Abstract
1. Hyperpolarizing responses up to 30 mV in amplitude were recorded from cones and from certain cells believed to be rods in the isolated retina of the swamp turtle, Pseudemys scripta elegans.
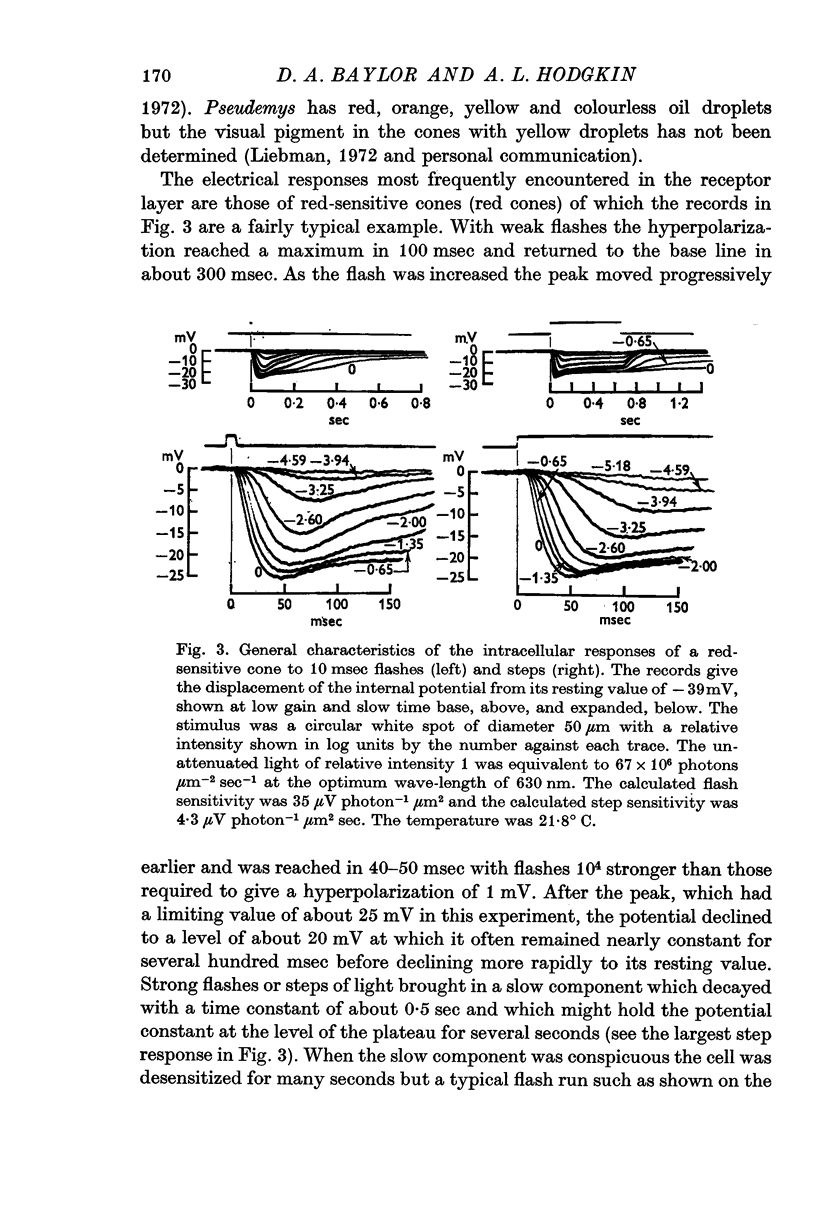
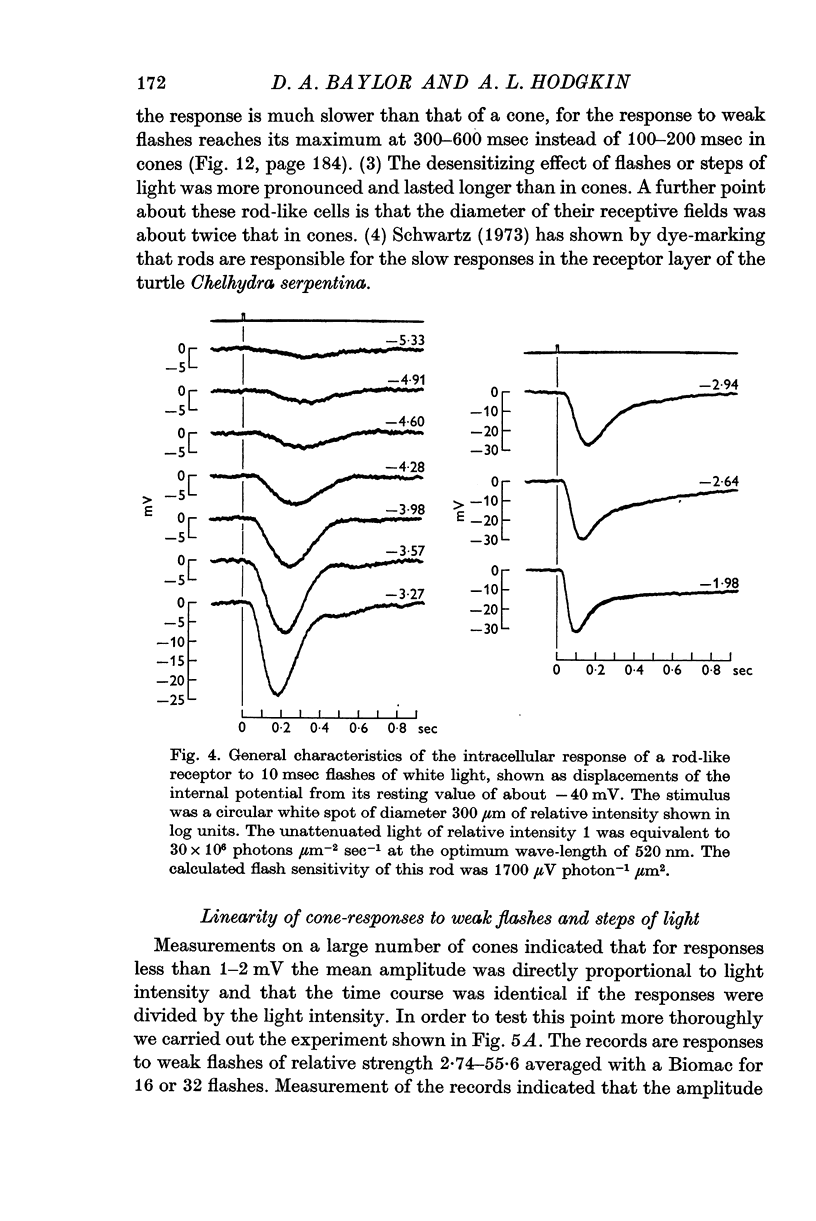
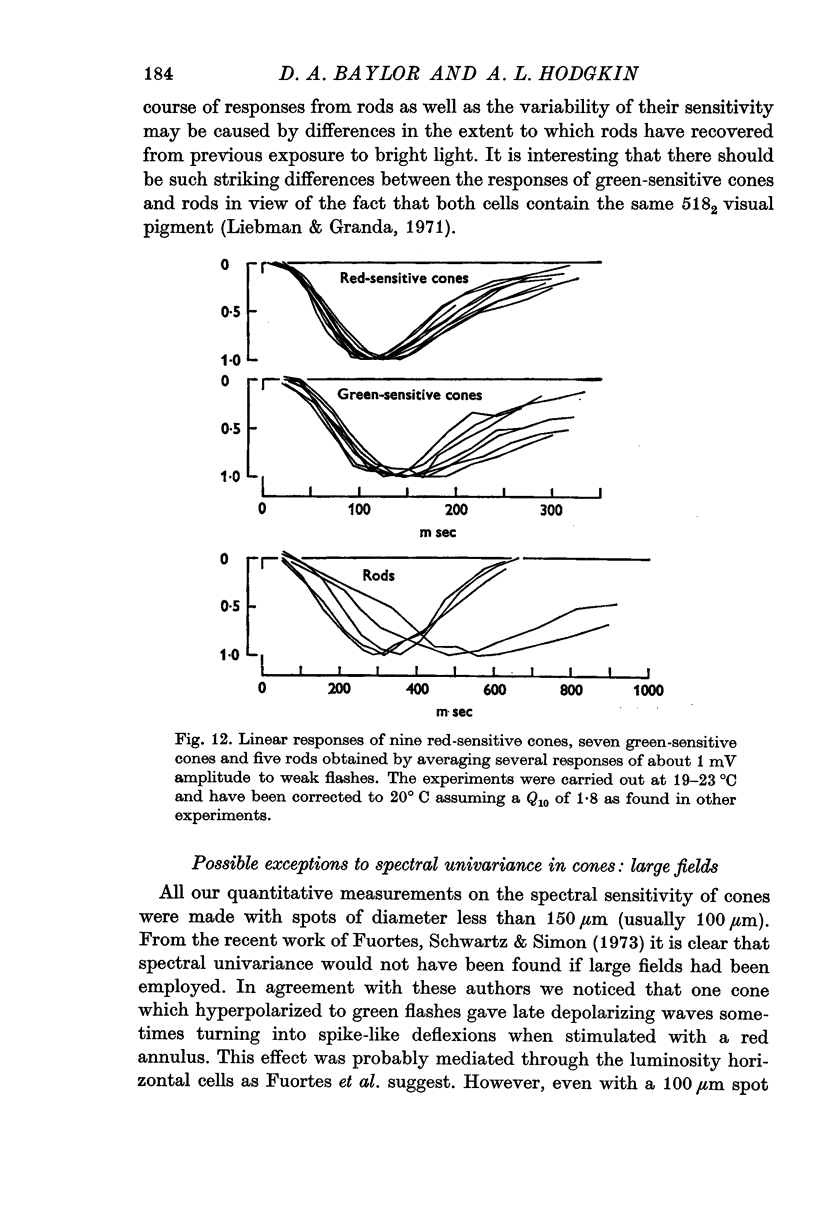
2. The responses evoked by weak flashes of light reach their maximum in 100-140 msec in red-sensitive cones, 140-180 msec in green-sensitive cones, and 300-600 msec in the rod-like cells (20° C).
