Abstract
1. The effects of changes of ionic environment upon corticosteroid production by rabbit adrenal glands have been investigated in vitro using a superfusion technique and on-line steroid analysis by an automated fluorescence method. In some experiments micro-electrode recordings of adrenocortical transmembrane potentials were made concomitantly with measurement of steroid output.
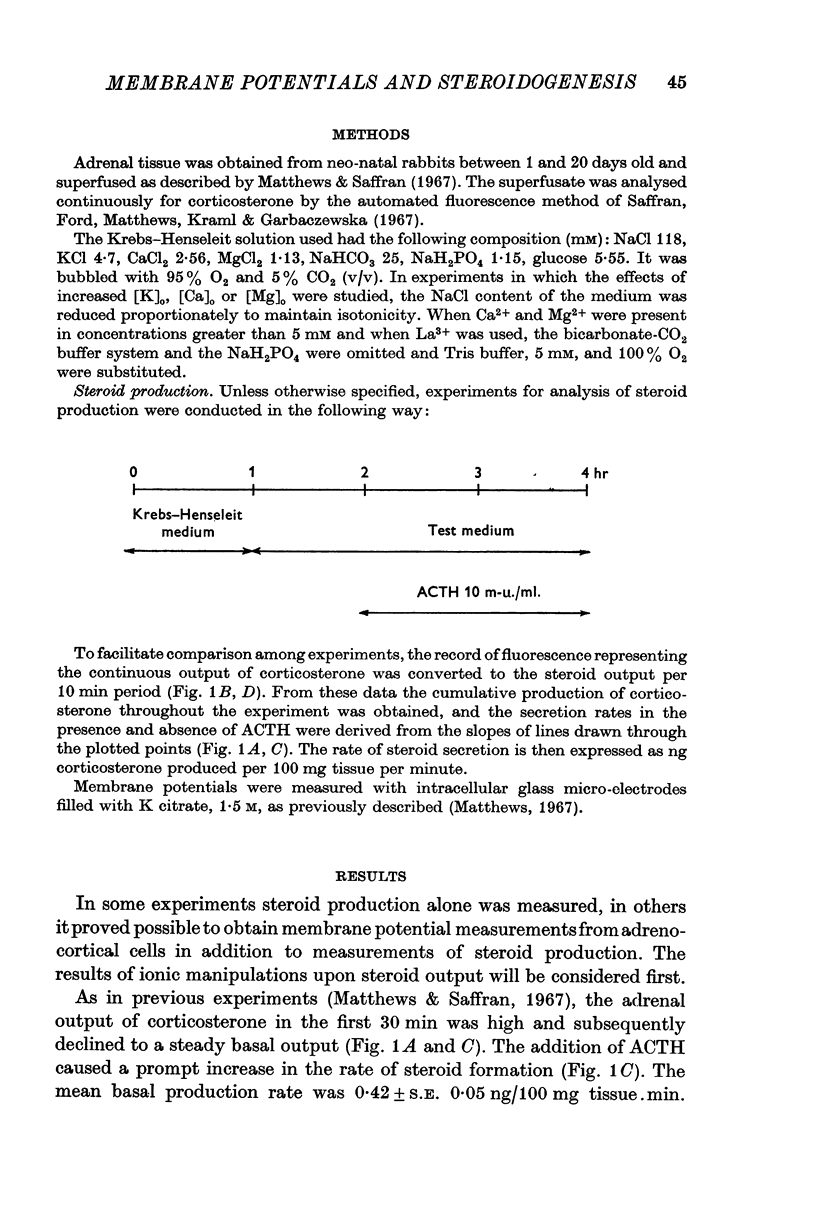
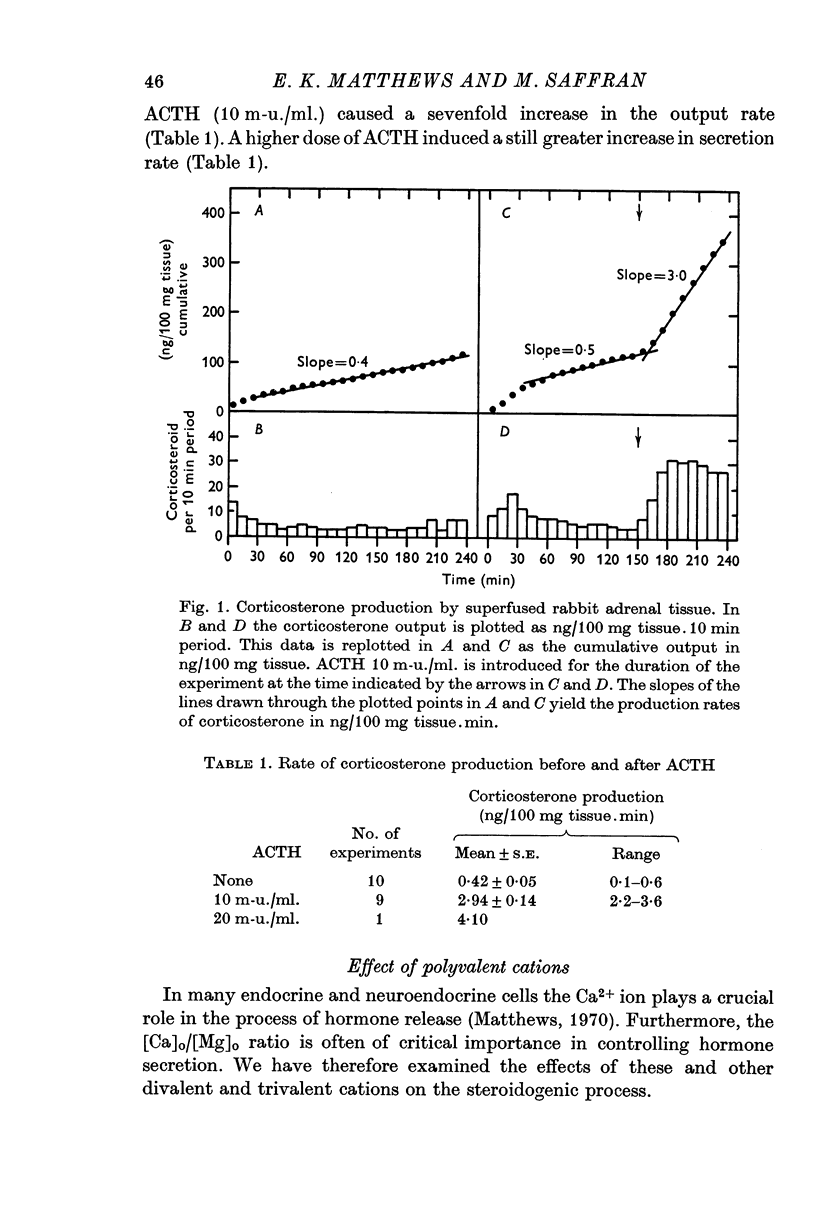
