Abstract
Pseudomonas aeruginosa is an important opportunistic pathogen that produces a variety of cell-associated and secreted virulence factors. P. aeruginosa infections are difficult to treat effectively because of the rapid emergence of antibiotic-resistant strains. In this study, we analyzed whether the amoeba Dictyostelium discoideum can be used as a simple model system to analyze the virulence of P. aeruginosa strains. The virulent wild-type strain PAO1 was shown to inhibit growth of D. discoideum. Isogenic mutants deficient in the las quorum-sensing system were almost as inhibitory as the wild type, while rhl quorum-sensing mutants permitted growth of Dictyostelium cells. Therefore, in this model system, factors controlled by the rhl quorum-sensing system were found to play a central role. Among these, rhamnolipids secreted by the wild-type strain PAO1 could induce fast lysis of D. discoideum cells. By using this simple model system, we predicted that certain antibiotic-resistant mutants of P. aeruginosa should show reduced virulence. This result was confirmed in a rat model of acute pneumonia. Thus, D. discoideum could be used as a simple nonmammalian host system to assess pathogenicity of P. aeruginosa.
The bacterium Pseudomonas aeruginosa is an important causative agent of nosocomial infections, including severe pneumonia (10) and bacteremia. This opportunistic pathogen also colonizes the lungs of cystic fibrosis patients and leads to progressive lung damage, respiratory failure, and eventually death (3, 12). The seriousness of P. aeruginosa infections is further exacerbated by the rapid selection of antibiotic-resistant strains following antibiotic treatment (14).
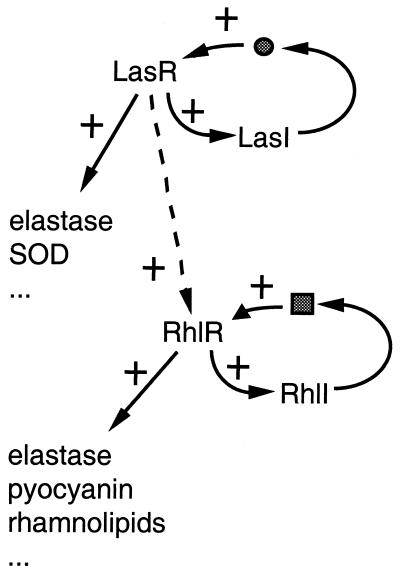
Studies in mammalian hosts have shown that quorum sensing is important for the virulence of P. aeruginosa (28, 37, 42). Secreted components essential for Pseudomonas virulence, such as proteases, rhamnolipids, pyocyanin, and exotoxin A, are under the control of two quorum-sensing systems, las and rhl (Fig. 1) (31, 43). When the bacterial cell density reaches a certain threshold, the accumulation in the medium of signaling autoinducer molecules (3-oxo-C12-homoserine lactone [HSL] and C4-HSL) induces the las and rhl pathways, respectively, leading to transcription of virulence genes. Both systems involve a transcriptional regulator (LasR and RhlR, respectively) and an autoinducer synthase (LasI and RhlI, respectively). The las quorum-sensing system can also induce the transcription of rhlR and consequently activate, to some degree, the rhl quorum-sensing system (21).
FIG. 1.
Quorum sensing in P. aeruginosa. In P. aeruginosa, secreted components such as proteases (LasB elastase), rhamnolipids, and pyocyanin are under the transcriptional control of two quorum-sensing systems, las and rhl. Both systems involve a transcriptional regulator (LasR and RhlR, respectively) and an autoinducer synthase (LasI and RhlI, respectively). When the bacterial density reaches a threshold, the accumulation in the medium of signaling autoinducer molecules (• and ▪) induces the las and rhl pathways, leading to transcription of virulence genes. The las quorum-sensing system can also induce, to some extent, the rhl quorum-sensing system. SOD, superoxide dismutase.
Strategies to develop innovative treatments against Pseudomonas infections rely on the elucidation of virulence and antibiotic resistance mechanisms. These studies involve the characterization of mutant strains and analysis of their virulence. However, the assessment of bacterial pathogenicity in mammalian hosts is time-consuming and expensive. Therefore, alternative yet equally relevant host systems would be extremely useful. Pseudomonas is remarkable in its ability to infect a number of alternative host systems. Plants (34), insects (5, 15), and nematodes (41) are susceptible to Pseudomonas infections, revealing the ubiquitous nature of a number of its virulence factors (33). In this study, a single-celled organism, the amoeba Dictyostelium discoideum, was used to examine P. aeruginosa virulence factors. A good correlation between results obtained in the Dictyostelium model and in a mammalian host system was observed, demonstrating the usefulness of this system as a novel tool for the analysis of virulence determinants of P. aeruginosa.
MATERIALS AND METHODS
Strains and culture conditions.
The D. discoideum wild-type strain DH1-10 used in this study is a subclone of DH1 (8). Cells were grown at 21°C in HL5 medium (14.3 g of peptone per liter [Oxoid], 7.15 g of yeast extract per liter, 18 g of maltose per liter, 0.64 g of Na2HPO4·2H2O per liter, 0.49 g of KH2PO4 per liter [pH 6.7]) (7) and subcultured twice a week. When indicated, Klebsiella pneumoniae was used as a growth substrate for D. discoideum (35).
P. aeruginosa strains used in this study are described in Table 1. PT5 is our laboratory wild-type PAO1 strain. PT502 was constructed by transducing the lasI::Tet mutation from strain PAO-JP1 (30) into the rhlI mutant strain PT454 by using the lipopolysaccharide-specific phage E79 tv2 (26). Tcr and Hgr transductants were checked by Southern hybridization as described previously (17).
