Abstract
1. The objective was to discover whether the extraction of sugars and iodide from the perfused cerebral ventricles is Na+-dependent.
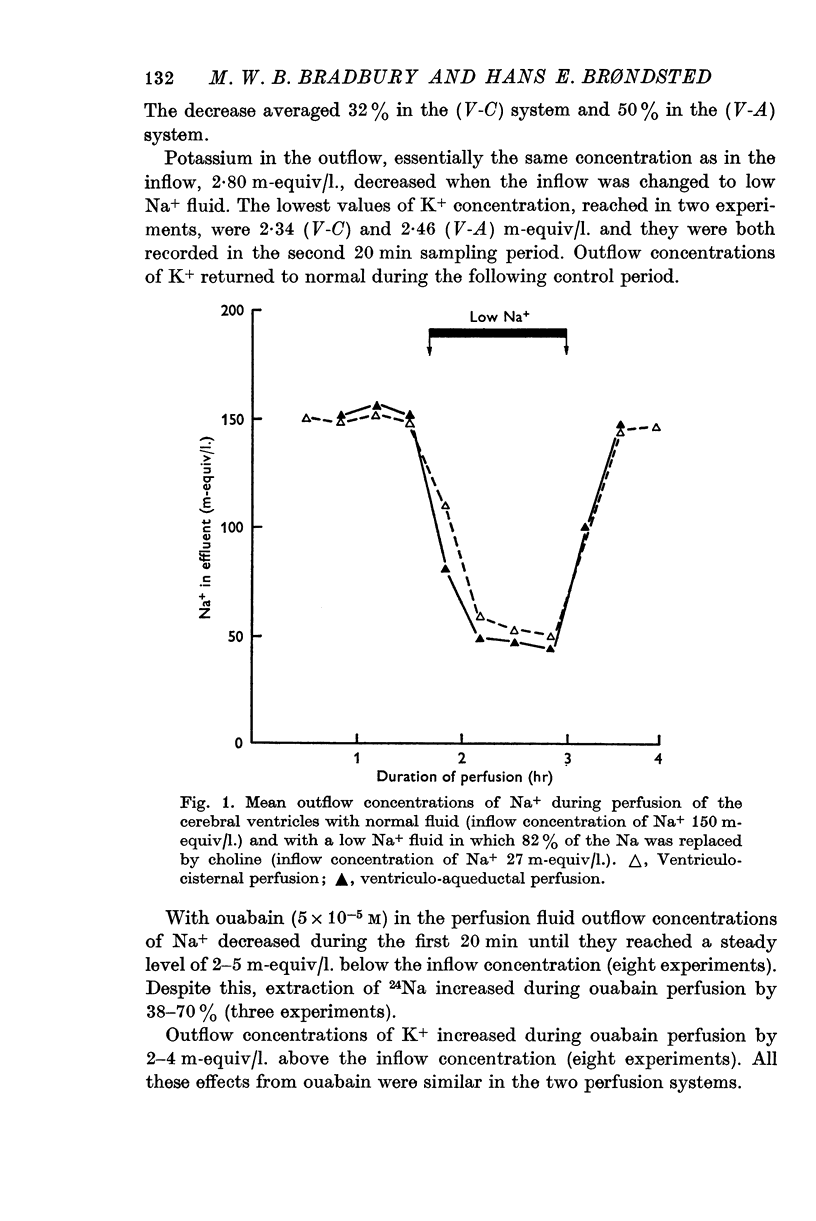
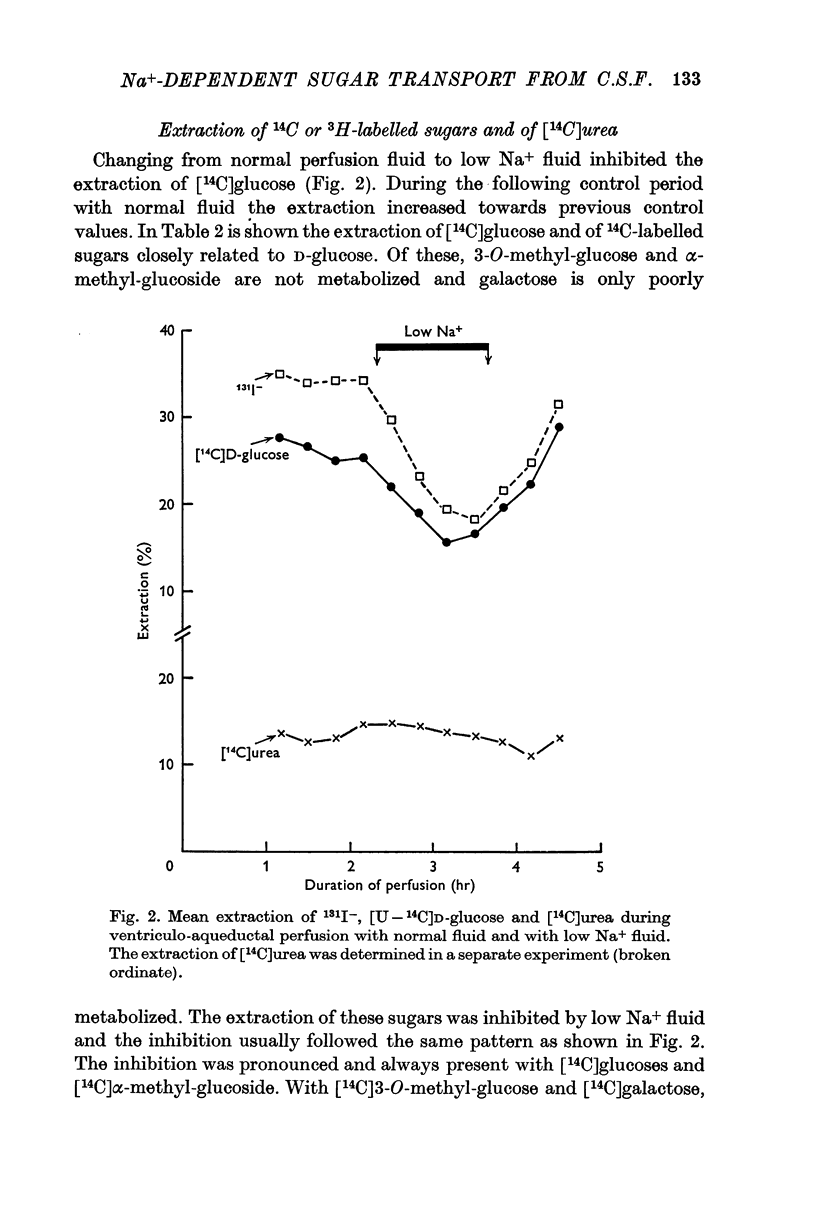
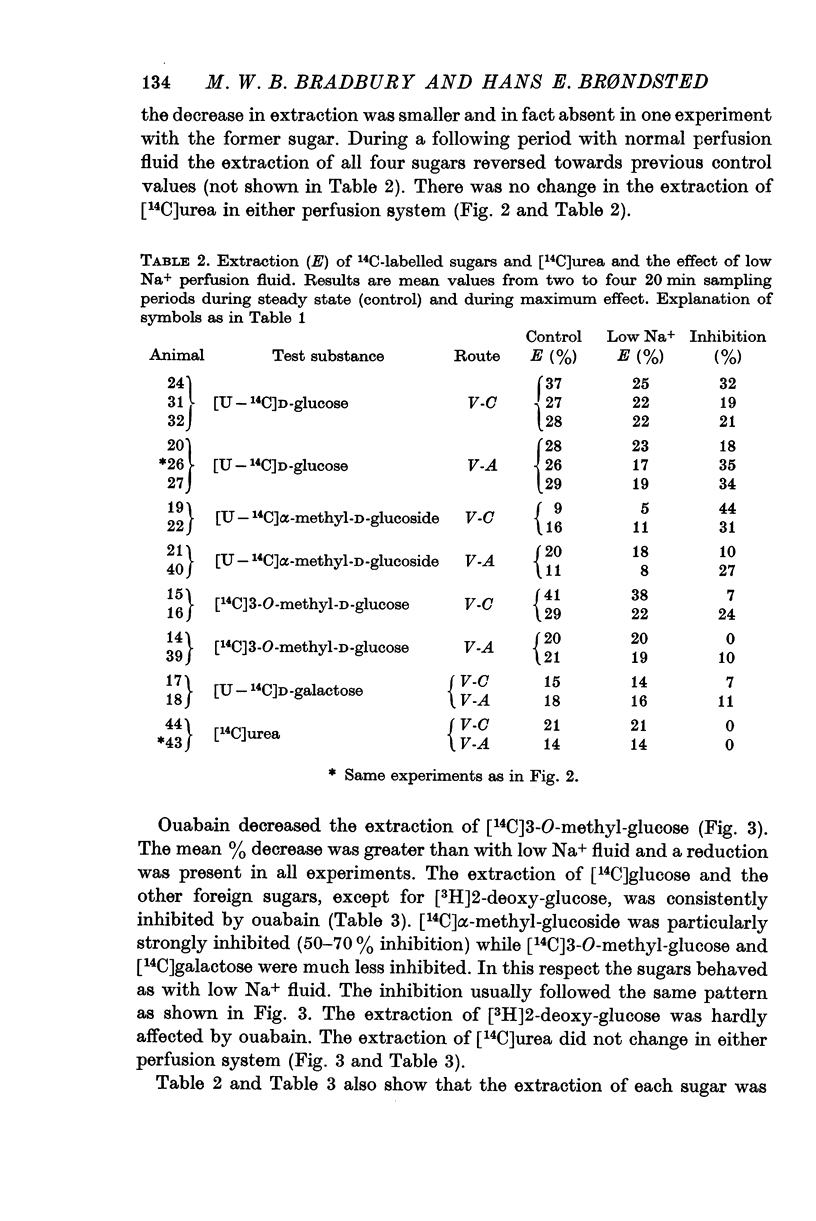
2. In the ventriculo-aqueductal and ventriculo-cisternal