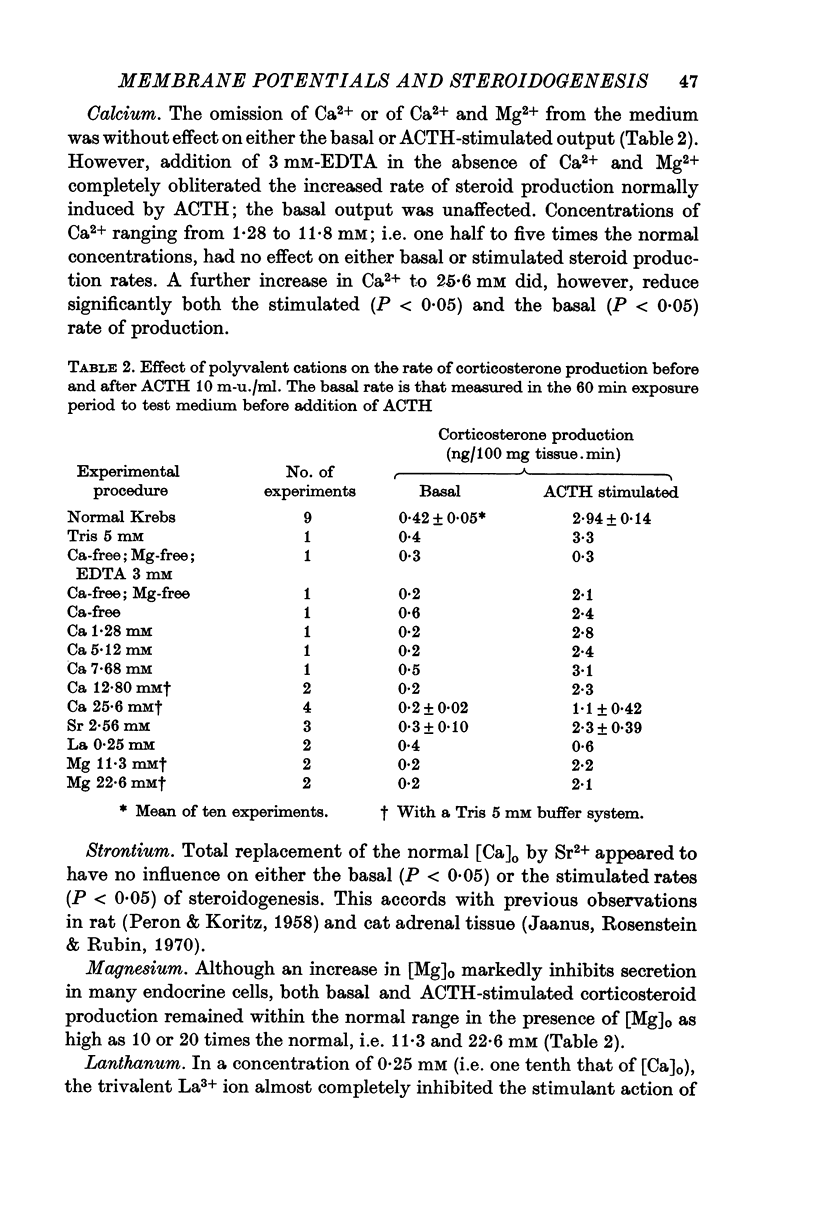
2. Adrenocorticotrophic hormone (ACTH), 10 m-u./ml., induced a sevenfold increase in corticosteroid production rate in normal Krebs solution.
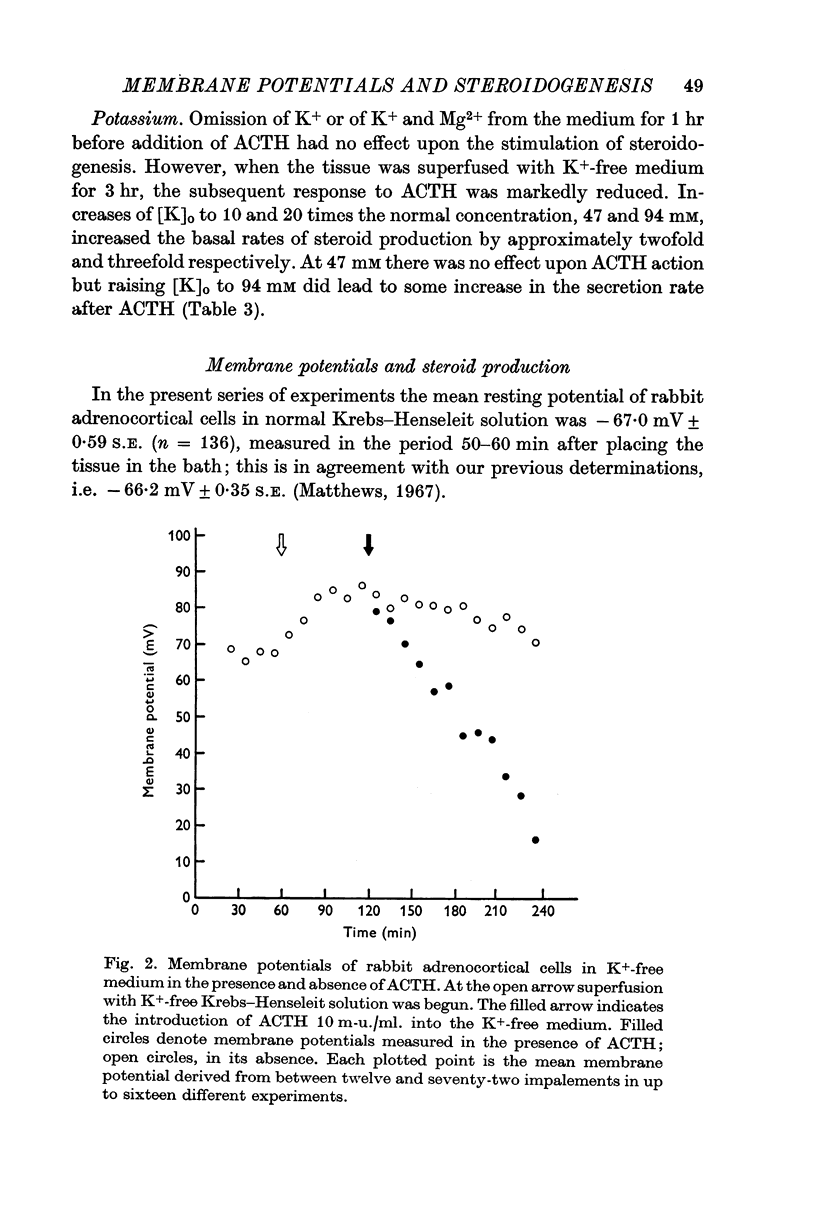
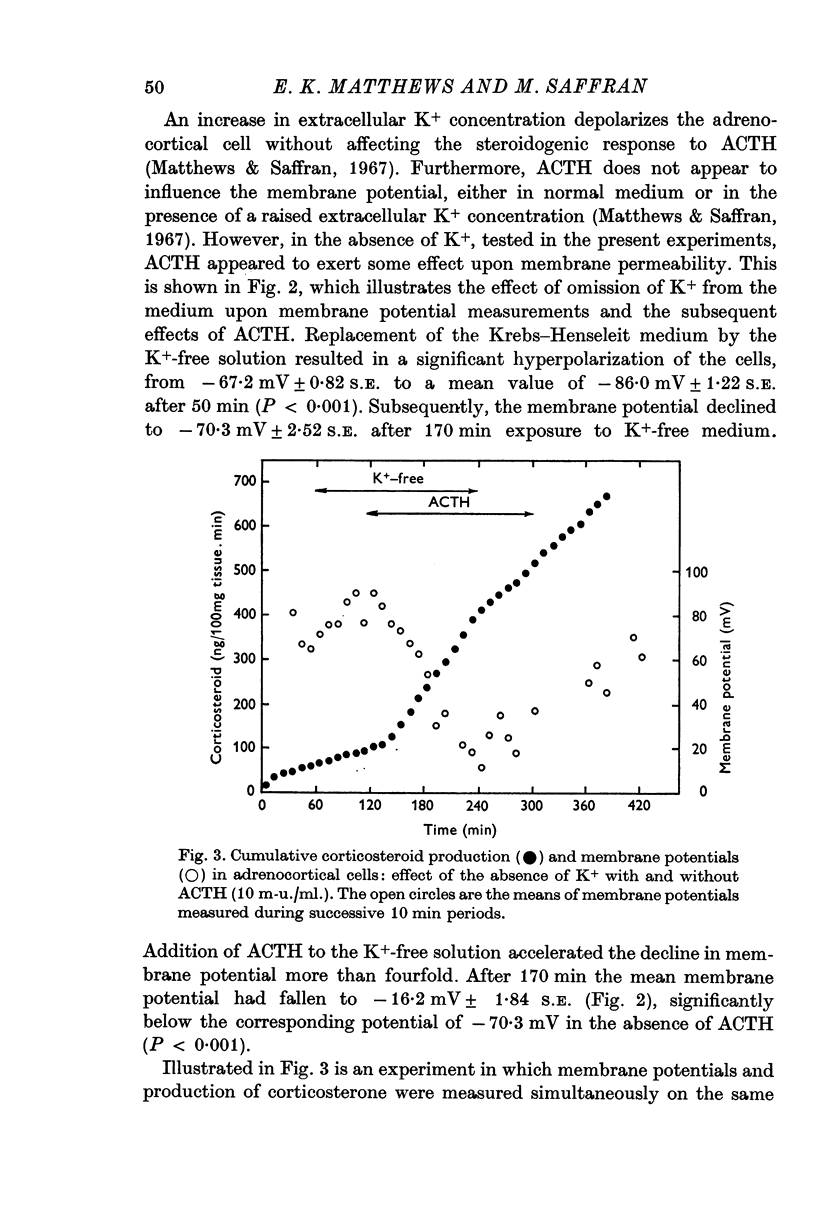
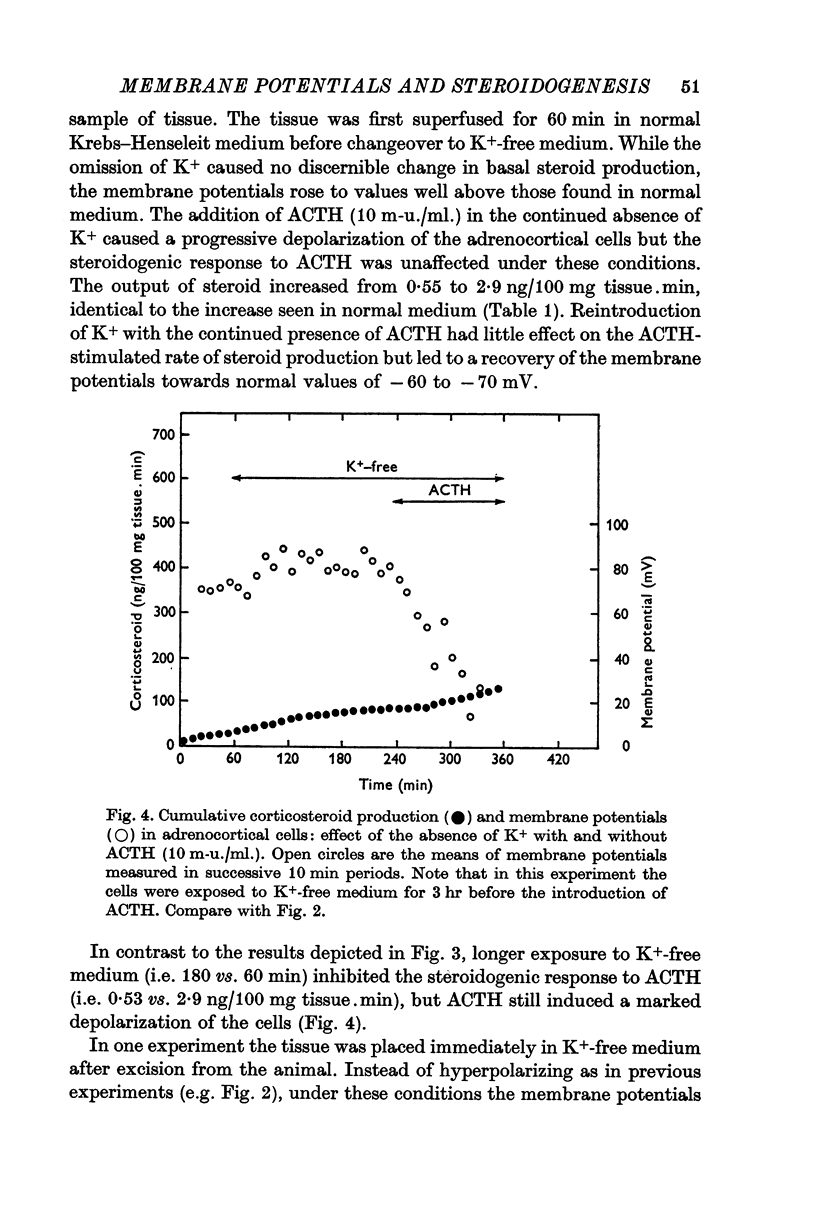
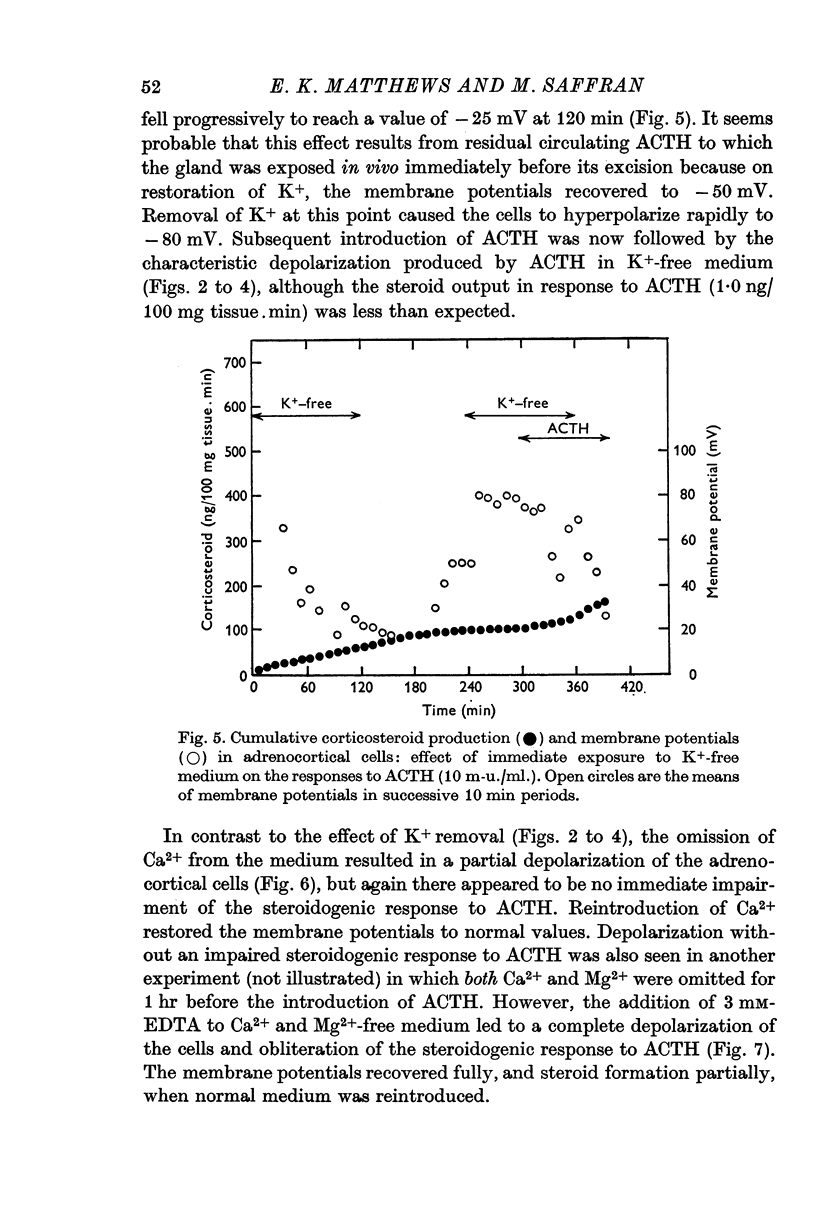
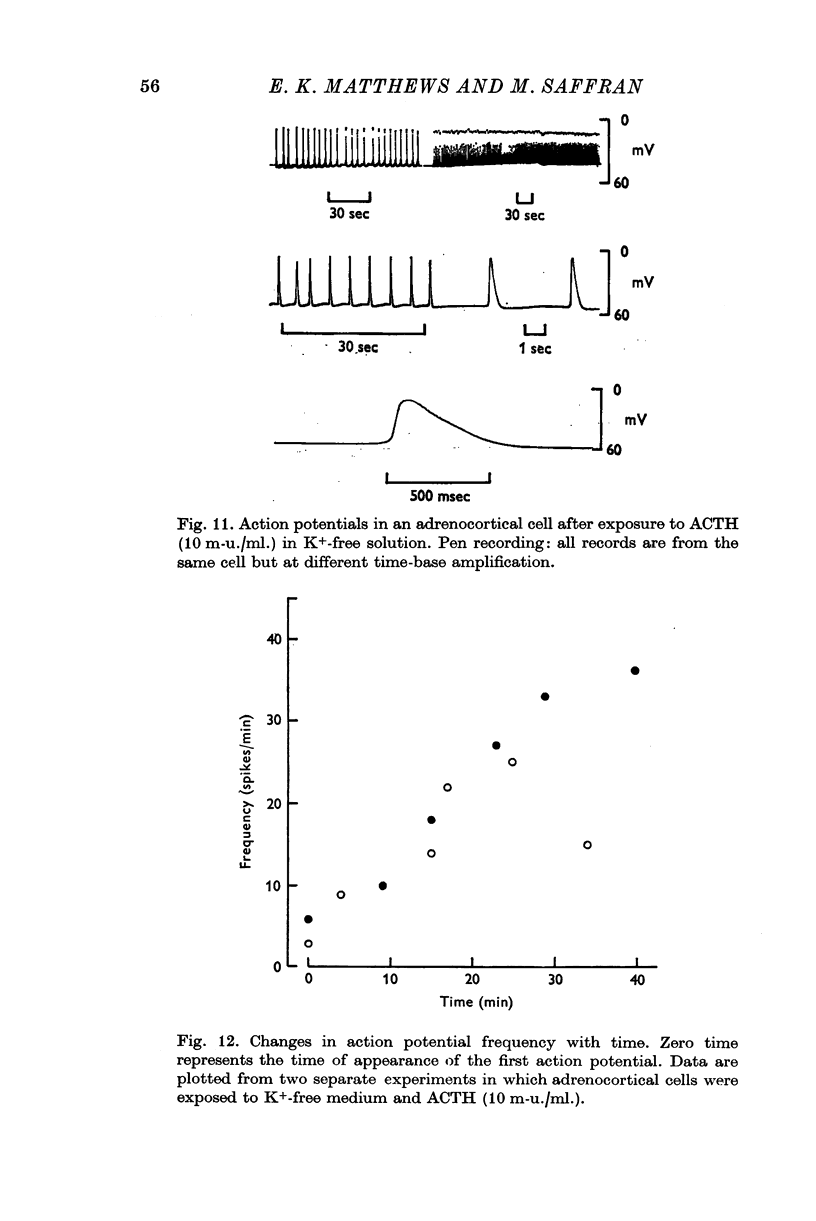