TABLE 1.
P. aeruginosa strains used in this study
| Strain | Phenotype or genotype | Source or reference |
|---|---|---|
| 35 | ||
| PT5 | PAO1 wild type | Laboratory collection |
| PT149 | PT5NfxC (previously called PAO-7H) | 18 |
| PT454 | PT5ΔrhlI::Tn501 Hgr | 17 |
| PT462 | PT5ΔrhlR::Tn501 Hgr | 17 |
| PT466 | PT5ΔlasI Tcr | 17 |
| PT498 | PT5ΔlasR Tcr | 17 |
| PT502 | PT5ΔrhlI::Tn501 ΔlasI Hgr Tcr | This study |
| PT531 | PT5rhlR::Tn501 ΔlasR Hgr Tcr | 17 |
| PT625 | PT5NalC | 19 |
| PT637 | PT149mexE::ΩHg | 20 |
| PT648 | PT5nfxB | 19 |
| PT712 | PT5rhlA::Gm | 17 |
The corresponding regulator genes of the three efflux pump-overexpressing mutants PT149 (MexEF-OprN overexpressor), PT625 (MexAB-OprM overexpressor), and PT648 (MexCD-OprJ overexpressor) were sequenced. The strains PT149 (NfxC) and PT637 (NfxC, mexE) were described recently in detail (16). Briefly, the mexT transcriptional activator gene (24) is interrupted by an 8-bp insertion in our P. aeruginosa wild-type strain, PT5. In the NfxC mutant PT149, the 8-bp insert is not present, yielding a functional mexT gene able to cause overexpression of the MexEF-OprN efflux system. To obtain PT637, the mexE gene was inactivated in PT149, restoring wild-type antibiotic susceptibility. PT625 did not contain any mutation in the mexR regulator gene of the mexAB-oprM efflux operon. However, since the strain was shown by Western blot analysis to overexpress the MexAB-OprM efflux pump (Köhler et al., unpublished observation), the strain was considered to be a NalC mutant (40). Strain PT648, which overexpresses the MexCD-OprJ system, was found to contain a 2-bp (AC) deletion at codon 19 of its cognate repressor gene, nfxB, resulting in overexpression of MexCD-OprJ.
Bacteria were grown overnight at 37°C on Luria-Bertani (LB) agar. Single colonies were inoculated into 5 ml of PB (2% [wt/vol] peptone, 0.3% [wt/vol] MgCl2·6H2O, 1% [wt/vol] K2SO4) (11) in a 50-ml flask and grown at 37°C for 8 h prior to use. Under these conditions, similar optical densities at 600 nm (OD600s) were obtained for each strain, and the induction of quorum sensing was maximal. The growth of various strains in rich medium (PB) and in defined M9 salts medium (23), supplemented with 0.2% glucose, 2 mM MgSO4, and trace elements, was tested by measuring the OD600.
Effect of P. aeruginosa on D. discoideum growth.
The inhibition of D. discoideum clonal growth by P. aeruginosa was assayed by mixing 200 D. discoideum cells with 300 μl (6 × 108 CFU) of K. pneumoniae culture and 10 μl (107 CFU) of P. aeruginosa culture and plating immediately on SM agar (7). The plates were then incubated for 5 days at 25°C to allow the growth of Dictyostelium clones.
Quantitative measurements of D. discoideum growth on a lawn of P. aeruginosa cells alone were obtained by first plating 200 μl (2 × 108 CFU) of P. aeruginosa culture on SM agar. The bacterial lawn was then spotted with eight 5-μl droplets containing serial dilutions of D. discoideum cells (50,000 cells in drop 1, 10,000 cells in drop 2, 2,000 cells in drop 3, etc., to 1 cell in drop 8). The plates were incubated for 5 days at 25°C, and the highest dilution at which D. discoideum growth was visible was recorded.
Effect of Pseudomonas supernatants on Dictyostelium cells.
Pseudomonas bacteria (PT5, PT531, or PT712) were grown in HL5 medium at 37°C overnight (16 h). The bacteria were then pelleted by centrifugation (10 min at 7,000 × g), and the supernatant was collected and filtered (0.22-μm pore size). Bacterial supernatants were applied to Dictyostelium cells observed in phase contrast with a Zeiss Axiovert 100 microscope, and pictures were recorded every 30 s with a Hamamatsu Orca camera and analyzed with OpenLab 3 software. Cells were counted, and their number was plotted as a function of time after addition of bacterial supernatant.
To prepare rhamnolipids, wild-type strain PT5 was grown in M9 medium supplemented with 0.2% glycerol, 2 mM MgSO4, trace elements, and 0.05% glutamate as a nitrogen source instead of NH4Cl. After growth for 48 h at 37°C, cultures were centrifuged at 10,000 × g for 10 min, and rhamnolipids were extracted from the supernatant with 2 volumes of diethyl ether. Pooled ether extracts were extracted once with 20 mM HCl, and the ether phase was evaporated. The residue was dissolved in water. Rhamnolipid concentration was determined by the orcinol assay (27) with rhamnose as a standard, considering that 1 mg of rhamnose corresponds to 2.5 mg of rhamnolipid (27).
Virulence test in rats.