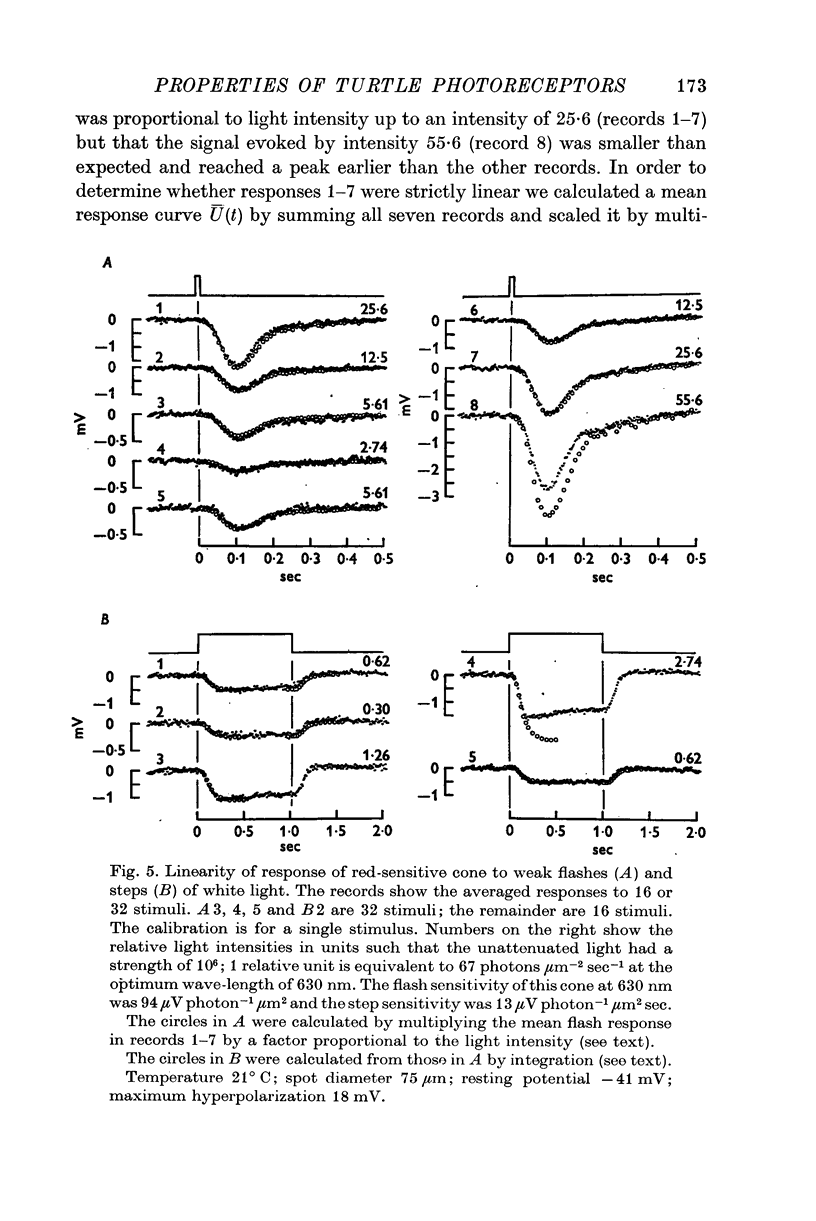
3. The cone response evoked by weak flashes of light is linearly related to light intensity and obeys the superposition principle in that the response to a very weak step of light is the integral of the response to a very weak flash.
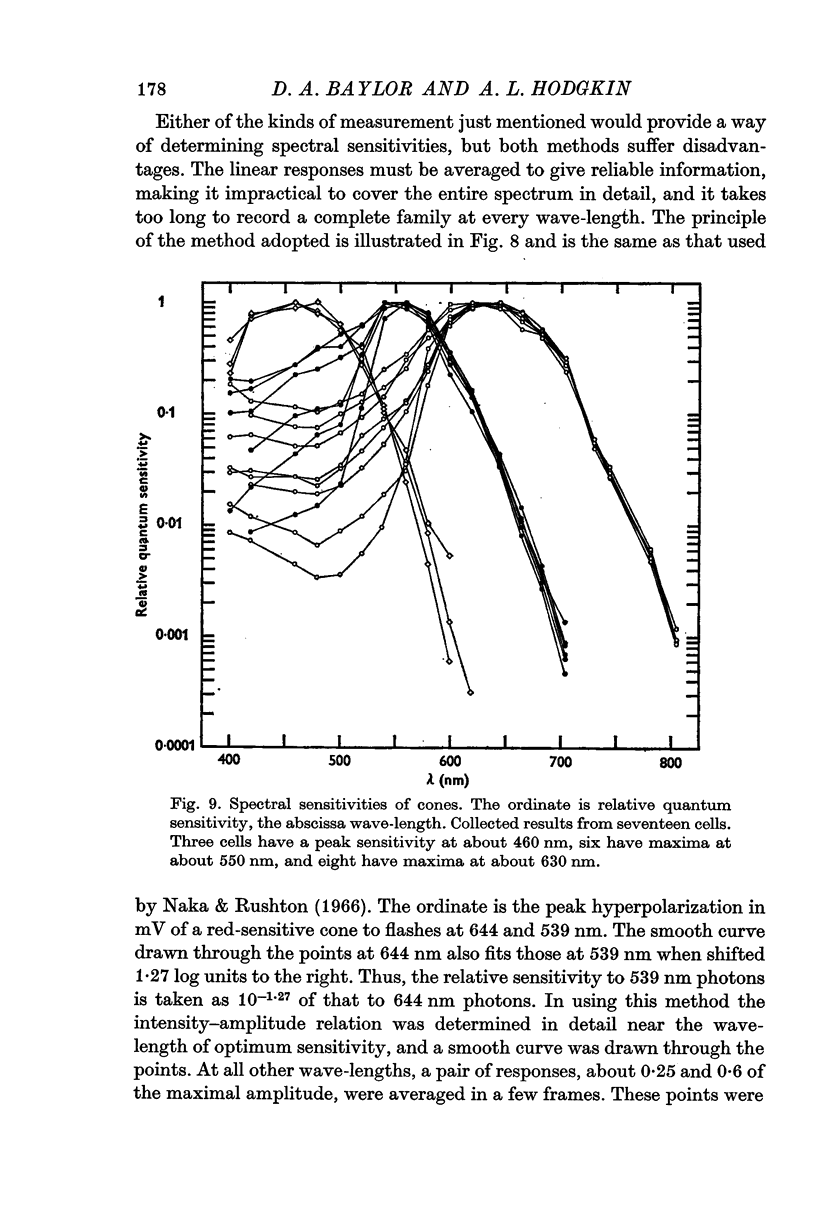
4. On the basis of their spectral sensitivities cones can be divided into three distinct classes, namely red-sensitive cones whose relative quantum sensitivity is maximal at 630 nm, green-sensitive cones with a maximal sensitivity at 550 nm and blue-sensitive cones with a maximum at 460 nm.