3. The steroidogenic response to ACTH was not impaired after omission of [K]o for 1 hr but was inhibited following exposure to K+-free medium for 3 hr. Increase of [K]o tenfold to 47 mM increased the basal but not the ACTH-stimulated output of corticosteroid whereas raising [K]o twentyfold to 94 mM enhanced both the basal and ACTH-stimulated steroid production rate. In K+-free solution the adrenocortical cells hyperpolarized from - 67 to - 86 mV; subsequently on addition of ACTH they depolarized. Reintroduction of K+ restored the membrane potential.
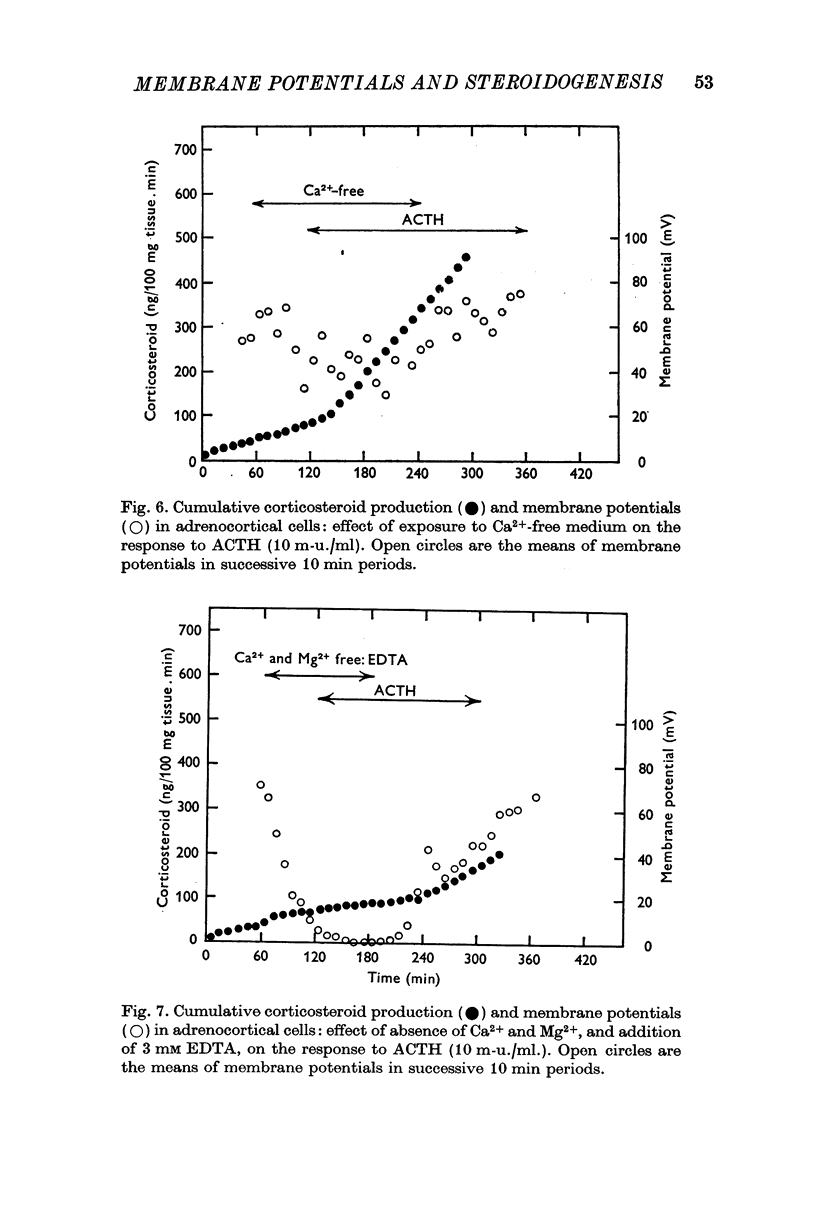
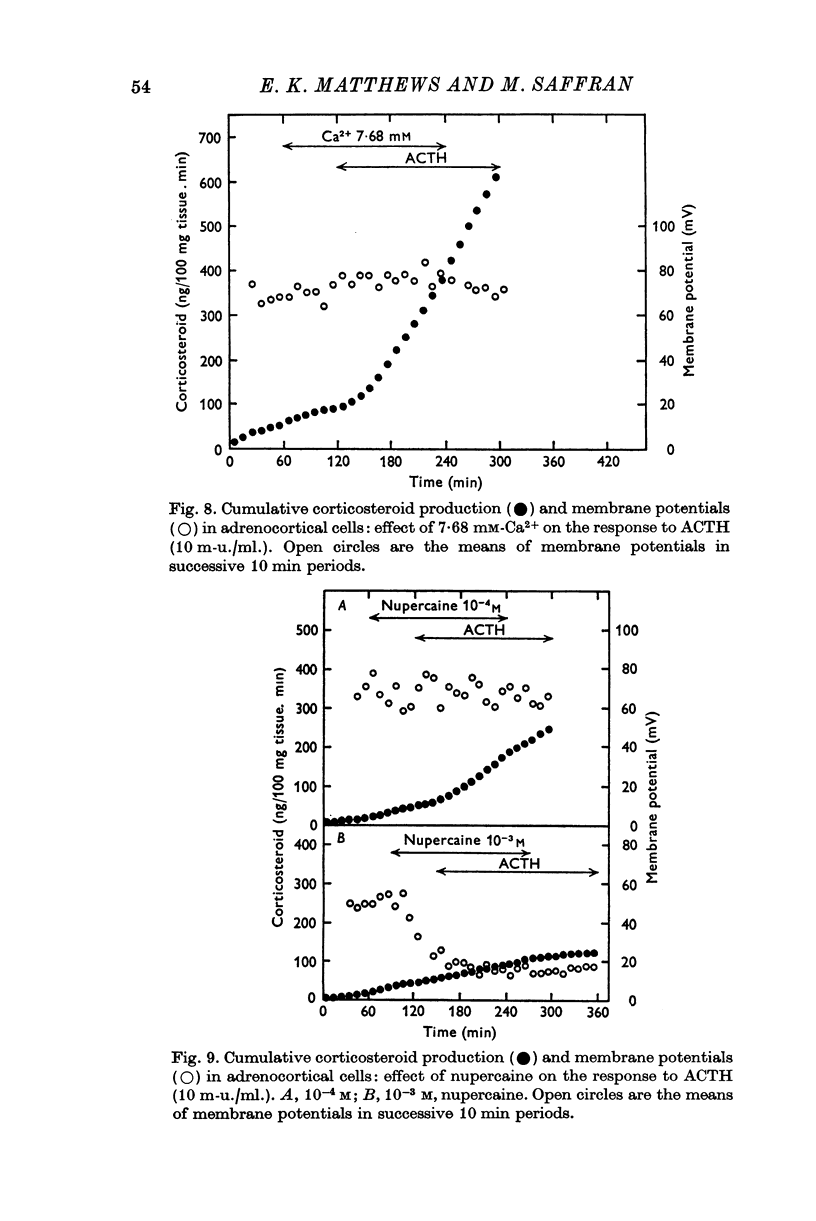
4. Omission of Ca2+ partially depolarized the cells but only affected the steroidogenic response to ACTH in the presence of EDTA. A threefold increase of [Ca]o, to 7·68 mM, had no effect on either membrane potentials or steroid formation, but increasing [Ca]o tenfold to 25·6 mM partially blocked ACTH action. Increasing [Mg]o twentyfold to 22·6 mM had