The model of acute P. aeruginosa pneumonia used in this study is based on the model by Cash et al. (4) and has been modified as described previously (16). Briefly, bacteria (106 CFU) were injected in agar-enmeshed beads into anesthetized male Sprague-Dawley rats (Charles River, Saint Aubin les Elbeufs, France) via the transtracheal route. Animals develop an acute bronchopneumonia characterized by an increase in lung weight. Control animals, which were inoculated with noninfective beads, all survived. Virulence of strains was determined by comparing mortality and time to death. For deceased animals, the bacterial load in the lungs was determined after tissue homogenization by plating dilutions of the homogenate on LB agar plates.
RESULTS
Inhibition of Dictyostelium growth by P. aeruginosa: role of quorum sensing.
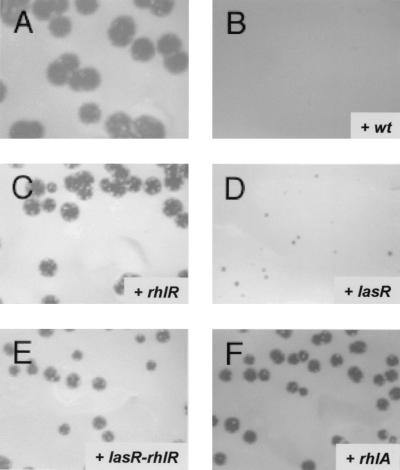
Dictyostelium amoebae are unicellular organisms that feed phagocytically upon bacteria, such as K. pneumoniae. When Dictyostelium cells are plated with K. pneumoniae bacteria, each amoebal cell creates a plaque in the bacterial lawn, where bacteria have been phagocytosed (Fig. 2A). We observed that when wild-type P. aeruginosa bacteria were added to the K. pneumoniae lawn, growth of Dictyostelium was completely inhibited (Fig. 2B). These results are in agreement with those of earlier studies that demonstrated that P. aeruginosa was a particularly inadequate bacterial growth substrate for D. discoideum (9, 36). We therefore investigated whether this growth inhibition was related to the production of virulence factors by P. aeruginosa, in particular those controlled by the las and rhl quorum-sensing systems. Isogenic derivatives of our wild-type strain, PT5, mutated in one of the quorum-sensing genes lasI, lasR, rhlI, or rhlR, were tested for growth inhibition of Dictyostelium cells. Interestingly, P. aeruginosa mutants affected in the rhl system, namely the rhlR (Fig. 2C) and rhlI (Table 2) mutants, were permissive—i.e., caused no inhibition of D. discoideum growth in this qualitative assay. The las system, however, did not seem to play an essential role, since both the lasR (Fig. 2D) and lasI mutants (Table 2) were still inhibitory for Dictyostelium growth. As would be expected, the rhlR-lasR double mutant was also permissive for Dictyostelium growth (Fig. 2E; Table 2).
FIG. 2.
Growth of D. discoideum clones in the presence of Klebsiella and P. aeruginosa. Approximately 200 D. discoideum cells were plated with a lawn of K. pneumoniae bacteria alone (A) or supplemented with P. aeruginosa strain PT5 (wild type [wt]) (B), PT462 (rhlR [C]), PT498 (lasR [D]), PT531 (lasR-rhlR [E]), or PT712 (rhlA [F]). Growth of D. discoideum created plaques in the bacterial lawn after 5 days of incubation at 25°C.
TABLE 2.
Growth of D. discoideum in the presence of various P. aeruginosa mutants
| Strain |
D. discoideum growth on substrate(s)a
|
|
|---|---|---|
| K. pneumoniae + P. aeruginosa | P. aeruginosab | |
| PT5 (wild type) | − | 0 |
| PT462 (rhlR) | + | 5 |
| PT498 (lasR) | − | 1 |
| PT531 (rhlR-lasR) | + | 8 |
| PT454 (rhlI) | + | 4 |
| PT466 (lasI) | − | 1 |
| PT502 (rhlI-lasI) | + | 8 |
| PT712 (rhlA) | + | 2 |
| PT149 (NfxC) | + | 4 |
| PT637 (NfxC, mexE) | − | 0 |
| PT625 (NalC) | − | 0 |
| PT648 (nfxB) | − | 1 |
Clonal growth of D. discoideum was tested on a mixture of K. pneumoniae and P. aeruginosa cells, as described in Fig. 2. +, growth of D. discoideum on the given substrate; −, no growth of D. discoideum.
The ability of D. discoideum cells to form plaques was tested on a lawn of pure P. aeruginosa cells as described in the legend to Fig. 3.