perfusion systems in the rabbit the extraction of 14C-labelled D-hexoses (glucose, 3-O-methyl-glucose, α-methyl-glucoside and galactose), 131I- and 24Na was inhibited when 82% of the Na+ in the perfusion fluid was replaced by choline. The extraction returned to control levels when the Na+ concentration in the perfusion fluid was returned to normal.
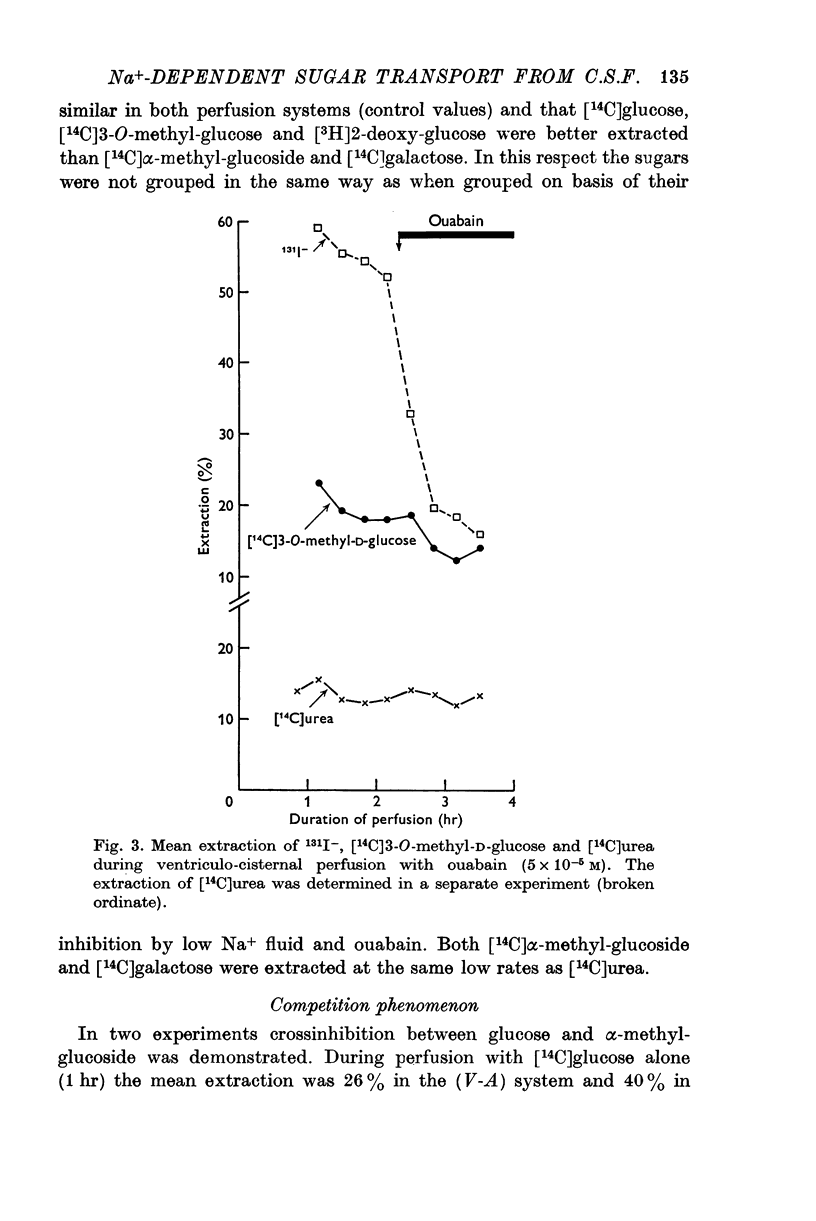
3. Ouabain, 5 × 10-5 M in the perfusion fluid inhibited the extraction of the above 14C sugars and 131I-, but hardly affected that of [3H]2-deoxy-D-glucose. It enhanced the extraction of 24Na. C.s.f. production was usually totally inhibited.