little effect on ACTH-stimulated corticosteroid output and Sr 2·56 mM, in substitution for Ca2+, supported ACTH action, but La, 0·25 mM, completely blocked the steroidogenic effect of ACTH.
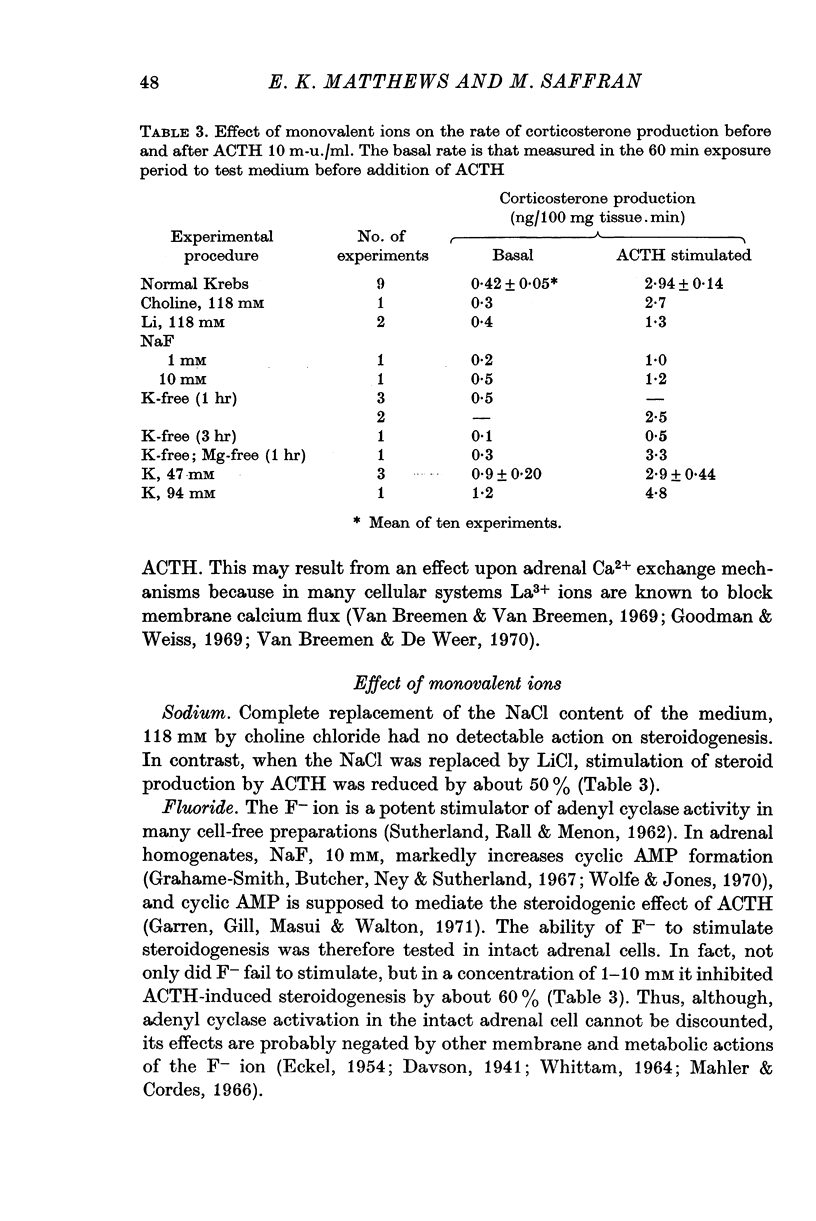
5. Replacement of NaCl, 118 mM by choline chloride, 118 mM, was without effect on ACTH-induced steroidogenesis, whereas LiCl, 118 mM, reduced it by 50%. NaF, 1 and 10 mM, inhibited ACTH-induced steroidogenesis by approximately 60%.