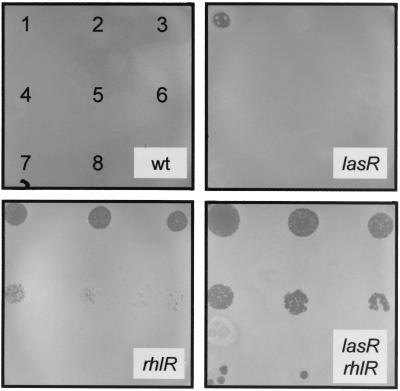
In these experiments, Dictyostelium was grown, however, in the presence of both Klebsiella and Pseudomonas bacteria. While the presence of Klebsiella ensures that an adequate food supply for Dictyostelium is present, it also makes the results more complex to interpret. For example, Pseudomonas bacteria might directly inhibit Dictyostelium growth, or they might inhibit the growth of the nutrient lawn of Klebsiella and thus indirectly inhibit Dictyostelium growth. To rule out the latter possibility, the ability of D. discoideum cells to grow on a lawn of pure P. aeruginosa bacteria was tested. To obtain quantitative results, a test was developed that allowed us to determine how many amoebal cells are necessary to create a plaque in a lawn of pure P. aeruginosa bacteria. In this test, 5-μl droplets were applied to a lawn of P. aeruginosa, with each droplet containing a defined number of Dictyostelium cells (droplet 1, 50,000 cells; droplet 2, 10,000 cells; droplet 3, 2,000 cells; etc., to droplet 8, 1 cell). Under these conditions, even 50,000 Dictyostelium cells failed to create a plaque in a lawn of wild-type P. aeruginosa PT5 cells (Fig. 3). In a scale measuring the growth of Dictyostelium, the wild-type strain was therefore scored as 0 (Table 2). When the lasR mutant was tested, 50,000 Dictyostelium cells created a plaque, while the next dilution (10,000 cells) failed to do so (Fig. 3). Consequently, the lasR mutant was scored as 1 (Table 2). The rhlR mutant permitted amoebal growth in the first five dilutions (Fig. 3) and was hence scored as 5 (Table 2). The rhlR-lasR double mutant was even more permissive for Dictyostelium growth, even at the highest dilution (Fig. 3), and thus obtained a score of 8 (Table 2). This new assay therefore defines a scale from 0 to 8, where the least permissive (fully inhibitory) strains are scored as 0 and the most permissive (least inhibitory) strains are scored as 8 (Table 2). With this test, it was apparent that the rhl quorum-sensing system was essential for efficient inhibition of Dictyostelium growth, since both rhlR and rhlI mutants were significantly more permissive (scores of 5 and 4, respectively) than the wild-type strain (score of 0). Surprisingly, lasR and lasI mutants (score of 1) were only marginally more permissive than the wild type, although when combined with an rhl mutation, lasR and lasI mutants resulted in fully permissive strains, obtaining a score of 8 (Table 2).
FIG. 3.
Quantitative assessment of D. discoideum growth in the presence of P. aeruginosa. Dictyostelium cells were applied as droplets onto a lawn of pure P. aeruginosa bacteria, as represented in the upper left panel. The numbers of Dictyostelium cells applied were 50,000 in drop number 1, 10,000 in 2, 2000 in 3, etc., as described in Materials and Methods. After 5 days at 25°C, the ability of Dictyostelium cells to create plaques in the bacterial lawn was recorded. The bacteria used here were PT5 (wild type [wt]), PT498 (lasR), PT462 (rhlR), and PT531 (lasR-rhlR). In a scale measuring growth of Dictyostelium cells, the results obtained were scored 0 for wild-type PT5, 1 for PT498, 5 for PT462, and 8 for PT531.
Effect of secreted virulence factors on D. discoideum cells.
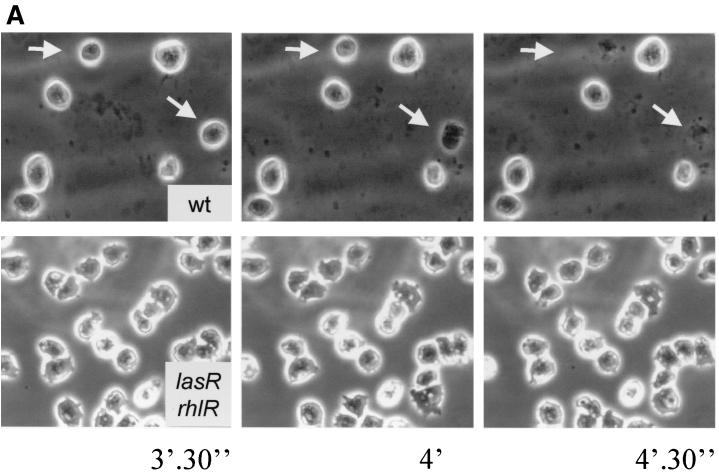
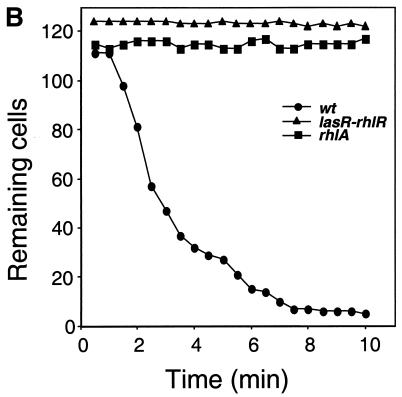
To test whether secreted factors are at least in part responsible for the growth inhibition of Dictyostelium, filtered culture supernatants of the wild-type strain and of the lasR-rhlR double mutant PT531 were incubated with Dictyostelium cells. Examination by phase-contrast microscopy showed a rapid lysis of Dictyostelium cells completed after a 10-min exposure to wild-type supernatants (Fig. 4A, upper panels). Under the same conditions, the supernatant of the lasR-rhlR double mutant PT531 did not induce significant lysis of Dictyostelium cells (Fig. 4A, lower panels). These results indicate that wild-type bacteria secrete, under the control of the quorum-sensing systems, one or several factors that disrupt the Dictyostelium cells and lead to fast lysis.
FIG. 4.
Lysis of Dictyostelium cells exposed to Pseudomonas supernatants. Dictyostelium cells were exposed to culture supernatants of wild-type (wt) strain PT5 (A, upper panels) and the double mutant PT531 (A, lower panels) and observed with a phase-contrast microscope. Arrows indicate individual Dictyostelium cells being lysed by wild-type supernatant during the indicated time frame. The kinetics of cell lysis induced by supernatants of the wild type (PT5), the lasR-rhlR double mutant (PT531) and the rhlA mutant (PT712) was determined by counting Dictoystelium cells under the microscope in a field containing approximately 100 cells at time zero (B).
