Abstract
1. The Ca-sensitive photoprotein aequorin was injected into squid axons and the light response to stimulation or depolarizing voltage clamp pulses recorded.
2. The effects of Mn2+, Co2+, Ni2+, La3+ and of the organic Ca antagonists D-600 and iproveratril on the early tetrodotoxin-sensitive and late tetrodotoxin-insensitive components of the light response were studied.
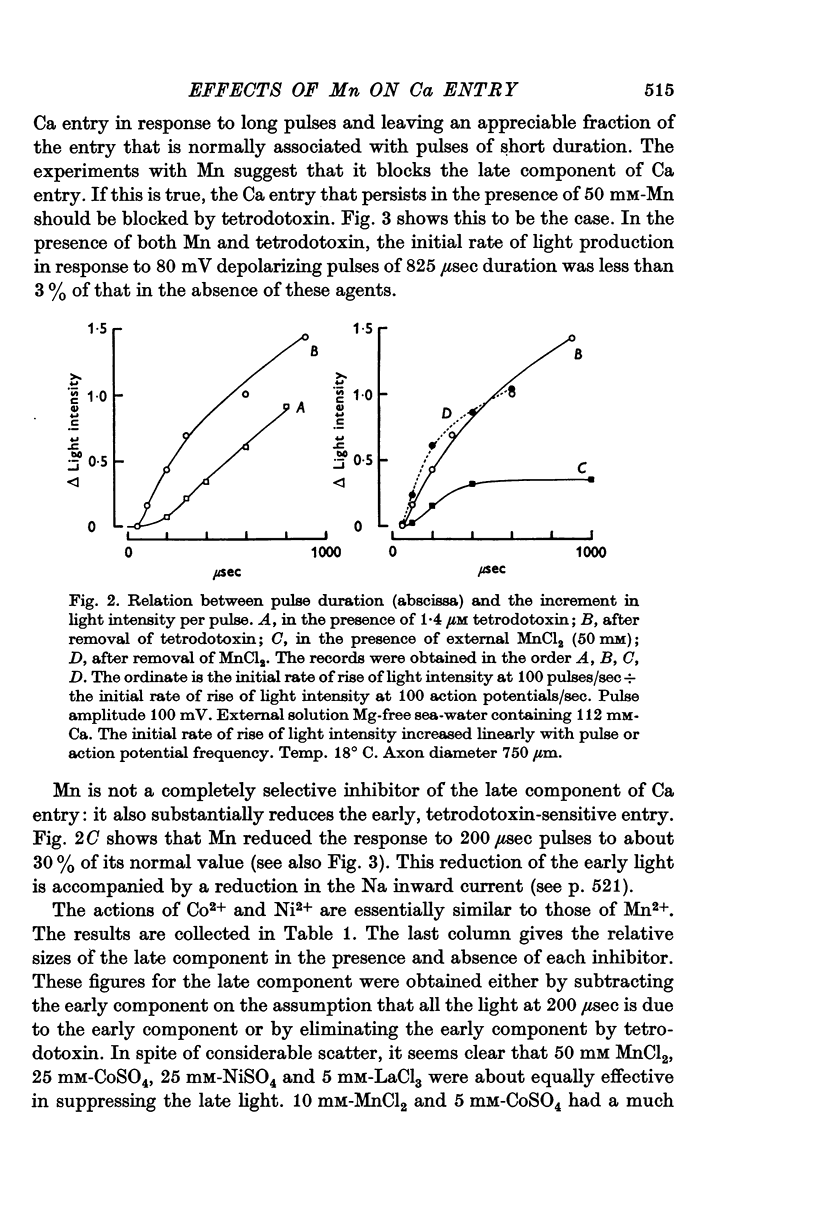
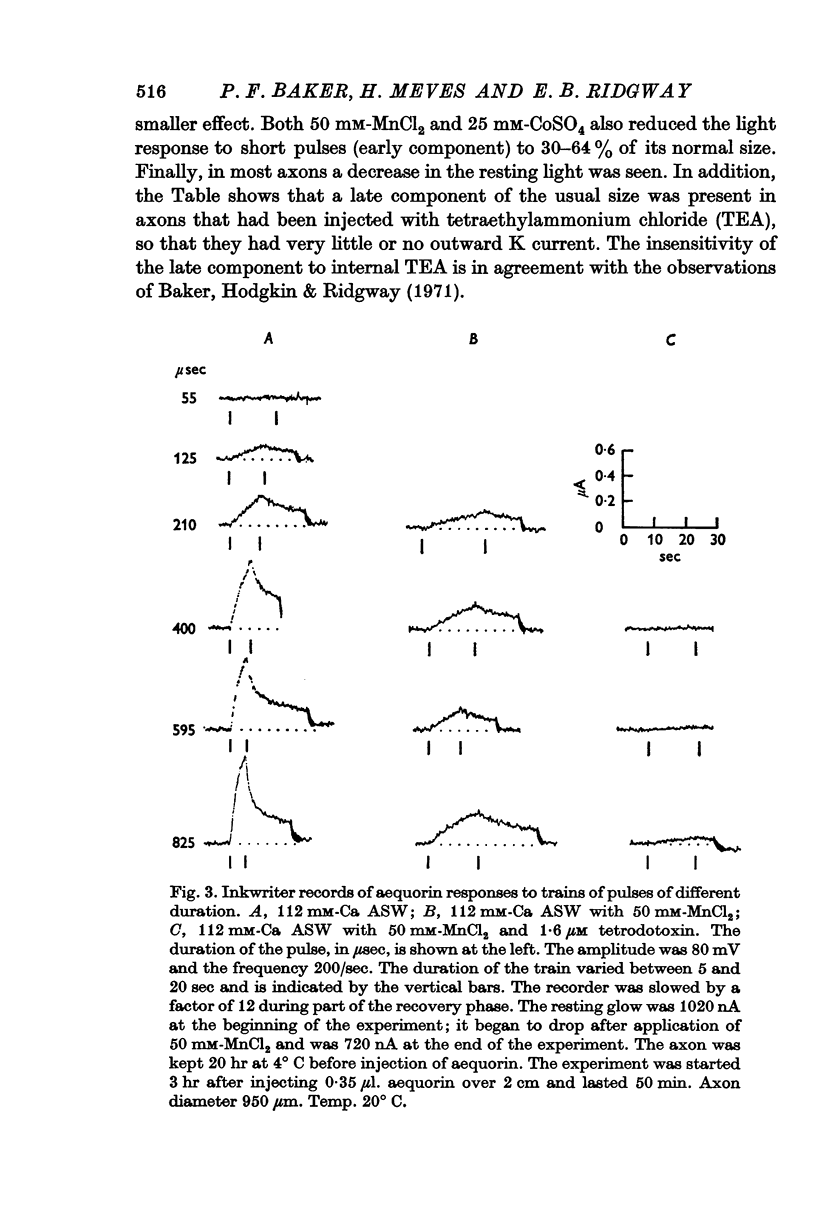
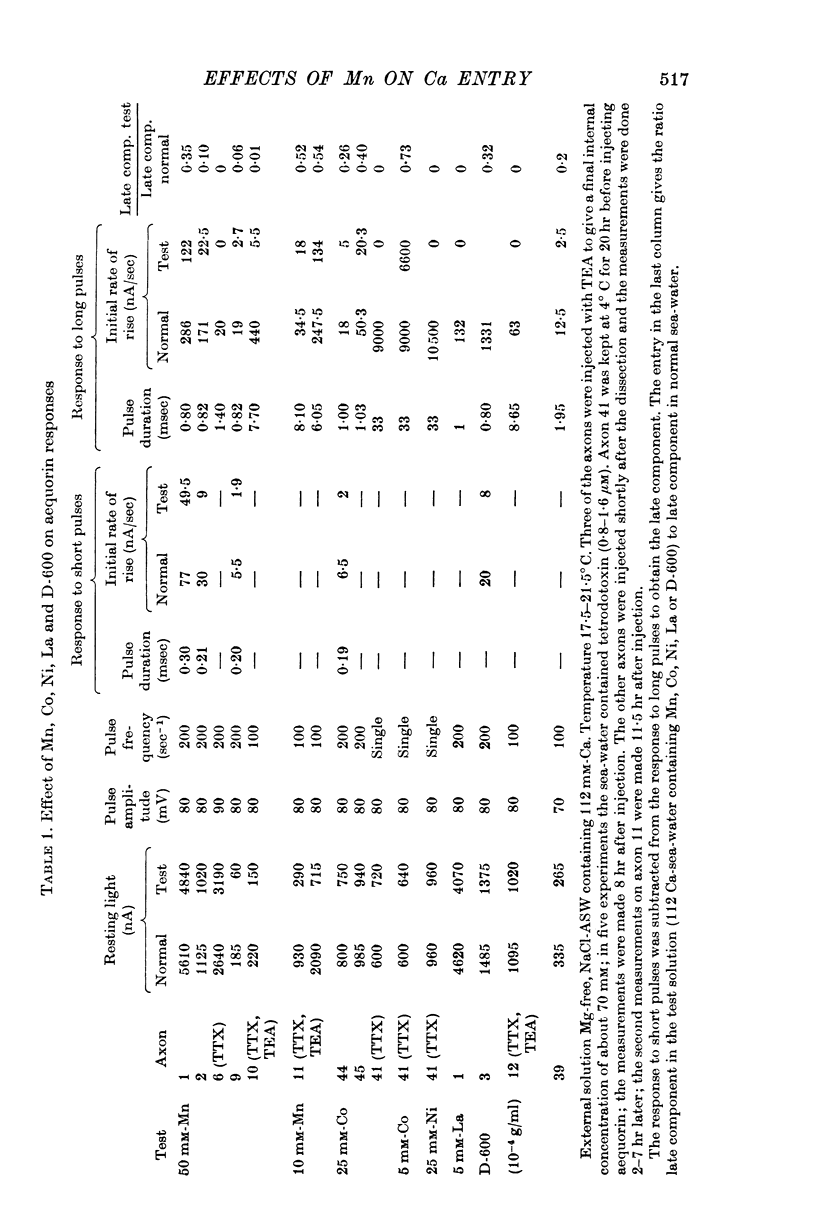
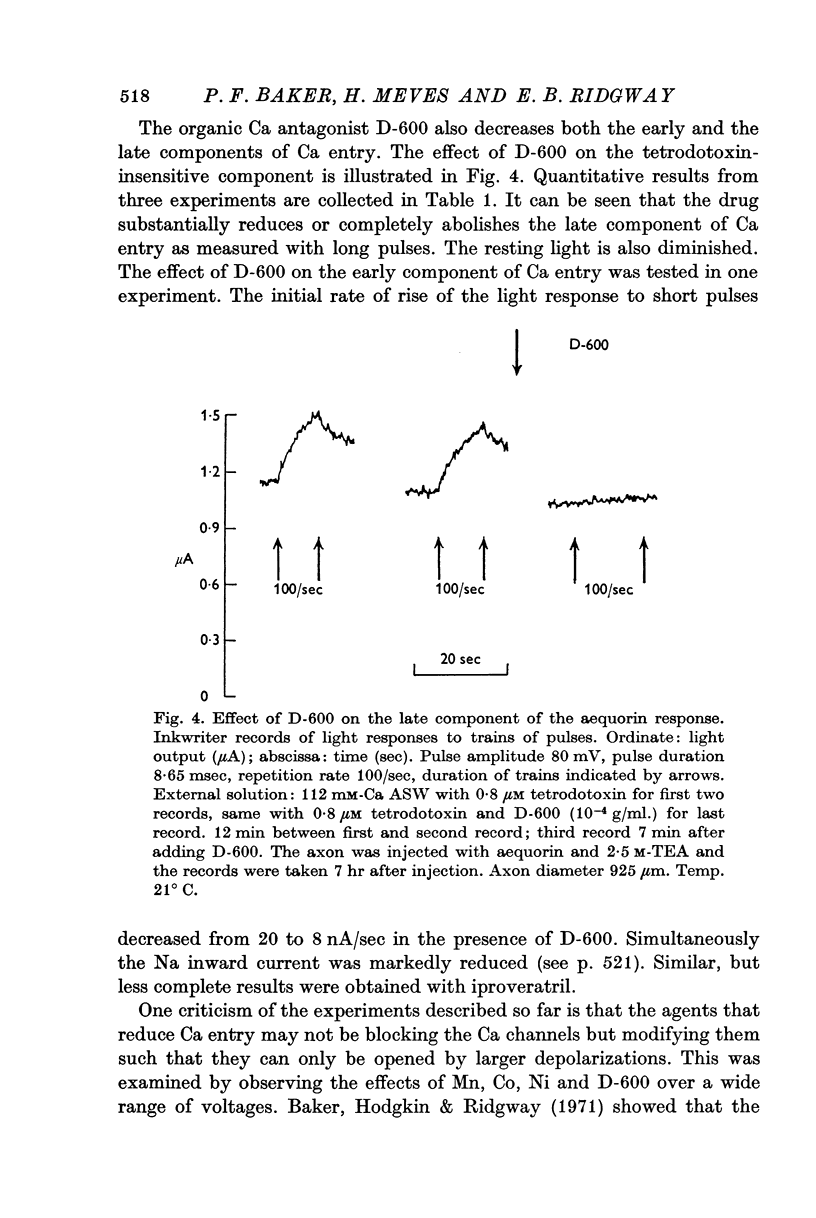