4. The extraction of [14C]urea remained unchanged during perfusion with low Na+ fluid or ouabain.
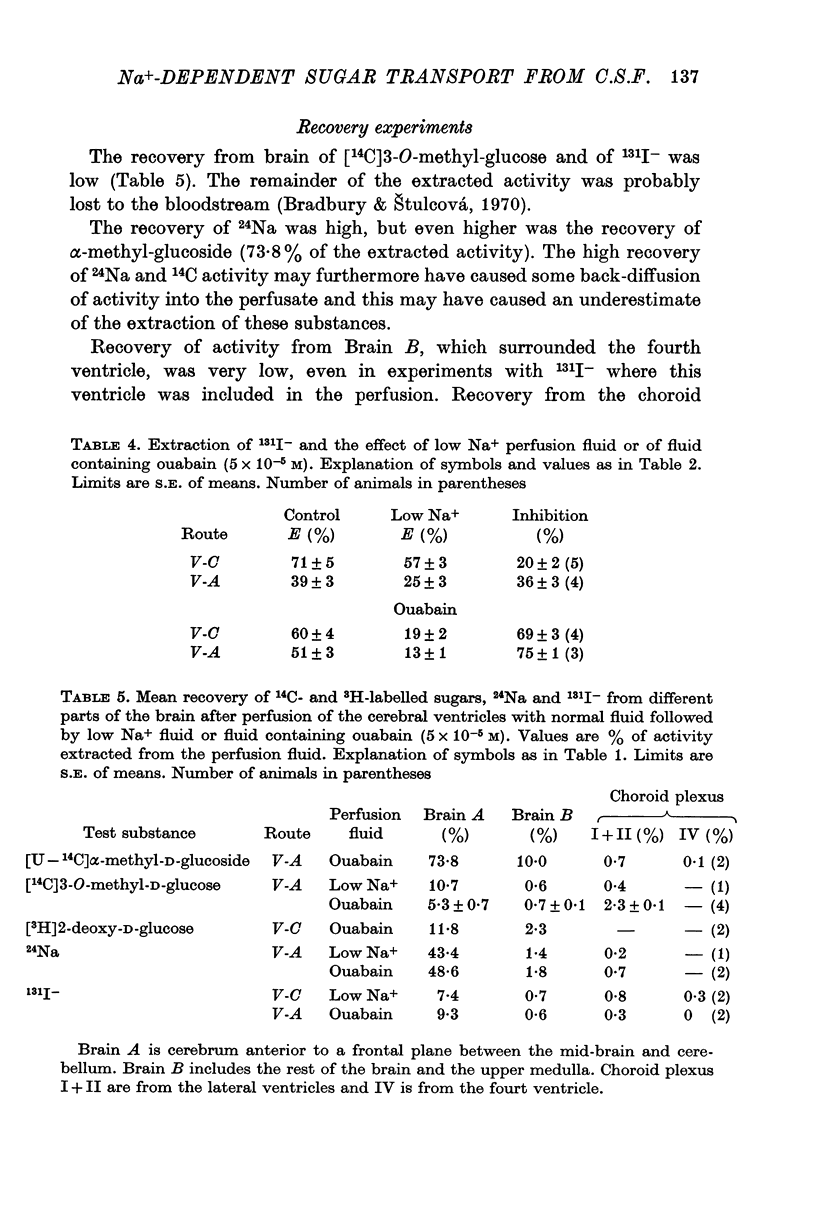
5. Recovery from brain of [14C]3-O-methyl-glucose, [3H]2-deoxy-glucose and 131I- was low while recovery of [14C]α-methyl-glucoside and 24Na was high. On an equal weight basis recovery of [14C]3-O-methyl-glucose was about twelve times higher from the choroid plexus than from the brain.