Since mutants in the rhl system were particularly permissive for Dictyostelium growth, we tested whether rhamnolipids, the synthesis of which depends mainly on the rhl system, were involved in the fast lysis of Dictyostelium cells. We therefore tested the effect of supernatants from the rhlA mutant PT712, which is specifically defective in rhamnolipid synthesis, but is not affected in the quorum-sensing circuit (17). Supernatant from this rhlA mutant did not cause lysis of Dictyostelium cells (Fig. 4B), suggesting that rhamnolipids are essential for this effect. We then purified rhamnolipids from the supernatants of the wild-type strain PT5 and showed that these rhamnolipids at a final concentration of 10 μg/ml were able to cause lysis of the Dictyostelium cells (data not shown).
Since rhamnolipids are only one of the numerous virulence factors controlled by the quorum-sensing circuit, we tested growth inhibition by the rhlA mutant PT712, which is specifically affected in rhamnolipid production. We found that this strain was indeed permissive for Dictyostelium growth in a qualitative test in the presence of Klebsiella bacteria (Fig. 2F). In the quantitative test with PT712 as the only food source, however, the rhlA mutant obtained a score of only 2 (Table 2). Since the rhlR mutant (score of 5) was much more permissive, these results suggest that rhamnolipid-induced lysis of Dictyostelium cells is not the only factor responsible for growth inhibition by the wild-type strain and that other products under the control of the rhl quorum-sensing system are essential for the inhibition of Dictyostelium growth.
Analysis of multidrug-resistant mutants of P. aeruginosa in the Dictyostelium model.
P. aeruginosa possesses several drug efflux pumps of broad specificity, which contribute to its intrinsic and acquired resistance to a wide range of antimicrobial agents. We recently observed that P. aeruginosa strains that overexpress one of the four multidrug efflux pumps, namely the MexEF-OprN efflux system, show reduced production of secreted virulence factors, including elastase, pyocyanin, and rhamnolipids (20). This was found to result from reduced expression of the rhlI gene responsible for the synthesis of the C4-HSL autoinducer in P. aeruginosa (20). Since rhl mutants were more permissive in our Dictyostelium host system than the wild type, we tested the behavior of multidrug efflux mutants in this model. We used isogenic derivatives of our wild-type strain PT5 overexpressing either the MexAB-OprM (NalC), MexCD-OprJ (nfxB), or MexEF-OprN (NfxC) efflux system. Interestingly, the multidrug-resistant MexEF-OprN overproducer (NfxC) was more permissive for Dictyostelium growth (score of 4) than the antibiotic-susceptible parental strain (score of 0) (Table 2). On the contrary, the MexAB-OprM (NalC) and MexCD-OprJ (nfxB) overproducers were as inhibitory as the wild-type strain (Table 2).
Overexpression of efflux pumps results in most cases from mutations occurring in the cognate regulator genes, which may encode a transcriptional repressor protein (MexR for MexAB-OprM and NfxB for MexCD-OprJ) or an activator protein (MexT for MexEF-OprN). In the wild-type strain PT5, MexEF-OprN is not expressed due to the insertion of 8 bp in the coding sequence of the mexT activator gene (20, 24). In the MexEF-OprN overproducer PT149, this insertion is removed, resulting in a functional activator protein, MexT. To test whether the permissivity of strain PT149 was due to the overexpression of the MexEF-OprN efflux pump per se or to possible pleiotropic effects caused by MexT, we tested strain PT637, which expresses a functional MexT activator, but is mutated in the mexE gene and is hence as susceptible to antibiotics as the wild-type strain (20). As shown previously (20), this strain expresses wild-type levels of pyocyanin, elastase, and rhamnolipids. Indeed in the Dictyostelium host system, PT637 (NfxC mexE) was as inhibitory as the isogenic wild-type strain (Table 2). This demonstrates that overexpression of the MexEF-OprN efflux pump per se in strain PT149 (NfxC) accounts for the reduced ability to inhibit Dictyostelium growth.
The Dictyostelium model is predictive for P. aeruginosa virulence in a rat model.
To establish the correlation between Dictyostelium and a mammalian host system, the three efflux pump mutants and the wild-type strain PT5 were tested in the well-established model of acute pneumonia in rats (4, 16). Bacteria were injected into rat trachea, and virulence of strains was determined by assessing mortality and time to death. In this model, the wild-type strain PT5 caused mortality in 72% of the infected rats. MexAB-OprM and MexCD-OprJ overproducers exhibited a slight decrease in virulence, which was not statistically significant compared to that of the wild type (Table 3). Interestingly, the MexEF-OprN overproducer PT149 (NfxC) did not cause any mortality (100% survival). When the mexE gene was inactivated in the latter strain, virulence was restored to almost wild-type levels (PT637) (Table 3), suggesting that MexEF-OprN overexpression is responsible for the reduced virulence of the NfxC mutant PT149. Analysis of the lungs of deceased animals confirmed that for all strains studied, death was accompanied by high bacterial loads (Table 3). All of the mutants used in this study showed growth curves that were similar to those of the wild type, in both rich and defined minimal media (data not shown), suggesting that the decreased virulence of the MexEF-OprN overproducer was not due to reduced growth. The results from the rat model correlate well with those obtained in the Dictyostelium model, which could therefore be used as a novel and simple assay for testing the virulence properties of P. aeruginosa strains.