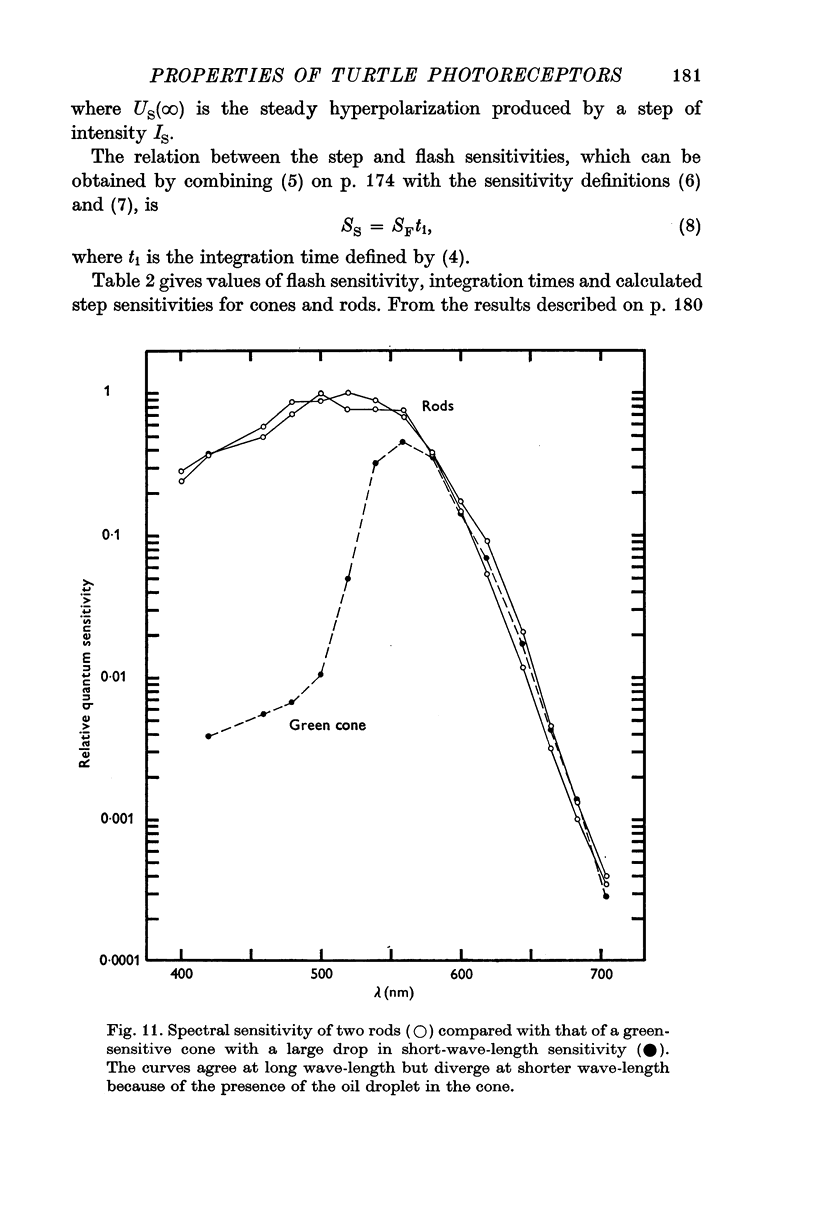
5. The difference between the spectral sensitivity of rods with a maximum at about 520 nm and green-sensitive cones (λmax = 550 nm) is consistent with the view that both receptors contain a 5182 retinal pigment as reported by Liebman & Granda, but that light is filtered by an orange oil droplet in green-sensitive cones.
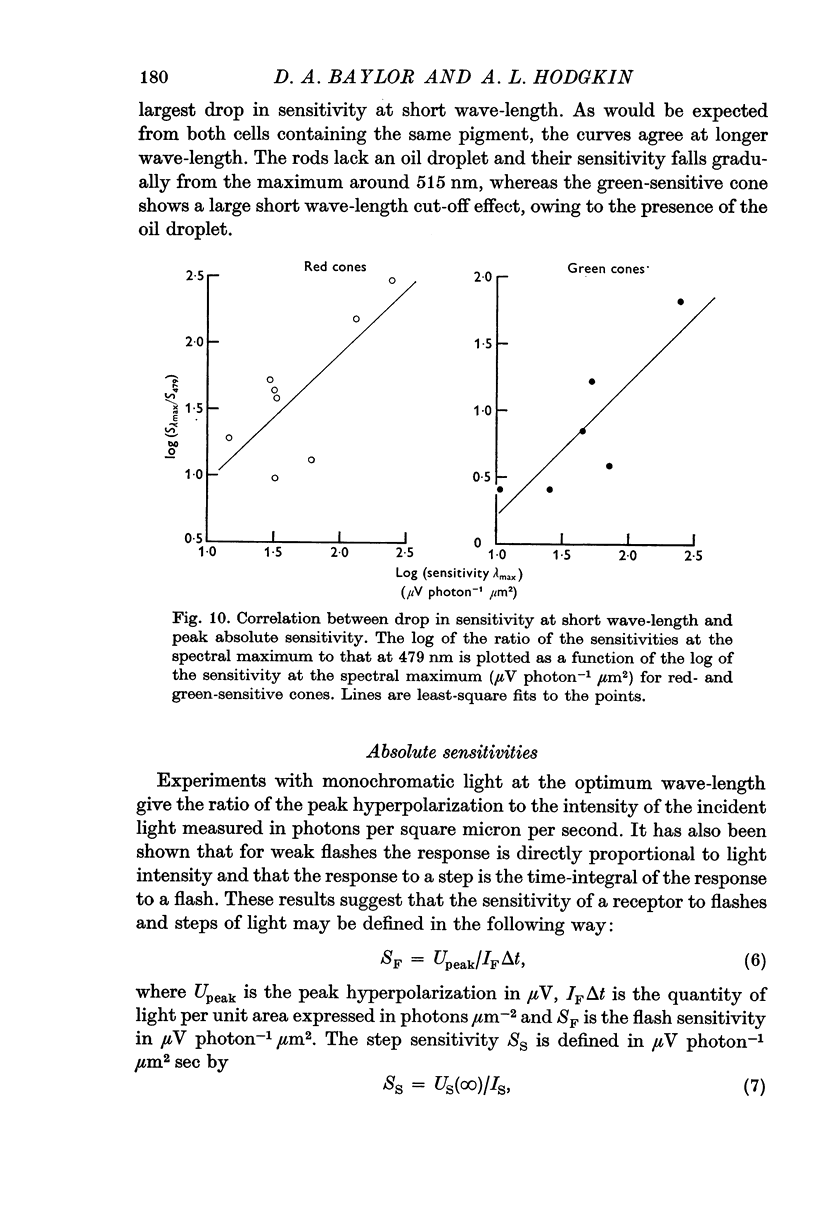
6. The spectral sensitivities of both red- and green-sensitive cones agree well amongst themselves at long wave-lengths but differ markedly in the extent of the reduction at short wave-lengths. This variation is attributed to differences in the extent to which light is filtered through the coloured oil droplets.
7. There is a significant positive correlation between the absolute sensitivity of red- and green-sensitive cones and the reduction in sensitivity at short wave-lengths. This would be explained if a greater fraction of the light passes through the oil droplet in the most sensitive cells.