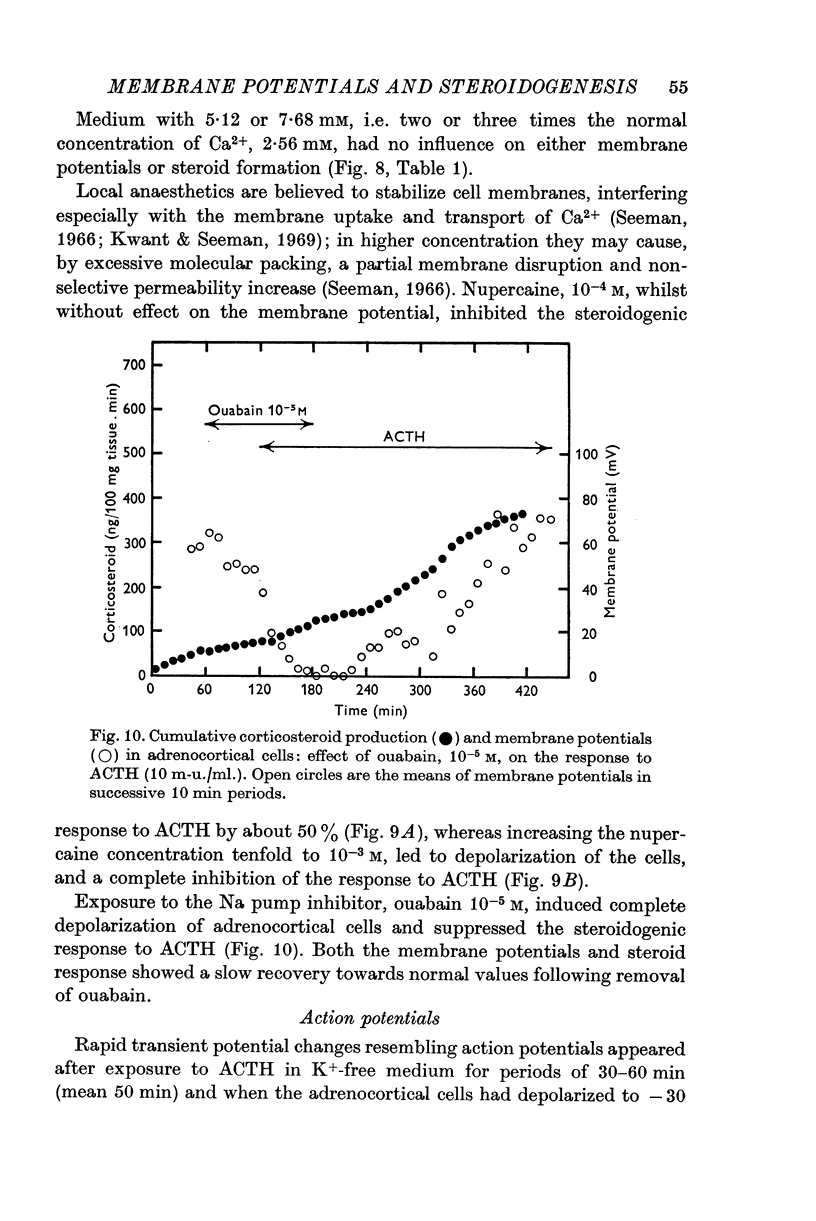
6. Nupercaine, 10-4 M, inhibited the steroid response to ACTH with no effect upon membrane potentials: increasing the nupercaine concentration to 10-3 M inhibited the steroid response and depolarized the cells. Ouabain, 10-5 M, induced complete depolarization and suppression of the steroidogenic response to ACTH.
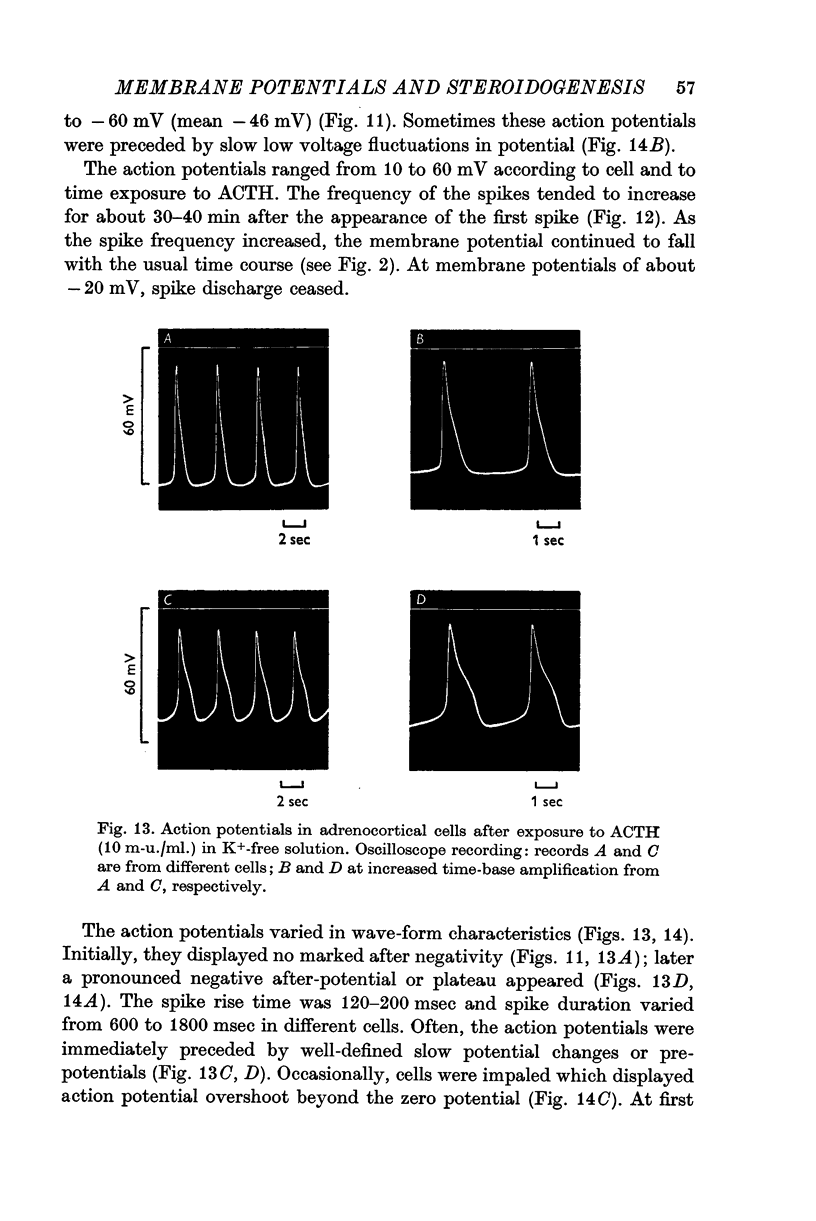
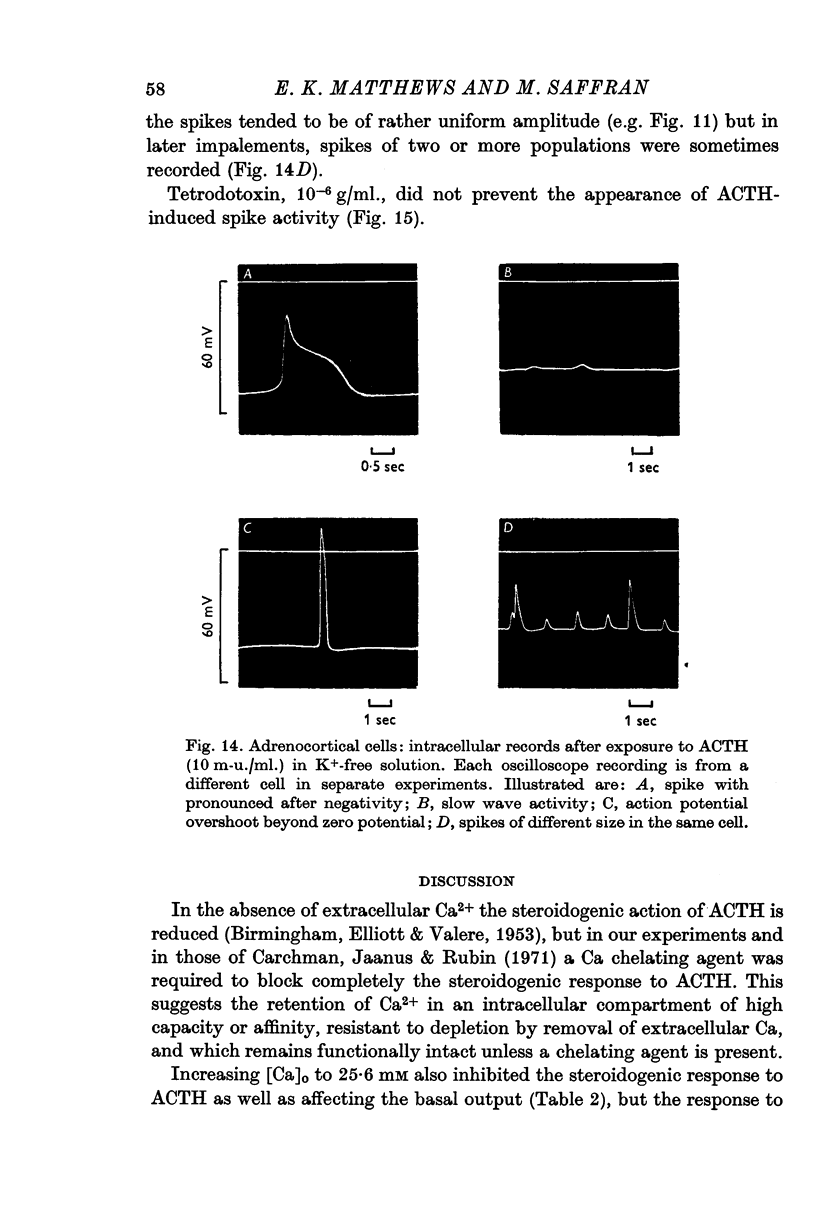