TABLE 3.
Virulence of P. aeruginosa efflux mutants in a rat model of acute pneumonia
| Strain | Inoculum (106 CFU) | n | % Mortalitya | P | Time to death (days) | Lung wt (g)b | Bacterial counts in lungs (log CFU/g) |
|---|---|---|---|---|---|---|---|
| PT5 (wild type) | 3.0 | 22 | 72 | 1.6 ± 0.63 | 3.98 ± 0.50 | 8.30 ± 0.54 | |
| PT625 (NalC) | 1.7 | 18 | 50 | 0.19 | 2.1 ± 1.0 | 3.84 ± 0.9 | 8.53 ± 0.38 |
| PT648 (nfxB) | 2.1 | 11 | 45 | 0.15 | 1.6 ± 0.89 | 4.83 ± 1.35 | 8.85 ± 0.28 |
| PT149 (NfxC) | 2.1 | 18 | 0 | <0.001 | NRc | NDd | ND |
| PT637 (NfxC, mexE) | 0.9 | 12 | 42 | 0.006 | 2.8 ± 1 | 4 ± 1.21 | 8.44 ± 0.48 |
Statistical significance (P < 0.05) was evaluated with a Student's t test calculated versus PT5, except for PT637, which was calculated against PT149.
Lung weight of uninfected rat, 1.0 ± 0.2 g.
NR, not relevant.
ND, not determined.
DISCUSSION
In the present study, D. discoideum was used to study the virulence of P. aeruginosa. With this unicellular system, the rhl quorum-sensing system of P. aeruginosa was found to be critical for inhibition of D. discoideum growth, while the las system appeared to be less important. In particular, rhamnolipids were shown to induce lysis of Dictyostelium cells. Furthermore, the model allowed us to predict the reduced virulence of a multidrug-resistant efflux mutant of P. aeruginosa, a result confirmed subsequently in a rat model of acute pneumonia.
Importance of quorum-sensing systems in mammalian and Dictyostelium host systems.
It was reported previously that mutations affecting the quorum-sensing circuitry resulted in less virulent strains in mammalian hosts (28, 29, 37, 42). However, in these experiments, the rhl system did not seem to play a more predominant role in virulence than the las system. Indeed, in the burn wound infection model and in the acute pneumonia model, las and rhl quorum-sensing mutants exhibited similar decreases in virulence (28, 29, 37). In these studies, however, a particular PAO1 isolate, referred to here as PAO-BI, was used as the wild-type strain. PAO-BI was reported earlier to exhibit particularly low pathogenicity in a mouse corneal infection model (32). We also observed in the pneumonia model a higher mortality rate with PT5 (70%) than that observed previously with strain PAO-BI (21%) (28). These observations correlate well with our recent finding that PAO-BI produces smaller amounts of extracellular virulence factors controlled by the rhl quorum-sensing system than our PAO1 strain PT5 (20). Four other PAO1 strains from four different laboratories were also tested in the Dictyostelium assay. Only PAO-BI was permissive for Dictyostelium growth (score of 4), while the other PAO1 strains were not permissive (score of 0). Since it was observed that in the PAO-BI strain, the rhl quorum system is attenuated (20), it would seem logical that in this situation, the las quorum-sensing system becomes more important for virulence, as was observed in mammalian systems. Interestingly, a lasR mutant in the PAO-BI background resulted in a Pseudomonas mutant fully permissive for Dictyostelium growth (score 8). In contrast, in the PT5 background, the lasR mutant remained inhibitory (score 1). As detailed below, these results suggest that the genetic backgrounds of the P. aeruginosa strains used might account for differences regarding the relative roles played by the las and rhl quorum-sensing systems in P. aeruginosa virulence. The fact that PAO-BI exhibits low virulence in both mammalian and Dictyostelium host systems and that, in this strain, the las quorum-sensing system is essential in both systems is further evidence for a good correlation between these two model systems.
Antibiotic resistance and virulence.
Analysis with the Dictyostelium model revealed that a multidrug-resistant MexEF-OprN-overproducing strain is less inhibitory than the isogenic wild-type strain. This strain was also less virulent in a rat model of acute pneumonia. This is in agreement with our recent report on reduced virulence factor production by the NfxC mutant PT149. Indeed, the MexEF-OprN overproducer showed drastic decreases in pyocyanin, rhamnolipid, and elastase production, which result from reduced levels of C4-HSL autoinducer produced by this strain (20). Interestingly, in this study, we also found that strain PAO-BI overproduced the MexEF-OprN efflux pump and showed decreased production of rhamnolipids and elastase compared to our wild-type PT5 strain (20). This might explain why both PT149 and PAO-BI show the same permissive phenotype (score of 4) in the Dictyostelium assay.
Several studies with other bacterial pathogens have shown that resistance to antibiotics can be associated with reduced virulence. For example, several antibiotic-resistant strains of Salmonella enterica serovar Typhimurium (1, 14) and Staphylococcus aureus (25) are less pathogenic. The lower infectivity of these antibiotic-resistant strains, however, could often be attributed to decreased growth rates and has thus been thought to result mostly from a decrease in bacterial fitness (2, 22, 38). In the case of the MexEF-OprN overproducer (NfxC), however, growth rates were identical to that of the wild-type strain (data not shown) and should therefore not account for its decrease in virulence. Instead, it appears that, in this particular case, acquisition of antibiotic resistance affects bacterial virulence by interfering with the P. aeruginosa quorum-sensing systems.