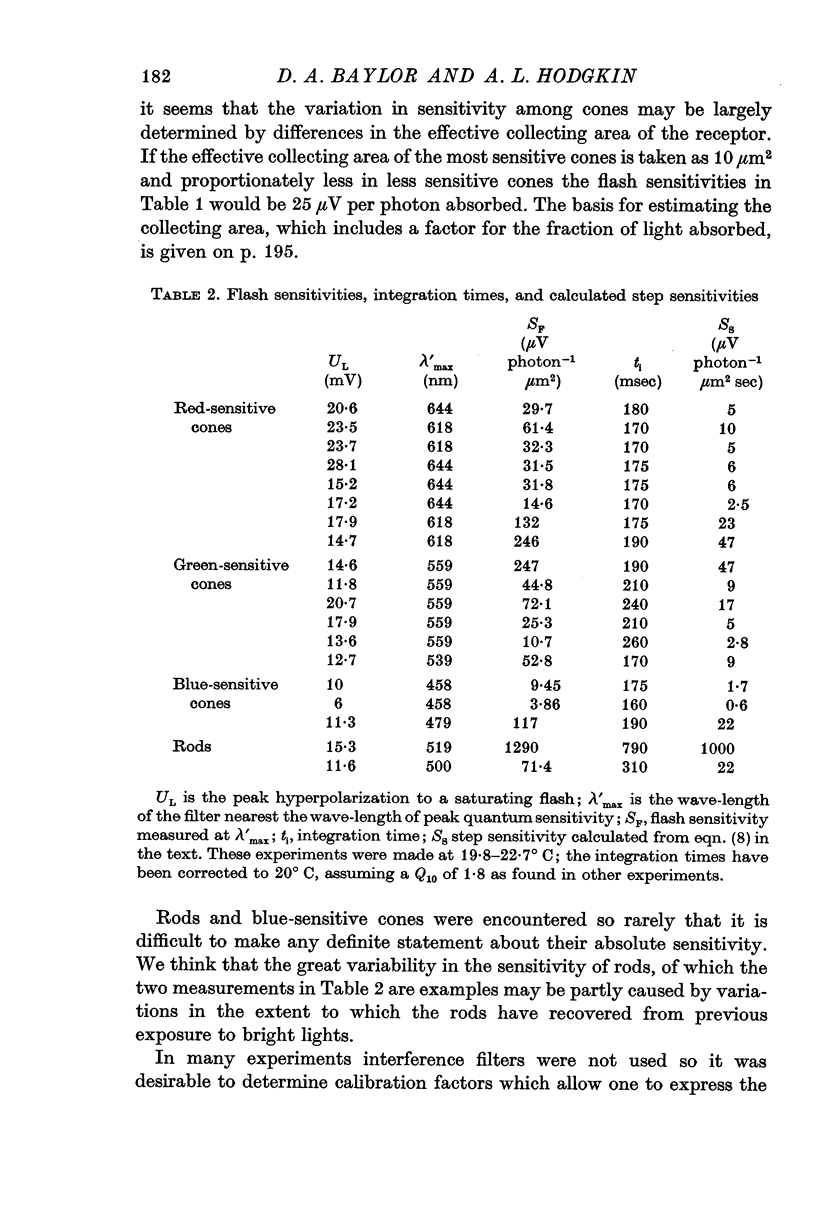
8. The absolute flash sensitivities of the most sensitive receptors were about 250 μV photon-1 μm2 in red- and green-sensitive cones, 120 μV photon-1 μm2 in blue-sensitive cones, and 1300 μV photon-1 μm2 in rods.
9. If the effective collecting area (which includes factors for absorption etc.) is taken as 10 μm2 in a red-sensitive cone the peak hyperpolarization produced by 1 photon would average 25 μV.