6. Part of the movement of 14C sugars may be explained on basis of a Na+-gradient hypothesis with involvement of the Na+ pump at the blood—c.s.f. or blood—brain barriers.
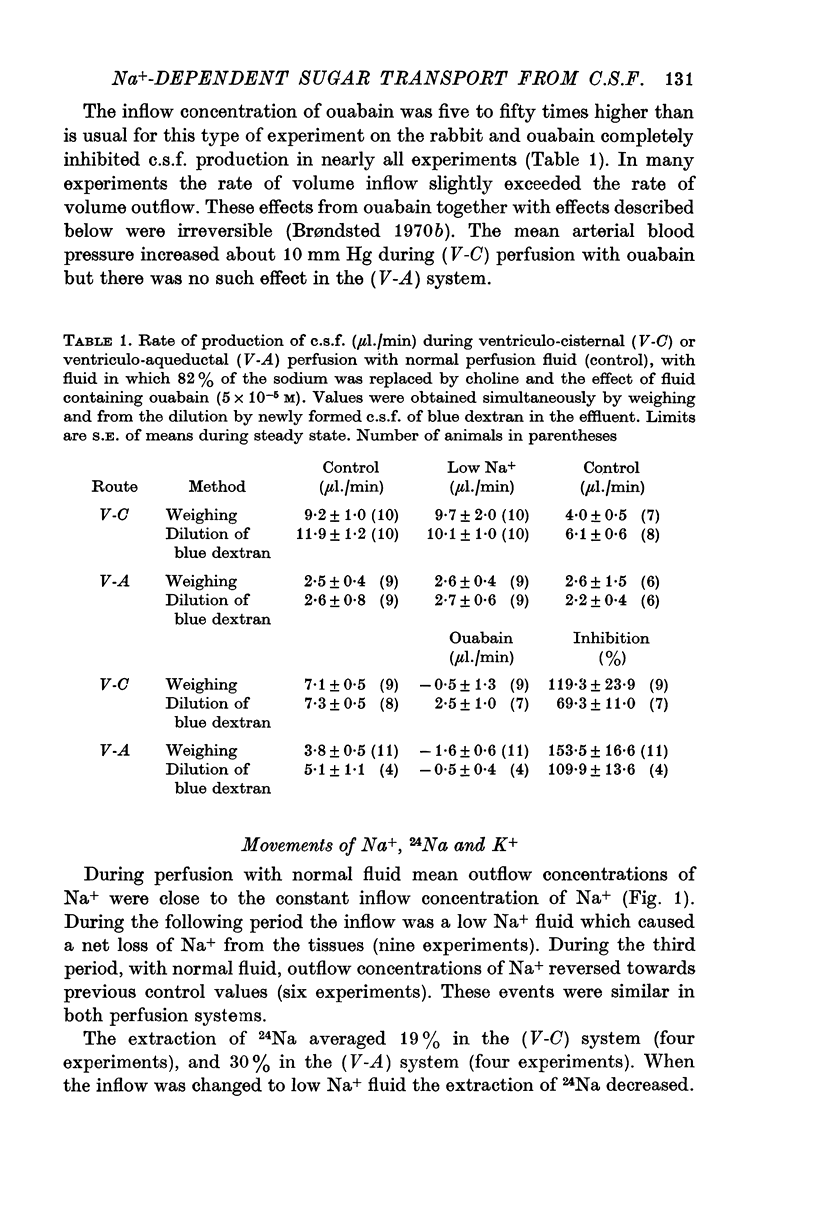
7. The rate of c.s.f. production from the first three ventricles comprised about 40% of the rate from all four ventricles. The extraction of sugars, urea and cations was similar in both perfusion systems while the extraction of 131I- was higher in the ventriculo-cisternal system than in the ventriculo-aqueductal system.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER W. D., WOLFF J. CATION REQUIREMENTS FOR IODIDE TRANSPORT. Arch Biochem Biophys. 1964 Jul 20;106:525–526. doi: 10.1016/0003-9861(64)90224-3. [DOI] [PubMed] [Google Scholar]
- Ahmed N., Van Harreveld A. The iodide space in rabbit brain. J Physiol. 1969 Sep;204(1):31–50. doi: 10.1113/jphysiol.1969.sp008896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRY R. J., DIKSTEIN S., MATTHEWS J., SMYTH D. H., WRIGHT E. M. ELECTRICAL POTENTIALS ASSOCIATED WITH INTESTINAL SUGAR TRANSFER. J Physiol. 1964 Jun;171:316–338. doi: 10.1113/jphysiol.1964.sp007379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADBURY M. W., DAVSON H. THE TRANSPORT OF UREA, CREATININE AND CERTAIN MONOSACCHARIDES BETWEEN BLOOD AND FLUID PERFUSING THE CEREBRAL VENTRICULAR SYSTEM OF RABBITS. J Physiol. 1964 Jan;170:195–211. doi: 10.1113/jphysiol.1964.sp007323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelard H. S. Specificity and kinetic properties of monosaccharide uptake into guinea pig cerebral cortex in vitro. J Neurochem. 1971 Feb;18(2):213–222. doi: 10.1111/j.1471-4159.1971.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Bradbury M. W., Stulcová B. Efflux mechanism contributing to the stability of the potassium concentration in cerebrospinal fluid. J Physiol. 1970 Jun;208(2):415–430. doi: 10.1113/jphysiol.1970.sp009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondsted H. E., Crowder J. Ventriculo-aqueductal perfusion in rabbits. J Physiol. 1972 Sep;225(2):13P–15P. [PubMed] [Google Scholar]
- Brondsted H. E. Ouabain-sensitive carrier-mediated transport of glucose from the cerebral ventricles to surrounding tissues in the cat. J Physiol. 1970 May;208(1):187–201. doi: 10.1113/jphysiol.1970.sp009113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSAKY T. Z., RIGOR B. M., Sr A CONCENTRATIVE MECHANISM FOR SUGARS IN THE CHOROID PLEXUS. Life Sci. 1964 Sep;3:931–936. doi: 10.1016/0024-3205(64)90101-8. [DOI] [PubMed] [Google Scholar]
