following simvastatin stimulation in the current study, the role of the COX-2/PGE2/collagen synthesis pathway appears plausible, even in the older animals used here.27
Plasma fibronectin, which provides a scaffold for cell migration and wound repair,28 was also found to be upregulated in the simvastatin group. Fibronectin also promotes osteoblast adhesion, proliferation, differentiation and bone formation.29,30 MMP-13 has been associated with osteoclastic bone resorption.31 Its upregulation by simvastatin would seem incongruent with the lack of osteoclasts on histologic evaluation. However, proteolysis, and in particular MMP-13, is required for matrix resorption in skeletal development,32 and MMP-13 is important in the recruitment and differentiation of osteoprogenitor cells.33 Therefore, the increase in MMP-13, fibronectin, and procollagen gene activation could foster the increased collagen synthesis and osteoblast recruitment associated with local simvastatin-induced bone growth.
Based on the results of the experiments in this study, it was concluded that: 1) locally-applied simvastatin in methylcellulose gel/PLA membrane can stimulate significant bone growth at an optimal dose of 0.5 mg where clinical inflammation is reduced; 2) the COX pathway of inflammation appears to play a partial role in simvastatin-induced bone growth in vivo, in that both NS-398 and especially indomethacin inhibited simvastatin/gel from causing significant bone growth; and 3) osteoblast activation and bone growth by simvastatin may be mediated by fibroblasts and collagen turnover.
Acknowledgments
The authors thank the following University of Nebraska Medical Center staff and faculty: Phyllis Kumm for her expertise in preparing histologic specimens, Dr. Dennis Robinson for consultation on drug-release systems, Kim Theesen for figure design, and Deb Dalton for preparing the manuscript. Simvastatin was provided by Merck Research Laboratories, Rahway, NJ. This work was supported by the National Institute of Dental and Craniofacial Research #DE15096 and the UNMC College of Dentistry.
Footnotes
Funded by: National Institute of Dental and Craniofacial Research (NIH) DE15096 and UNMC College of Dentistry
Merck, Rahway, NJ.
Harlan Teklad, Madison, WI.
Phoenix Pharmaceutical Co., St. Joseph, MO.
Sigma, St. Louis, MO.
RNA Midi & Mini Kits, Qiagen, Valencia, CA
GEArray Q Series Mouse Osteogenesis Gene Array; SuperArray Bioscience Co., Frederick, MD.
References
- 1.Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann Periodontol. 2003;8:227–65. doi: 10.1902/annals.2003.8.1.227. [DOI] [PubMed] [Google Scholar]
- 2.Cochran DL, Schenk R, Buser D, Wozney JM, Jones AA. Recombinant human bone morphogenetic protein-2 stimulation of bone formation around endosseous dental implants. J Periodontol. 1999;70:139–150. doi: 10.1902/jop.1999.70.2.139. [DOI] [PubMed] [Google Scholar]
- 3.Garrett IR, Gutierrez G, Mundy GR. Statins and bone formation. Curr Pharm Design. 2001;7:715–36. doi: 10.2174/1381612013397762. [DOI] [PubMed] [Google Scholar]
- 4.Mundy G, Garrett R, Harris S, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–49. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 5.Thylin MR, McConnell JC, Schmid MJ, et al. Effects of statin gels on murine calvarial bone. J Periodontol. 2002;73:1141–48. doi: 10.1902/jop.2002.73.10.1141. [DOI] [PubMed] [Google Scholar]
- 6.Masferrer JL, Zweifel BS, Manning PT, et al. Selective inhibition of inducible cyclooxygenase 2 in vivo is anti-inflammatory and nonulcerogenic. Proc Natl Acad Sci USA. 1994;91:3228–32. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paya M, Garcia Pastor P, Coloma J, Alcaraz MJ. Nitric oxide synthase and cyclooxygenase pathways in the inflammatory response induced by zymosan in the rat air pouch. Br J Pharmacol. 1997;120:1445–52. doi: 10.1038/sj.bjp.0701073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bezerra MM, deLima V, Alencar VBM, et al. Selective cyclooxygenase-2 inhibition prevents alveolar bone loss in experimental periodontitis in rats. J Periodontol. 2000;71:1009–14. doi: 10.1902/jop.2000.71.6.1009. [DOI] [PubMed] [Google Scholar]
- 9.Boppart MD, Kimmel DB, Yee JA, Cullen DM. Time course of osteoblast appearance after in vivo mechanical loading. Bone. 1998;23:409–15. doi: 10.1016/s8756-3282(98)00119-7. [DOI] [PubMed] [Google Scholar]
- 10.Tanner SJ, Yee JA, Cullen DM. Pre-osteoblast proliferation following in vivo mechanical loading. J Bone Miner Res. 2001;16:S203. [Google Scholar]
- 11.Mundy GR. Bone resorbing cells. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Raven Press; New York: 1993. pp. 25–31. [Google Scholar]
- 12.Oxlund H, Dalstra M, Andreassen T. Statin given perorally to adult rats increases cancellous bone mass and compressive strength. Calcif Tiss Int. 2001;69:299–304. doi: 10.1007/s00223-001-2027-5. [DOI] [PubMed] [Google Scholar]