Dictyostelium as a host model for bacterial pathogens.
Dictyostelium amoebae feed upon bacteria in the soil, and it is not surprising to observe that certain bacteria have developed strategies to resist this predator. Our results suggest that in the case of Pseudomonas, similar mechanisms are crucial for virulence in mammalian systems and in amoebae. The role of quorum-sensing systems was particularly important in our experiments. Many other virulence mechanisms have also been described for Pseudomonas, and their role in a Dictyostelium model was not tested here. It will be interesting in the future to determine whether other virulence mechanisms, including cytotoxic mechanisms, can also be analyzed with the Dictyostelium host system.
Interestingly, Legionella pneumophila was shown recently to replicate intracellularly in amoebae by mechanisms similar to those used for growth inside macrophages (13, 39). In particular, dot/icm mutants of L. pneumophila have lost the ability to replicate intracellularly in both mammalian and Dictyostelium cells (39). It is therefore likely that many bacterial virulence mechanisms can be analyzed by using Dictyostelium amoebae as a host system.
The use of Dictyostelium as a host model has several advantages. First, the simplicity and reproducibility of the Dictyostelium system surpass those of other mammalian as well as nonmammalian systems. Second, D. discoideum represents a powerful genetic system to analyze host-pathogen relationships. Indeed, efficient genetic tools are available to allow the isolation of Dictyostelium mutants with increased or decreased sensitivity to pathogens and the identification of the corresponding genes. Mutants affected in the organization of the endocytic and phagocytic pathways have also been isolated and characterized (6, 8). It will be interesting to determine how alterations of the phagocytic machinery modify the relationship of Dictyostelium cells with various pathogens.
Acknowledgments
The laboratory of P.C. is funded by a START Fellowship of the Fonds National Suisse de la Recherche Scientifique (FNS) and a grant from the Fondation Gabriella Giorgi-Cavaglieri. The laboratory of T.K. is funded by a grant of the FNS (no. 3100-055961.98). C.V. was funded by grants of the FNS (no. 32-51940.97 and 32-52189-97).
We thank Olivier Brun for assistance with confocal and live microscopy, Eric Chamot for statistical analysis, and Sophie Cornillon for reading the manuscript.
P.C. and L.Z. contributed equally to this work.
REFERENCES
- 1.Björkman, J., D. Hughes, and D. I. Andersson. 1998. Virulence of antibiotic-resistant Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 95:3949-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouma, J. E., and R. E. Lenski. 1988. Evolution of a bacteria/plasmid association. Nature 335:351-352. [DOI] [PubMed] [Google Scholar]
- 3.Burns, J. L., B. W. Ramsey, and A. L. Smith. 1993. Clinical manifestations and treatment of pulmonary infections in cystic fibrosis. Adv. Pediatr. Infect. Dis. 8:53-66. [PubMed] [Google Scholar]
- 4.Cash, H. A., D. E. Woods, B. McCullough, W. G. J. Johanson, and J. A. Bass. 1979. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 119:453-459. [DOI] [PubMed] [Google Scholar]
- 5.Chugani, S. A., M. Whiteley, K. M. Lee, D. D'Argenio, C. Manoil, and E. P. Greenberg. 2001. QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 98:2752-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, C. J., R. Bacon, M. Clarke, K. Joiner, and I. Mellman. 1994. Dictyostelium discoideum mutants with conditional defects in phagocytosis. J. Cell Biol. 126:955-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornillon, S., C. Foa, J. Davoust, N. Buonavista, J. D. Gross, and P. Golstein. 1994. Programmed cell death in Dictyostelium. J. Cell Sci. 107:2691-2704. [DOI] [PubMed] [Google Scholar]
- 8.Cornillon, S., E. Pech, M. Benghezal, K. Ravanel, E. Gaynor, F. Letourneur, F. Bruckert, and P. Cosson. 2000. Phg1p is a nine-transmembrane protein superfamily member involved in Dictyostelium adhesion and phagocytosis. J. Biol. Chem. 275:34287-34292. [DOI] [PubMed] [Google Scholar]
- 9.Depraitère, C., and M. Darmon. 1978. Growth of “Dictyostelium discoideum” on different species of bacteria. Ann. Microbiol. (Paris) 129B:451-461. [PubMed] [Google Scholar]
- 10.Dunn, M., and R. G. Wunderink. 1995. Ventilator-associated pneumonia caused by Pseudomonas infection. Clin. Chest Med. 16:95-109. [PubMed] [Google Scholar]
- 11.Essar, D. W., L. Eberly, and I. P. Crawfords. 1990. Identification and characterization of genes for a second anthranilate synthetase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 172:884-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Govan, J. R. W., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hägele, S., R. Köhler, H. Merkert, M. Schleicher, J. Hacker, and M. Steinert. 2000. Dictyostelium discoideum: a new host model system for intracellular pathogens of the genus Legionella. Cell Microbiol. 2:165-171. [DOI] [PubMed] [Google Scholar]
- 14.Hoiby, N. 1993. Antibiotic therapy for chronic infection of Pseudomonas in the lung. Annu. Rev. Med. 44:1-10. [DOI] [PubMed] [Google Scholar]