- Crone C. Facilitated transfer of glucose from blood into brain tissue. J Physiol. 1965 Nov;181(1):103–113. doi: 10.1113/jphysiol.1965.sp007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVSON H., POLLAY M. Influence of various drugs on the transport of 131-I and PAH across the cerebrospinal-fluid-blood barrier. J Physiol. 1963 Jul;167:239–246. doi: 10.1113/jphysiol.1963.sp007144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelberg E., Fishman J., Hams M. L. Penetration of sugars across the blood-brain barrier. J Physiol. 1967 Jul;191(1):47–57. doi: 10.1113/jphysiol.1967.sp008236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochwald G. M., Sahar A. Effect of spinal fluid pressure on cerebrospinal fluid formation. Exp Neurol. 1971 Jul;32(1):30–40. doi: 10.1016/0014-4886(71)90162-2. [DOI] [PubMed] [Google Scholar]
- IFF H. W., WILBRANDT W. DIE ABHAENGIGKEIT DER JODAKKUMULATION IN SCHILDDRUESENSCHNITTEN VON DER IONALEN ZUSAMMENSETZUNG DES INKUBATIONSMEDIUMS UND IHRE BEEINFLUSSUNG DURCH HERZGLYKOSIDE. Biochim Biophys Acta. 1963 Dec 13;78:711–725. doi: 10.1016/0006-3002(63)91037-0. [DOI] [PubMed] [Google Scholar]
- Kleinzeller A., Kolínská J., Benes I. Transport of monosaccharides in kidney-cortex cells. Biochem J. 1967 Sep;104(3):852–860. doi: 10.1042/bj1040852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFEVRE P. G., MARSHALL J. K. Conformational specificity in a biological sugar transport system. Am J Physiol. 1958 Aug;194(2):333–337. doi: 10.1152/ajplegacy.1958.194.2.333. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol. 1971 Dec;221(6):1629–1639. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H., Davson H. Brain extracellular space and the sink action of cerebrospinal fluid. Measurement of rabbit brain extracellular space using sucrose labeled with carbon 14. Arch Neurol. 1967 Aug;17(2):196–205. doi: 10.1001/archneur.1967.00470260086010. [DOI] [PubMed] [Google Scholar]
- POLLAY M., DAVSON H. The passage of certain substances out of the cerebrosphinal fluid. Brain. 1963 Mar;86:137–150. doi: 10.1093/brain/86.1.137. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]