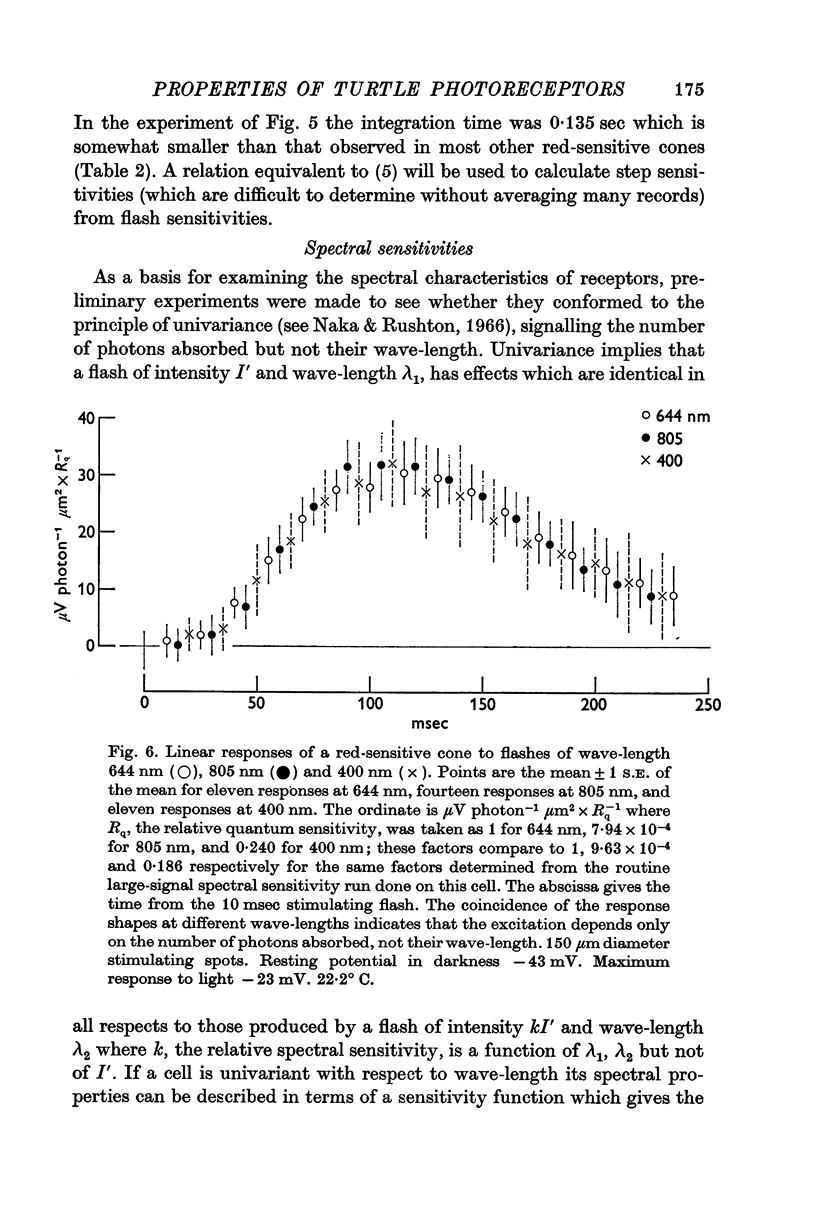
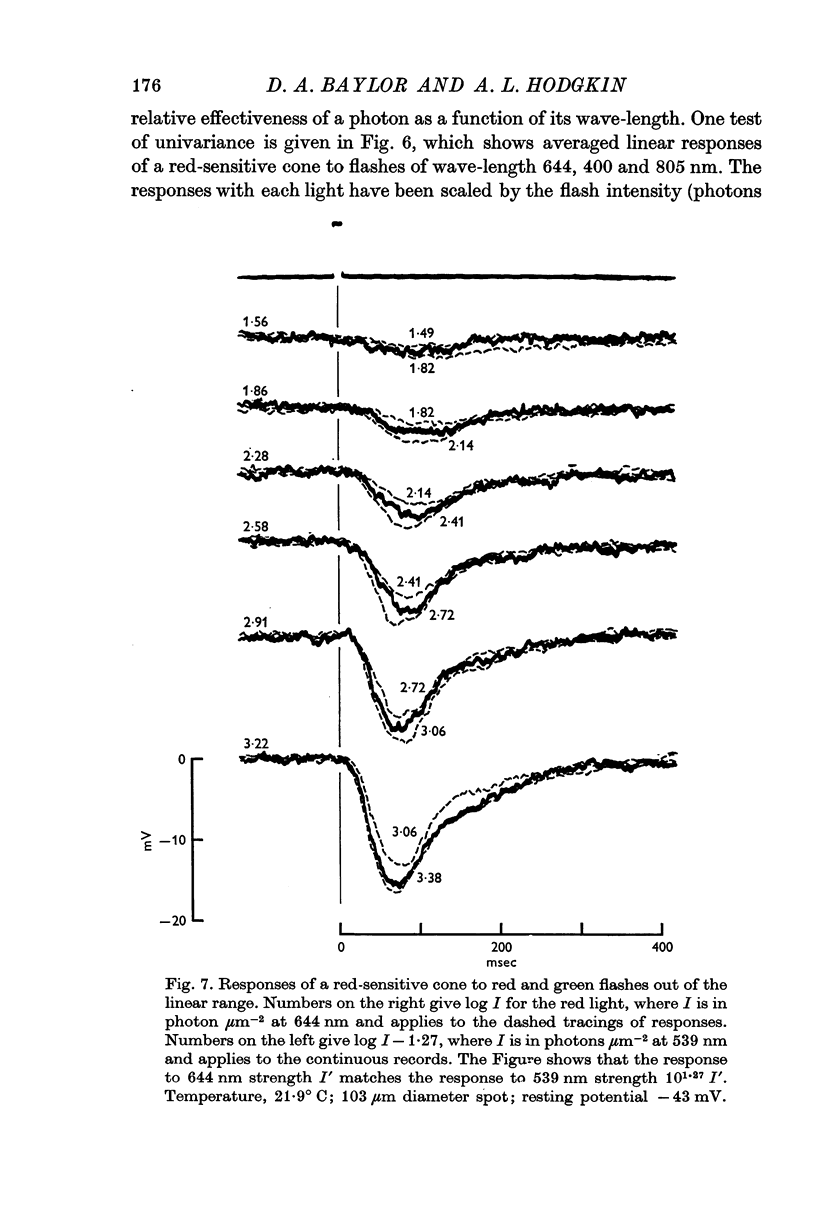
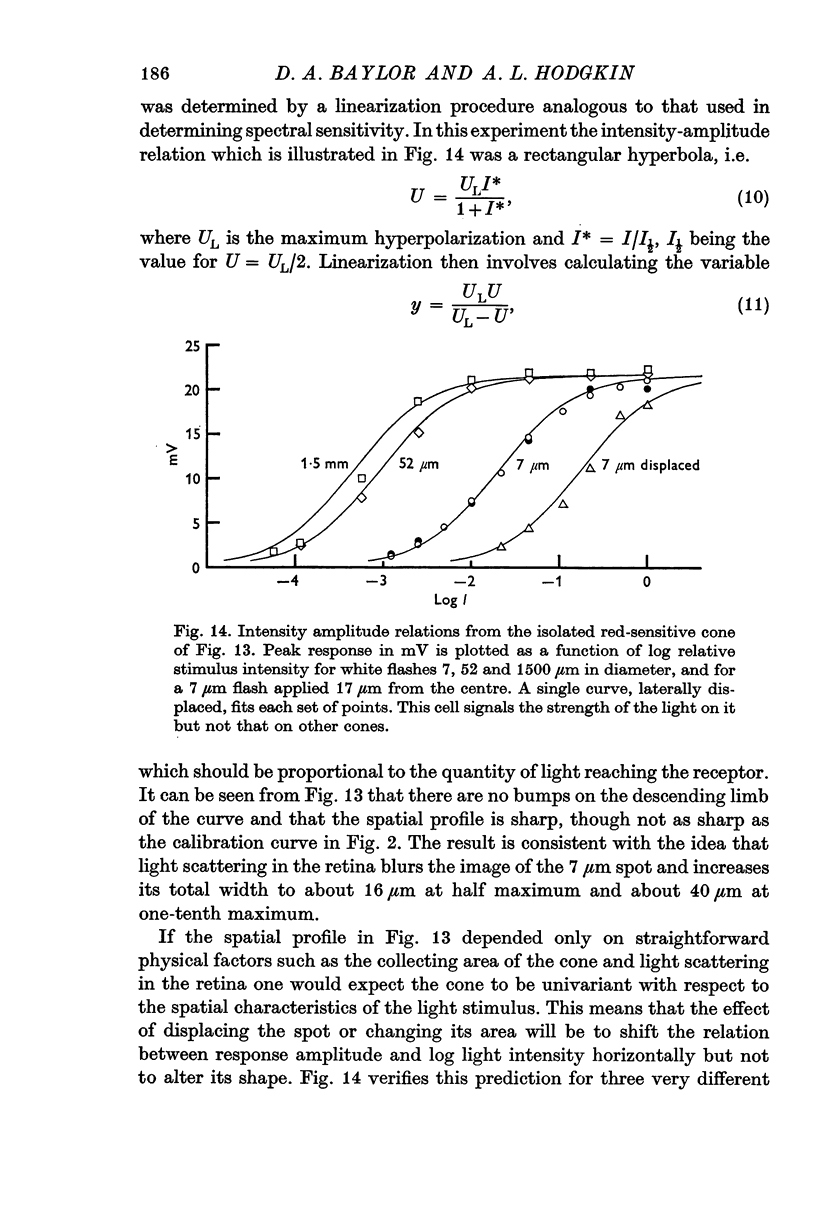
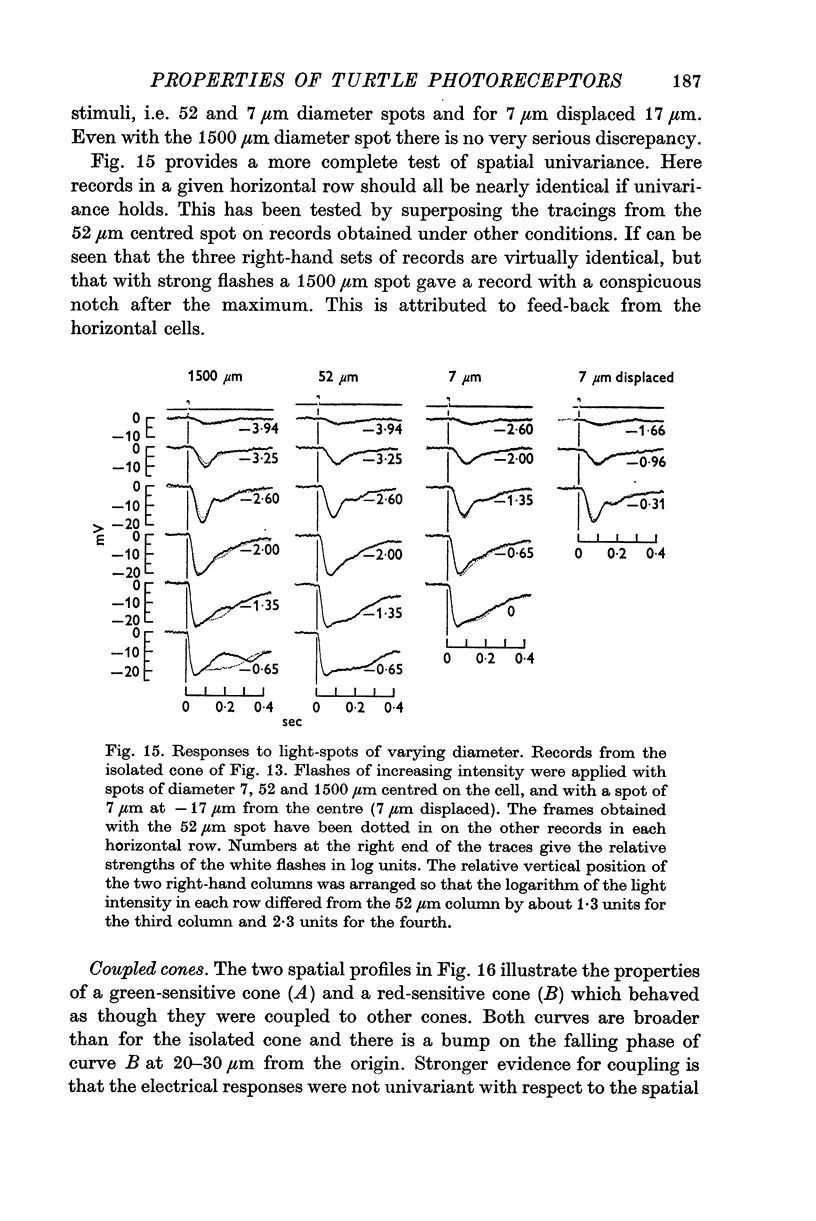
10. Provided that small spots of light are used, individual receptors obey the `univariance principle' and the response produced by light of strength I′, and wave-length λ1 can be matched by a light of strength kI′ and wave-length λ2, where k is the same for all values of I′.