- 15.Jander, G., L. G. Rahme, and F. M. Ausubel. 2000. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J. Bacteriol. 182:3843-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Join-Lambert, O. F., M. Michéa-Hamzehpour, T. Köhler, F. Chau, F. Faurisson, S. Dautrey, C. Vissuzaine, C. Carbon, and J.-C. Pechere. 2001. Differential selection of multidrug efflux mutants by trovafloxacin and ciprofloxacin in an experimental model of Pseudomonas aeruginosa acute pneumonia in rats. Antimicrob. Agents Chemother. 45:571-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Köhler, T., L. Kocjancic-Curty, F. Barja, C. van Delden, and J.-C. Pechère. 2000. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 182:5990-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Köhler, T., M. Michea-Hamzehpour, U. Henze, N. Gotoh, L. Kocjancic-Curty, and J. C. Pechère. 1997. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol. Microbiol. 23:345-354. [DOI] [PubMed] [Google Scholar]
- 19.Köhler, T., M. Michea-Hamzehpour, P. Plésiat, A.-L. Kahr, and J. C. Pechere. 1997. Differential selection of multidrug efflux systems by quinolones in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 41:2540-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Köhler, T., C. van Delden, L. Kocjancic-Curty, M. M. Hamzehpour, and J.-C. Pechère. 2001. Overexpression of the MexEF-OprN multidrug efflux system affects cell-to-cell signaling in Pseudomonas aeruginosa. J. Bacteriol. 183:5213-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latifi, A., M. Foglino, K. Tanaka, P. Williams, and A. Lazdunski. 1996. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 21:1137-1146. [DOI] [PubMed] [Google Scholar]
- 22.Lenski, R. E., S. C. Simpson, and T. T. Nguyen. 1994. Genetic analysis of a plasmid-encoded, host genotype-specific enhancement of bacterial fitness. J. Bacteriol. 176:3140-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 24.Maseda, H., K. Saito, A. Nakajima, and T. Nakae. 2000. Variation of the mexT gene, a regulator of the MexEF-OprN efflux pump expression in wild-type strains of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 192:107-112. [DOI] [PubMed] [Google Scholar]
- 25.Mizobuchi, S., J. Minami, F. Jin, O. Matsushita, and A. Okabe. 1994. Comparison of the virulence of methicillin-resistant and methicillin-sensitive Staphylococcus aureus. Microbiol. Immunol. 38:599-605. [DOI] [PubMed] [Google Scholar]
- 26.Morgan, A. F. 1979. Transduction of Pseudomonas aeruginosa with a mutant of bacteriophage E79. J. Bacteriol. 139:137-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochsner, U. A. 1993. Genetics and biochemistry of Pseudomonas aeruginosa rhamnolipid biosurfactant synthesis. Ph.D. dissertation. Swiss Federal Institute of Technology, Zürich, Switzerland.
- 28.Pearson, J. P., M. Feldman, B. H. Iglewski, and A. Prince. 2000. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect. Immun. 68:4331-4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson, J. P., E. C. Pesci, and B. H. Iglewski. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 179:5756-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearson, J. P., C. van Delden, and B. H. Iglewski. 1999. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 181:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pesci, E. C., J. P. Pearson, P. C. Seed, and B. H. Iglewski. 1997. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179:3127-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preston, M. J., S. M. J. Fleiszig, T. S. Zaidi, J. B. Goldberg, V. D. Shortridge, M. L. Vasil, and G. B. Pier. 1995. Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect. Immun. 63:3497-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahme, L. G., F. M. Ausubel, H. Cao, E. Drenkard, B. C. Goumnerov, G. W. Lau, S. Mahajan-Miklos, J. Plotnikova, M. W. Tan, J. Tsongalis, C. L. Walendziewicz, and R. G. Tompkins. 2000. Plants and animals share functionally common bacterial virulence factors. Proc. Natl. Acad. Sci. USA 97:8815-8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahme, L. G., M. W. Tan, L. Le, S. M. Wong, R. G. Tompkins, S. B. Calderwood, and F. M. Ausubel. 1997. Use of model plant hosts to identify Pseudomonas aeruginosa virulence factors. Proc. Natl. Acad. Sci. USA 94:13245-13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raper, K. B. 1984. The dictyostelids. Princeton University Press, Princeton, N.J.
- 36.Raper, K. B., and N. R. Smith. 1939. The growth of Dictyostelium discoideum upon pathogenic bacteria. J. Bacteriol. 38:431-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rumbaugh, K. P., J. A. Griswold, B. H. Iglewski, and A. N. Hamood. 1999. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections. Infect. Immun. 67:5854-5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schrag, S. J., and V. Perrot. 1996. Reducing antibiotic resistance. Nature 381:120-121. [DOI] [PubMed] [Google Scholar]
- 39.Solomon, J. M., A. Rupper, J. A. Cardelli, and R. R. Isberg. 2000. Intracellular growth of Legionella pneumophila in Dictyostelium discoideum, a system for genetic analysis of host-pathogen interactions. Infect. Immun. 68:2939-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srikumar, R., C. J. Paul, and K. Poole. 2000. Influence of mutations in the mexR repressor gene on expression of the MexA-MexB-OprM multidrug efflux system of Pseudomonas aeruginosa. J. Bacteriol. 182:1410-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan, M. W., L. G. Rahme, J. A. Sternberg, R. G. Tompkins, and F. M. Ausubel. 1999. Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc. Natl. Acad. Sci. USA 96:2408-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang, H. B., E. DiMango, R. Bryan, M. Gambello, B. H. Iglewski, J. B. Goldberg, and A. Prince. 1996. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infect. Immun. 64:37-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whiteley, M., K. M. Lee, and E. P. Greenberg. 1999. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:13904-13909. [DOI] [PMC free article] [PubMed] [Google Scholar]