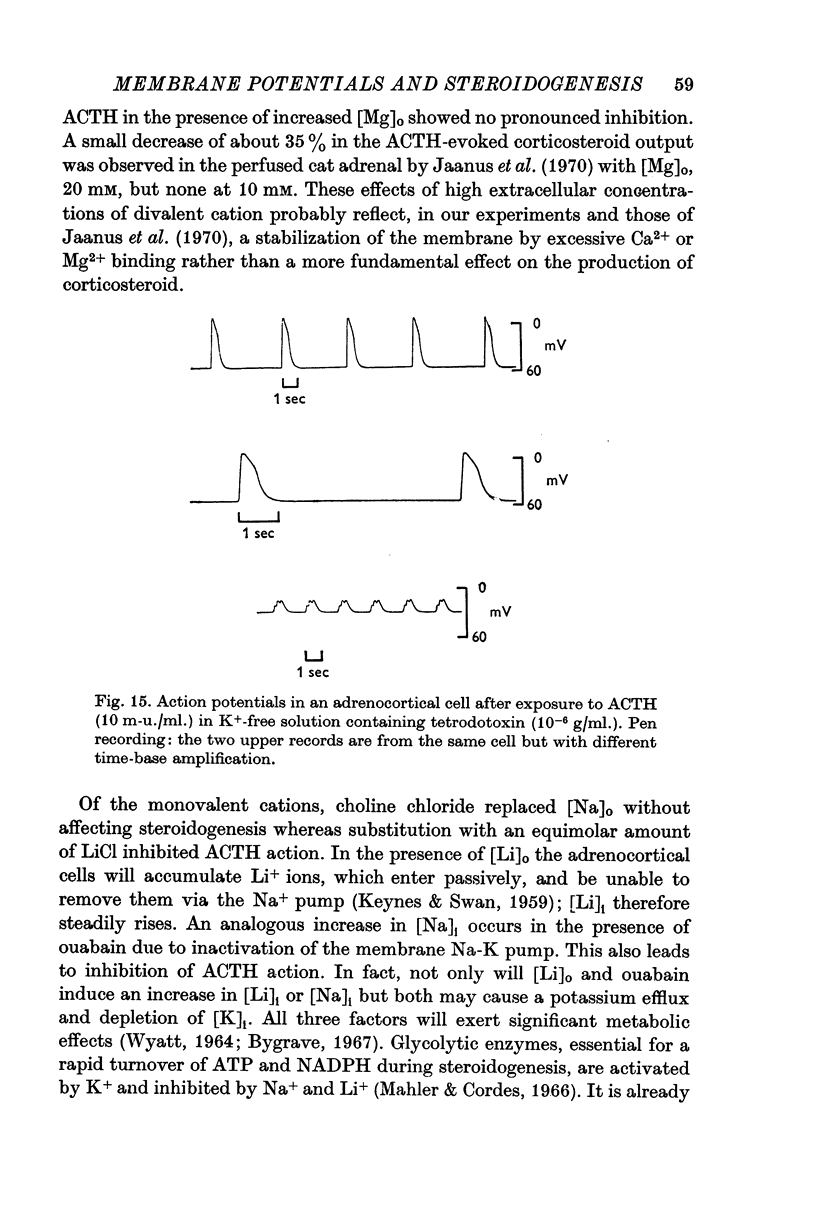
7. Action-potential-like changes in membrane potential appeared in cells exposed to ACTH in a K+-free medium. The amplitude of the action potentials ranged from 10 to 60 mV according to cell, with a frequency up to 36/min; the frequency tended to increase with time. Tetrodotoxin, 10-6 g/ml., did not inhibit ACTH-induced action potentials in K+-free medium.