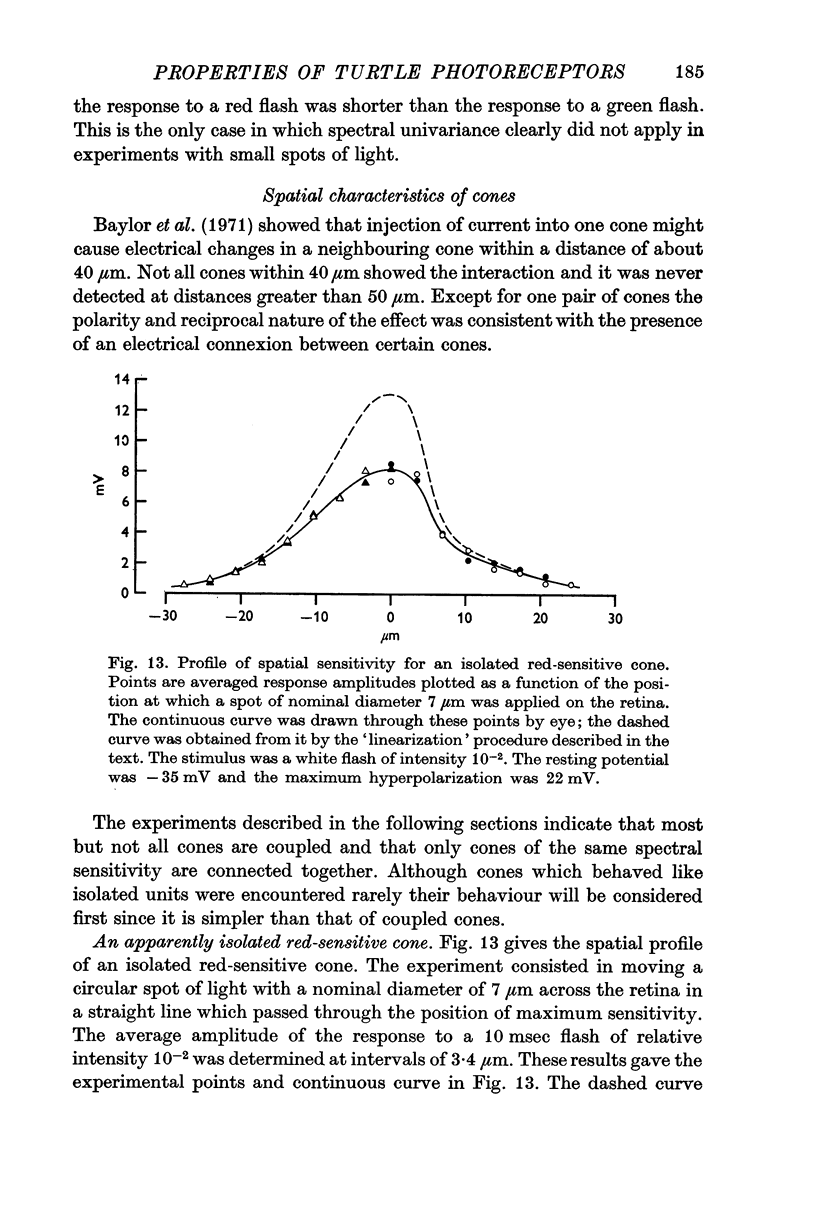
11. A small proportion of cones behave like isolated units in that they have very sharp sensitivity-profiles and obey the univariance principle with respect to the position as well as to the wave-length of light.
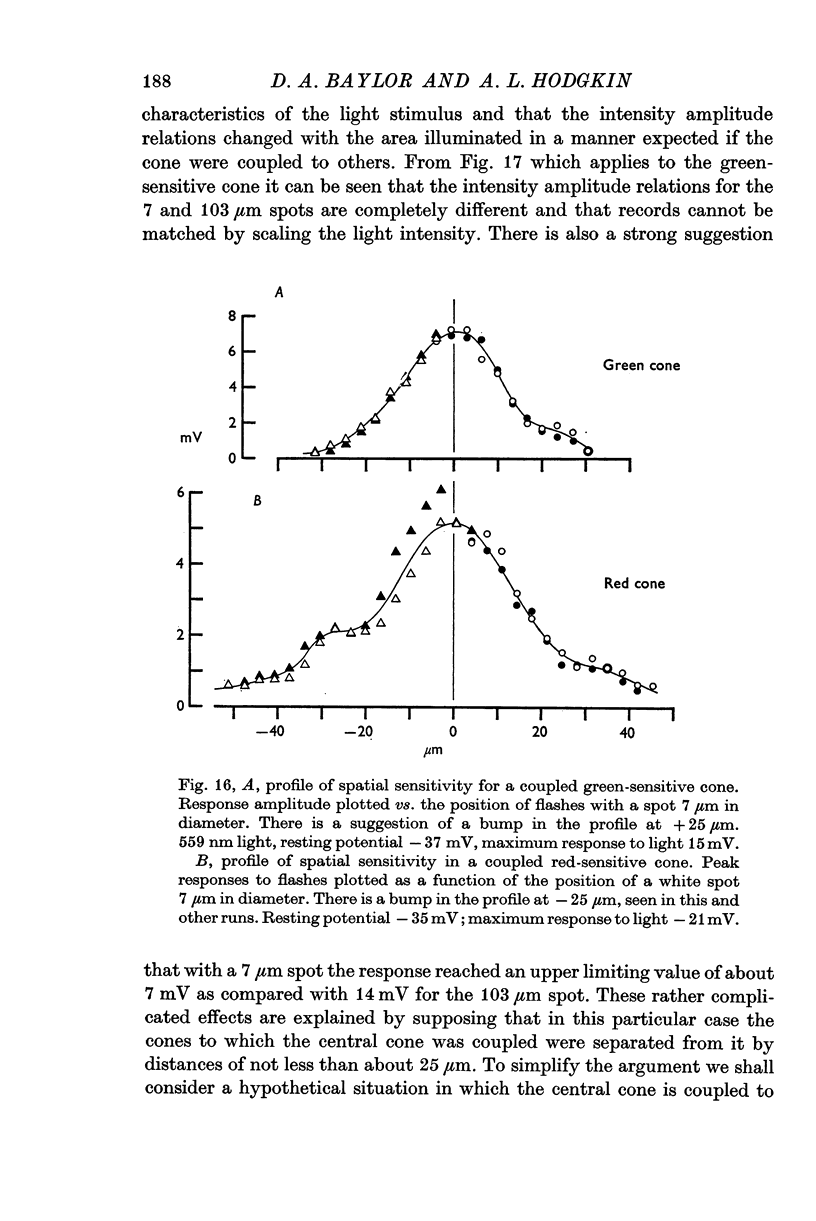
12. The majority of red and green cones have more diffuse sensitivity-profiles, sometimes with bumps on the descending limb, and behave as though cones with the same spectral sensitivity were electrically coupled to one another.