- 13.Ayukawa Y, Okamura A, Koyano K. Simvastatin promotes osteogenesis around titanium implants. Clin Oral Impl Res. 2004;15:346–50. doi: 10.1046/j.1600-0501.2003.01015.x. [DOI] [PubMed] [Google Scholar]
- 14.Rogers MJ. Statins: Lower lipids and better bones? Nature Medicine. 2000;6:21–23. doi: 10.1038/71484. [DOI] [PubMed] [Google Scholar]
- 15.Sugiyama M, Kodama T, Konisihi K, Abe K, Asami S, Oikawa S. Compactin and simvastatin, but not provastatin, induce bone morphogentic-2 in human osteosarcoma cells. Biochem Biophys Res Comm. 2000;271:688–92. doi: 10.1006/bbrc.2000.2697. [DOI] [PubMed] [Google Scholar]
- 16.Pytlik M, Janiec W, Misiarz-Myrta M, Gubala I. Effects of simvastatin on the development of osteopenia caused by ovariectomy in rats. Pol J Pharmacol. 2003;55:63–71. [PubMed] [Google Scholar]
- 17.Raab DM, Crenshaw TD, Kimmel DB, Smith EL. A histomorphometric study of cortical bone activity during increased weight bearing exercise. J Bone Miner Res. 1991;7:741–49. doi: 10.1002/jbmr.5650060712. [DOI] [PubMed] [Google Scholar]
- 18.Brown KM, Saunders MM, Kirsch T, Donahue HJ, Reid JS. Effect of COX-2-specific inhibition on fracture-healing in the rat femur. J Bone Joint Surg Am. 2004;86-A:116–23. doi: 10.2106/00004623-200401000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Fransen M, Neal B. Non-steroidal anti-inflammatory drugs for preventing heterotopic bone formation after hip arthroplasty. Cochrane Database Syst Rev. 2004;CD001160 doi: 10.1002/14651858.CD001160.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O'Keefe RJ. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109:1405–15. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans CE, Butcher C. The influence on human osteoblasts in vitro of non-steroidal anti-inflammatory drugs which act on different cyclooxygenase enzymes. J Bone Joint Surg Br. 2004;86:444–9. doi: 10.1302/0301-620x.86b3.14592. [DOI] [PubMed] [Google Scholar]
- 22.Maeda T, Kawane T, Horiuchi N. Statins augment vascular endothelial growth factor expression in osteoblastic cells via inhibition of protein prenylation. Endocrinology. 2003;144:681–92. doi: 10.1210/en.2002-220682. [DOI] [PubMed] [Google Scholar]
- 23.Meinel L, Zoidis E, Zapf J, et al. Localized insulin-like growth factor I delivery to enhance new bone formation. Bone. 2003;33:660–72. doi: 10.1016/s8756-3282(03)00207-2. [DOI] [PubMed] [Google Scholar]
- 24.Lynch MP, Stein JL, Stein GS, Lian JB. The influence of type I collagen on the development and maintenance of the osteoblast phenotype in primary and passaged rat calvarial osteoblasts: modification of expression genes supporting cell growth, adhesion, and extracellular matrix mineralization. Exp Cell Res. 1995;216:35–45. doi: 10.1006/excr.1995.1005. [DOI] [PubMed] [Google Scholar]
- 25.Hakeda Y, Nakatani Y, Kurihara N, Ikeda E, Maeda N, Kumegawa M. Prostaglandin E2 stimulates collagen and non-collagen protein syntheses and prolyl hydroxylase activity in osteoblastic clone MC3T3-E1 cells. Biochem Biophys Res Commun. 1985;126:340–5. doi: 10.1016/0006-291x(85)90611-4. [DOI] [PubMed] [Google Scholar]
- 26.Shamir D, Keila S, Weinreb M. A selective EP4 receptor antagonist abrogates the stimulation of osteoblast recruitment from bone marrow stromal cells by prostaglandin E2 in vivo and in vitro. Bone. 2004;34:157–62. doi: 10.1016/j.bone.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Cui L, Ma YF, Yao W, et al. Cancellous bone of aged rats maintains its capacity to respond vigorously to the anabolic effects of prostaglandin E2 by modeling-dependent bone gain. J Bone Miner Metab. 2001;19:29–37. doi: 10.1007/s007740170057. [DOI] [PubMed] [Google Scholar]
- 28.Corbett SA, Schwarzbauer JE. Fibronectin-fibrin cross-linking; a regulator of cell behavior. Trends Cardiovasc Med. 1998;8:357–62. doi: 10.1016/s1050-1738(98)00028-0. [DOI] [PubMed] [Google Scholar]
- 29.Moursi AM, Darnsky CH, Lull J, et al. Fibronectin regulates calvarial osteoblast differentiation. J Cell Sci. 1996;109:1369–80. doi: 10.1242/jcs.109.6.1369. [DOI] [PubMed] [Google Scholar]
- 30.Kim TI, Jang JH, Chung CP, Ku Y. Fibronectin fragment promotes osteoblast-associated gene expression and biological activity of human osteoblast-like cell. Biotechnol Lett. 2003;25:2007–11. doi: 10.1023/b:bile.0000004393.02839.d8. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura H, Sato G, Hirata A, Yamamoto T. Immunolocalization of matrix metalloproteinase-13 on bone surface under osteoclasts in rat tibia. Bone. 2004;34:48–56. doi: 10.1016/j.bone.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Wu W, Mwale F, Tchetina E, Kojima T, Yasuda T, Poole AR. Cartilage matrix resorption in skeletogenesis. Novartis Found Symp. 2001;232:158–66. doi: 10.1002/0470846658.ch11. [DOI] [PubMed] [Google Scholar]
- 33.Ortega N, Behonick D, Stickens D, Werb Z. How proteases regulate bone morphogenesis. Ann NY Acad Sci. 2003;995:109–16. doi: 10.1111/j.1749-6632.2003.tb03214.x. [DOI] [PubMed] [Google Scholar]