8. These observations are discussed in relation to the ionic requirements for the steroidogenic action of ACTH. The results further emphasize the dissociation of membrane polarization and the secretion of steroid. The mechanism of output of steroid hormone from the adrenocortical cell may thus differ fundamentally from the secretory mechanisms in other, particle-storing cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELMAN W. J., Jr, MOORE J. W. Action of external divalent ion reduction on sodium movement in the squid giant axon. J Gen Physiol. 1961 Sep;45:93–103. doi: 10.1085/jgp.45.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRMINGHAM M. K., ELLIOTT F. H., VALERE P. H. The need for the presence of calcium for the stimulation in vitro of rat adrenal glands by adrenocorticotrophic hormone. Endocrinology. 1953 Dec;53(6):687–689. doi: 10.1210/endo-53-6-687. [DOI] [PubMed] [Google Scholar]
- Brady A. J., Woodbury J. W. The sodium-potassium hypothesis as the basis of electrical activity in frog ventricle. J Physiol. 1960 Dec;154(2):385–407. doi: 10.1113/jphysiol.1960.sp006586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygrave F. L. The ionic environment and metabolic control. Nature. 1967 May 13;214(5089):667–671. doi: 10.1038/214667a0. [DOI] [PubMed] [Google Scholar]
- Carchman R. A., Jaanus S. D., Rubin R. P. The role of adrenocorticotropin and calcium in adenosine cyclic 3', 5'-phosphate production and steroid release from the isolated, perfused cat adrenal gland. Mol Pharmacol. 1971 Sep;7(5):491–499. [PubMed] [Google Scholar]
- Curry D. L., Bennett L. L., Grodsky G. M. Requirement for calcium ion in insulin secretion by the perfused rat pancreas. Am J Physiol. 1968 Jan;214(1):174–178. doi: 10.1152/ajplegacy.1968.214.1.174. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. CALCIUM MOVEMENT IN THE NEUROHYPOPHYSIS OF THE RAT AND ITS RELATION TO THE RELEASE OF VASOPRESSIN. J Physiol. 1964 Jul;172:19–30. doi: 10.1113/jphysiol.1964.sp007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese R. V. Calcium as a mediator of adrenocorticotrophic hormone action on adrenal protein synthesis. Science. 1971 Jul 30;173(3995):447–450. doi: 10.1126/science.173.3995.447. [DOI] [PubMed] [Google Scholar]
- Friend D. S., Gilula N. B. A distinctive cell contact in the rat adrenal cortex. J Cell Biol. 1972 Apr;53(1):148–163. doi: 10.1083/jcb.53.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The behaviour of the sodium pump in red cells in the absence of external potassium. J Physiol. 1967 Sep;192(1):159–174. doi: 10.1113/jphysiol.1967.sp008294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garren L. D., Gill G. N., Masui H., Walton G. M. On the mechanism of action of ACTH. Recent Prog Horm Res. 1971;27:433–478. doi: 10.1016/b978-0-12-571127-2.50035-3. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith D. G., Butcher R. W., Ney R. L., Sutherland E. W. Adenosine 3',5'-monophosphate as the intracellular mediator of the action of adrenocorticotropic hormone on the adrenal cortex. J Biol Chem. 1967 Dec 10;242(23):5535–5541. [PubMed] [Google Scholar]
- HOLZBAUER M. The corticosterone content of rat adrenals under different experimental conditions. J Physiol. 1957 Dec 3;139(2):294–305. doi: 10.1113/jphysiol.1957.sp005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C. N., Milner R. D. Cations and the secretion of insulin from rabbit pancreas in vitro. J Physiol. 1968 Nov;199(1):177–187. doi: 10.1113/jphysiol.1968.sp008647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A. M., Macintosh F. C. Calcium and synaptic transmission in a sympathetic ganglion. J Physiol. 1940 Jan 15;97(3):408–416. doi: 10.1113/jphysiol.1940.sp003818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokin L. E. Effects of calcium omission on acetylcholine-stimulated amylase secretion and phospholipid synthesis in pigeon pancreas slices. Biochim Biophys Acta. 1966 Jan 25;115(1):219–221. doi: 10.1016/0304-4165(66)90066-3. [DOI] [PubMed] [Google Scholar]
- Jaanus S. D., Rosenstein M. J., Rubin R. P. On the mode of action of ACTH on the isolated perfused adrenal gland. J Physiol. 1970 Aug;209(3):539–556. doi: 10.1113/jphysiol.1970.sp009178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The permeability of frog muscle fibres to lithium ions. J Physiol. 1959 Oct;147:626–638. doi: 10.1113/jphysiol.1959.sp006265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev. 1966 Jun;18(2):997–1049. [PubMed] [Google Scholar]
- Katz B., Miledi R. The timing of calcium action during neuromuscular transmission. J Physiol. 1967 Apr;189(3):535–544. doi: 10.1113/jphysiol.1967.sp008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Misu Y. Release of noradrenaline by splenic nerve stimulation and its dependence on calcium. J Physiol. 1967 Jan;188(2):219–234. doi: 10.1113/jphysiol.1967.sp008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwant W. O., Seeman P. The displacement of membrane calcium by a local anesthetic (chlorpromazine). Biochim Biophys Acta. 1969;193(2):338–349. doi: 10.1016/0005-2736(69)90194-1. [DOI] [PubMed] [Google Scholar]
- MAIZELS M. Calcium ions and the permeability of human red cells. Nature. 1959 Aug 1;184(Suppl 6):366–366. doi: 10.1038/184366a0. [DOI] [PubMed] [Google Scholar]
- MORRILL G. A., KABACK H. R., ROBBINS E. EFFECT OF CALCIUM ON INTRACELLULAR SODIUM AND POTASSIUM CONCENTRATIONS IN PLANT AND ANIMAL CELLS. Nature. 1964 Nov 14;204:641–642. doi: 10.1038/204641a0. [DOI] [PubMed] [Google Scholar]
- Matthews E. K. Membrane potential measurement in cells of the adrenal gland. J Physiol. 1967 Mar;189(1):139–148. doi: 10.1113/jphysiol.1967.sp008159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K., Saffran M. Effect of ACTH on the electrical properties of adrenocortical cells. Nature. 1968 Sep 28;219(5161):1369–1370. doi: 10.1038/2191369a0. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Saffran M. Steroid production and membrane potential measurement in cells of the adrenal cortex. J Physiol. 1967 Mar;189(1):149–161. doi: 10.1113/jphysiol.1967.sp008160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERON F. G., KORITZ S. B. On the exogenous requirements for the action of ACTH in vitro on rat adrenal glands. J Biol Chem. 1958 Jul;233(1):256–259. [PubMed] [Google Scholar]
- SKOU J. C. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957 Feb;23(2):394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]
- Saffran M., Ford P., Matthews E. K., Kraml M., Garbaczewska L. Automated method for following the production of corticosteroids by adrenal tissue in vitro. Can J Biochem. 1967 Dec;45(12):1901–1907. doi: 10.1139/o67-223. [DOI] [PubMed] [Google Scholar]
- Samli M. H., Geschwind I. I. Some effects of energy-transfer inhibitors and of Ca++-free or K+-enhanced media on the release of luteinizing hormone (LH) from the rat pituitary gland in vitro. Endocrinology. 1968 Feb;82(2):225–231. doi: 10.1210/endo-82-2-225. [DOI] [PubMed] [Google Scholar]
- Seeman P. M. Membrane stabilization by drugs: tranquilizers, steroids, and anesthetics. Int Rev Neurobiol. 1966;9:145–221. doi: 10.1016/s0074-7742(08)60138-5. [DOI] [PubMed] [Google Scholar]
- Vale W., Burgus R., Guillemin R. Presence of calcium ions as a requisite for the in vitro stimulation of TSH-release by hypothalamic TRF. Experientia. 1967 Oct 15;23(10):853–855. doi: 10.1007/BF02146887. [DOI] [PubMed] [Google Scholar]
- Vale W., Guillemin R. Potassium-induced stimulation of thyrotropin release in vitro. Requirement for presence of calcium and inhibition by thyroxine. Experientia. 1967 Oct 15;23(10):855–857. doi: 10.1007/BF02146888. [DOI] [PubMed] [Google Scholar]
- Van Breemen D., Van Breemen C. Calcium exchange diffusion in a porous phospholipid ion-exchange membrane. Nature. 1969 Aug 30;223(5209):898–900. doi: 10.1038/223898a0. [DOI] [PubMed] [Google Scholar]
- Vogt M. The output of cortical hormone by the mammalian suprarenal. J Physiol. 1943 Dec 31;102(3):341–356. doi: 10.1113/jphysiol.1943.sp004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J., Jones A. B. Inhibition of hormone-sensitive adenyl cyclase by phenothiazines. Proc Natl Acad Sci U S A. 1970 Feb;65(2):454–459. doi: 10.1073/pnas.65.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt H. V. Cations, enzymes and control of cell metabolism. J Theor Biol. 1964 May;6(3):441–470. doi: 10.1016/0022-5193(64)90058-x. [DOI] [PubMed] [Google Scholar]
- van Breemen C., De Weer P. Lanthanum inhibition of 45Ca efflux from the squid giant axon. Nature. 1970 May 23;226(5247):760–761. doi: 10.1038/226760a0. [DOI] [PubMed] [Google Scholar]