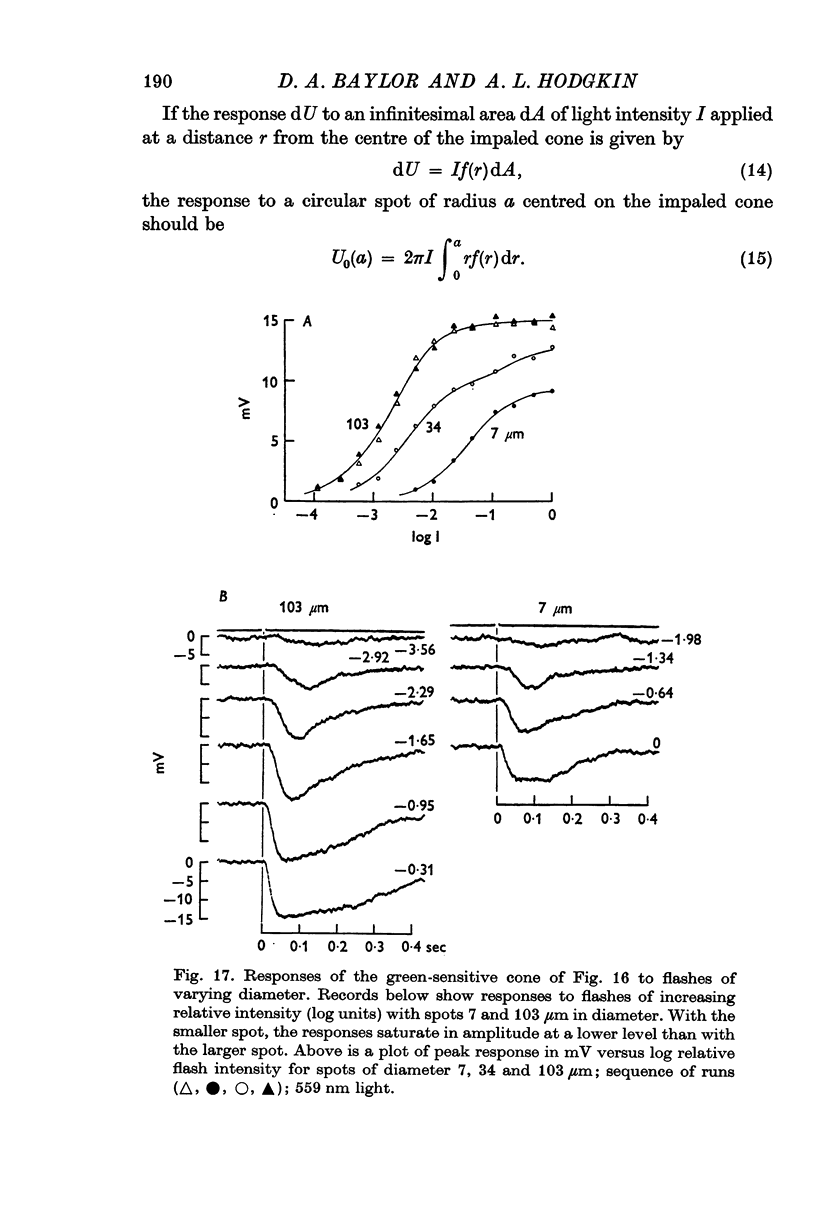
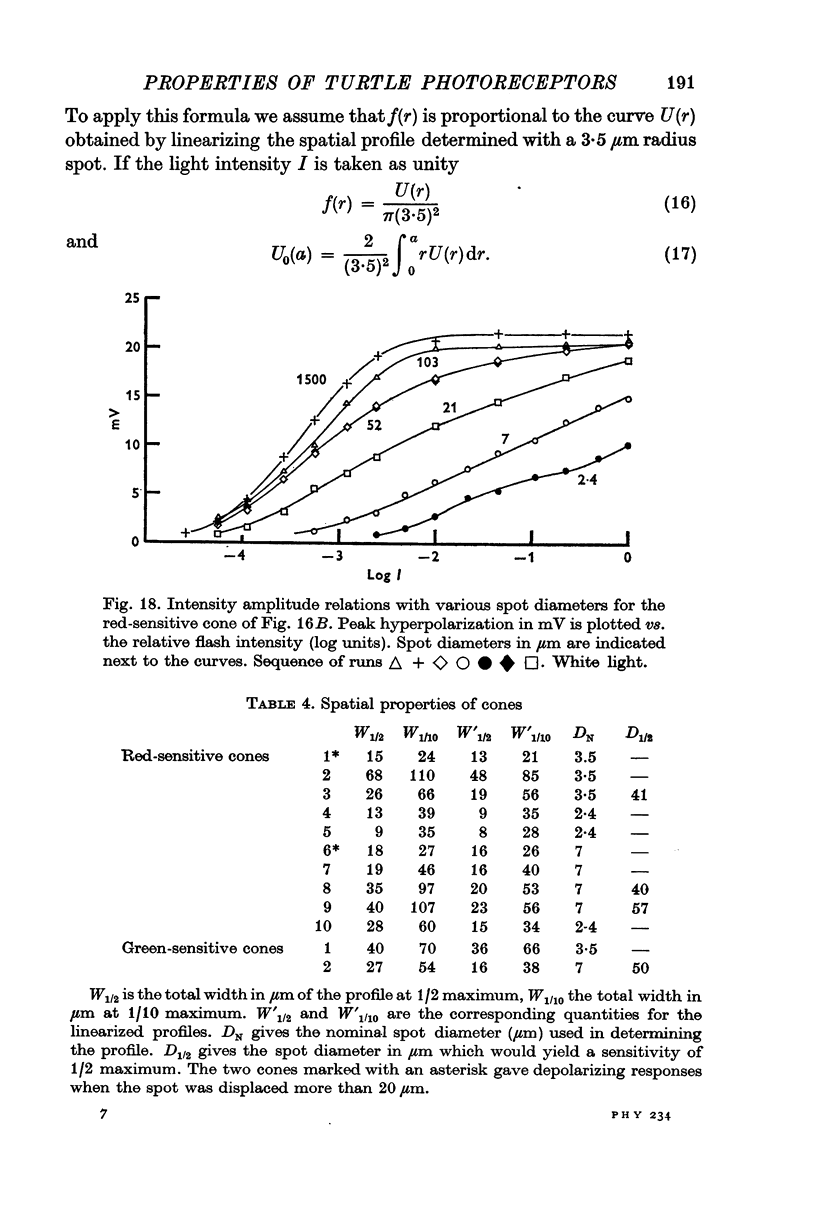
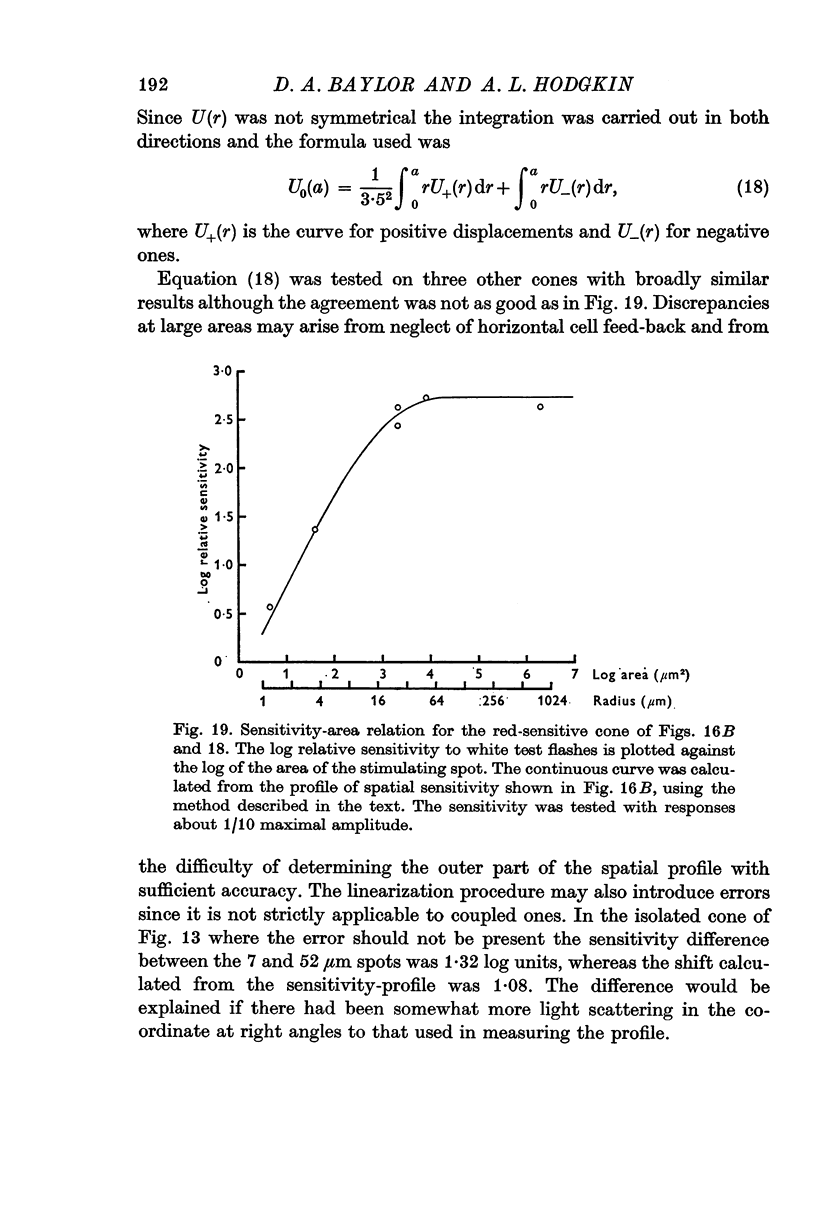
13. The relation between the area of illumination and flash sensitivity agreed approximately with that calculated from the spatial profile.
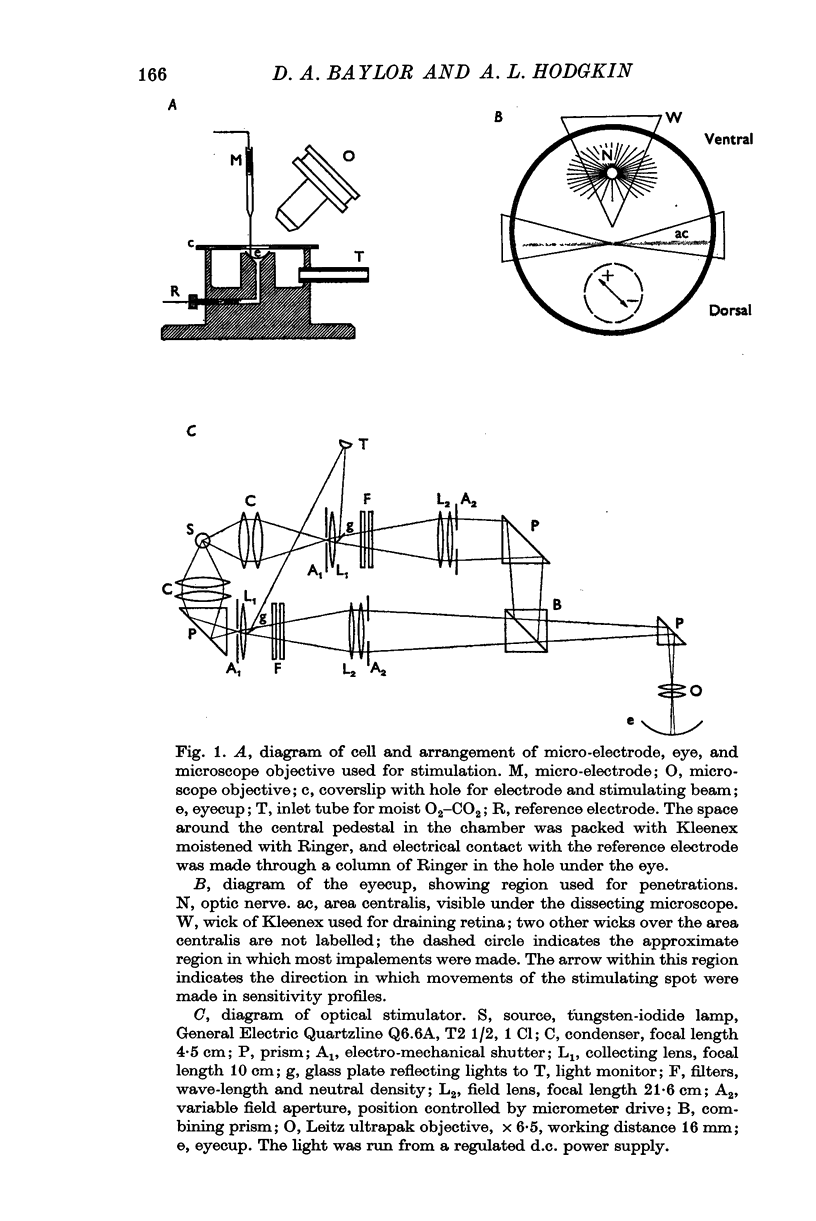
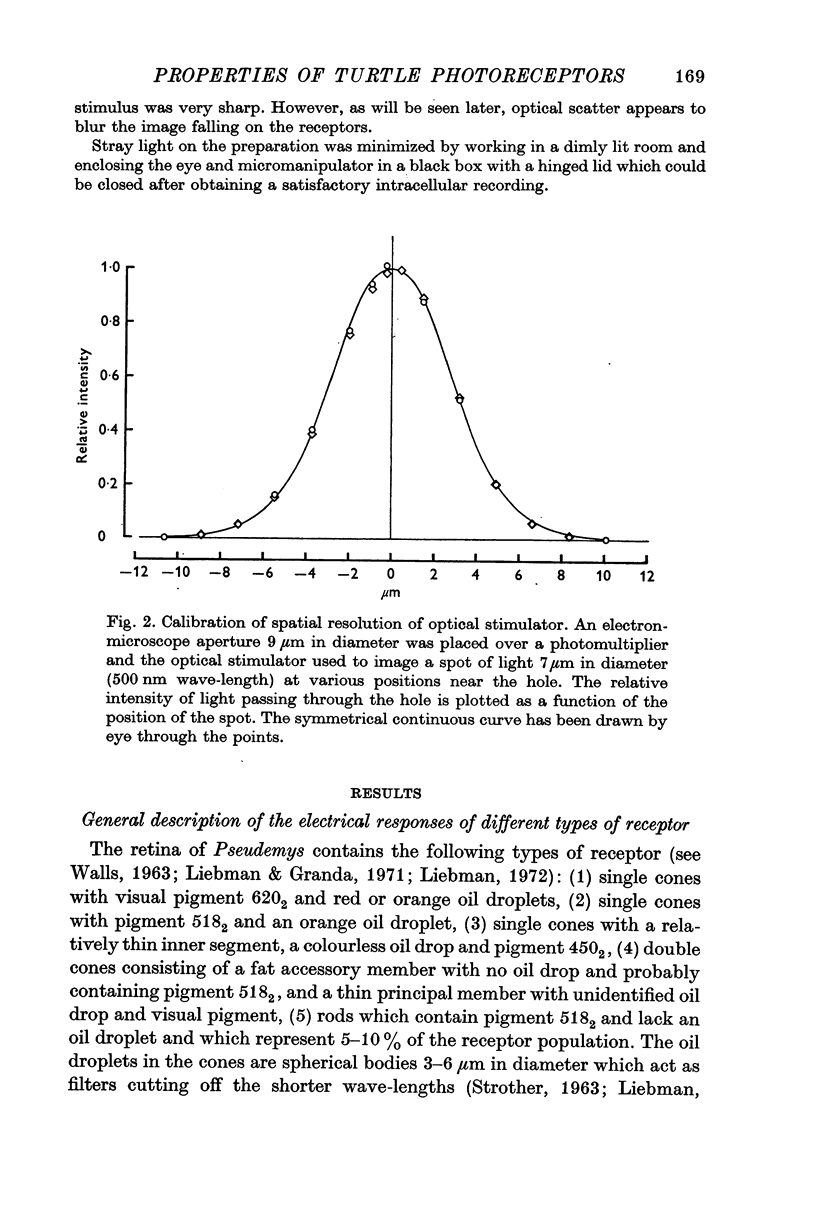
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges C. D. Spectroscopic properties of porphyropsins. Vision Res. 1967 May;7(5):349–369. doi: 10.1016/0042-6989(67)90044-2. [DOI] [PubMed] [Google Scholar]
- Fuortes M. G., Schwartz E. A., Simon E. J. Colour-dependence of cone responses in the turtle retina. J Physiol. 1973 Oct;234(1):199–216. doi: 10.1113/jphysiol.1973.sp010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Hashimoto H. Recording site of the single cone response determined by an electrode marking technique. Vision Res. 1967 Nov;7(11):847–851. doi: 10.1016/0042-6989(67)90005-3. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot J. I., Cone R. A. Dark ionic flux and the effects of light in isolated rod outer segments. J Gen Physiol. 1972 Jul;60(1):20–45. doi: 10.1085/jgp.60.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Granda A. M. Microspectrophotometric measurements of visual pigments in two species of turtle, Pseudemys scripta and Chelonia mydas. Vision Res. 1971 Feb;11(2):105–114. doi: 10.1016/0042-6989(71)90227-6. [DOI] [PubMed] [Google Scholar]
- Munz F. W., Schwanzara S. A. A nomogram for retinene-2-based visual pigments. Vision Res. 1967 Mar;7(3):111–120. doi: 10.1016/0042-6989(67)90078-8. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROTHER G. K. Absorption spectra of retinal oil globules in turkey, turtle and pigeon. Exp Cell Res. 1963 Jan;29:349–355. doi: 10.1016/0014-4827(63)90389-6. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of single rods in the retina of the turtle. J Physiol. 1973 Aug;232(3):503–514. doi: 10.1113/jphysiol.1973.sp010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrical activity of vertebrate photoreceptors. Q Rev Biophys. 1970 May;3(2):179–222. doi: 10.1017/s0033583500004571. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]