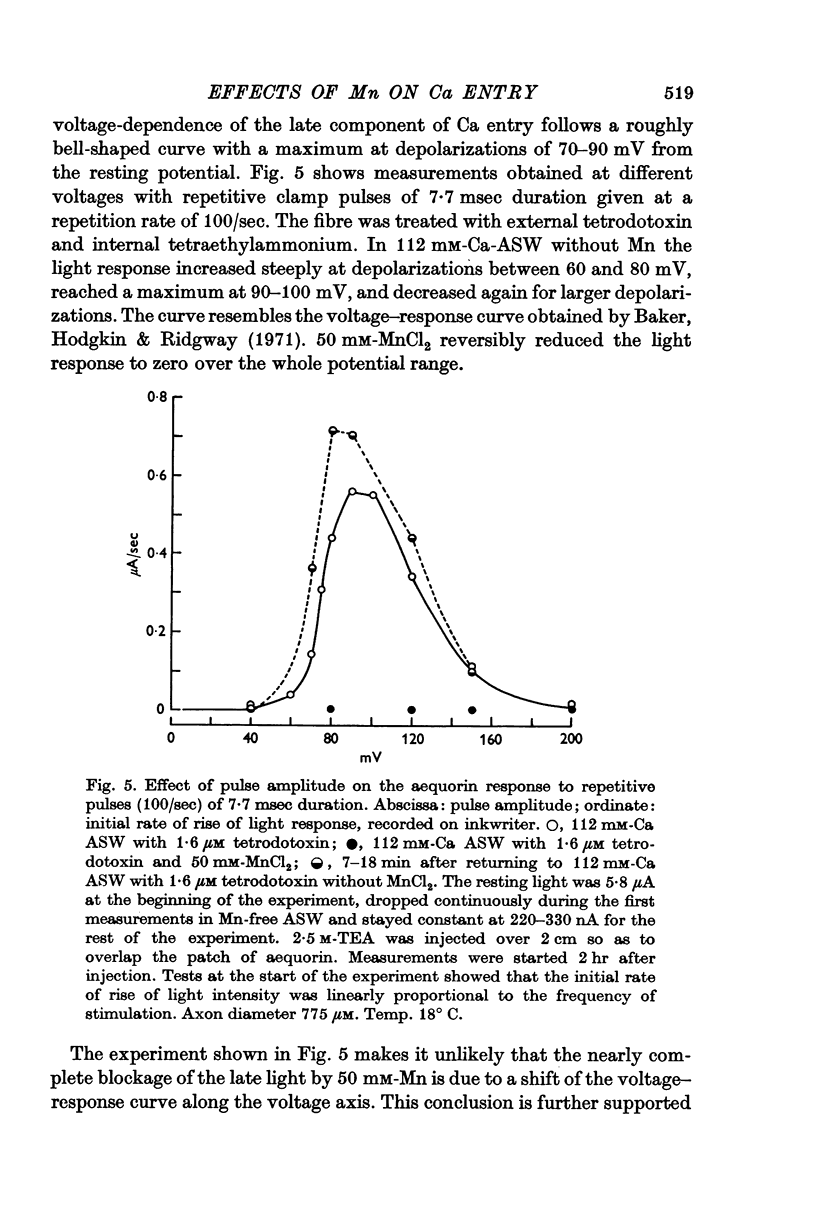
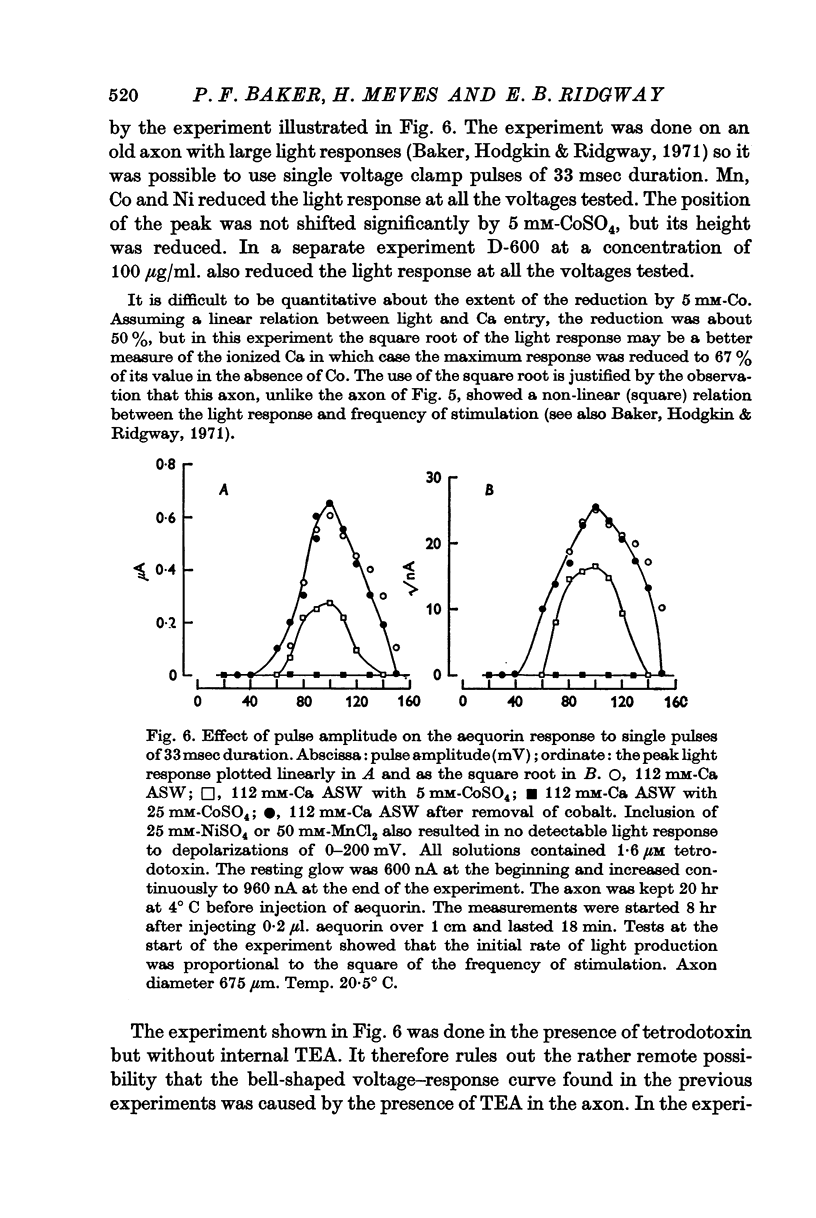
3. The late tetrodotoxin-insensitive component can be blocked, reversibly, by concentrations of Mn, Co and Ni that reduce but do not block the tetrodotoxin-sensitive component. The late component can also be blocked by La3+ and the organic Ca antagonists D-600 and iproveratril.
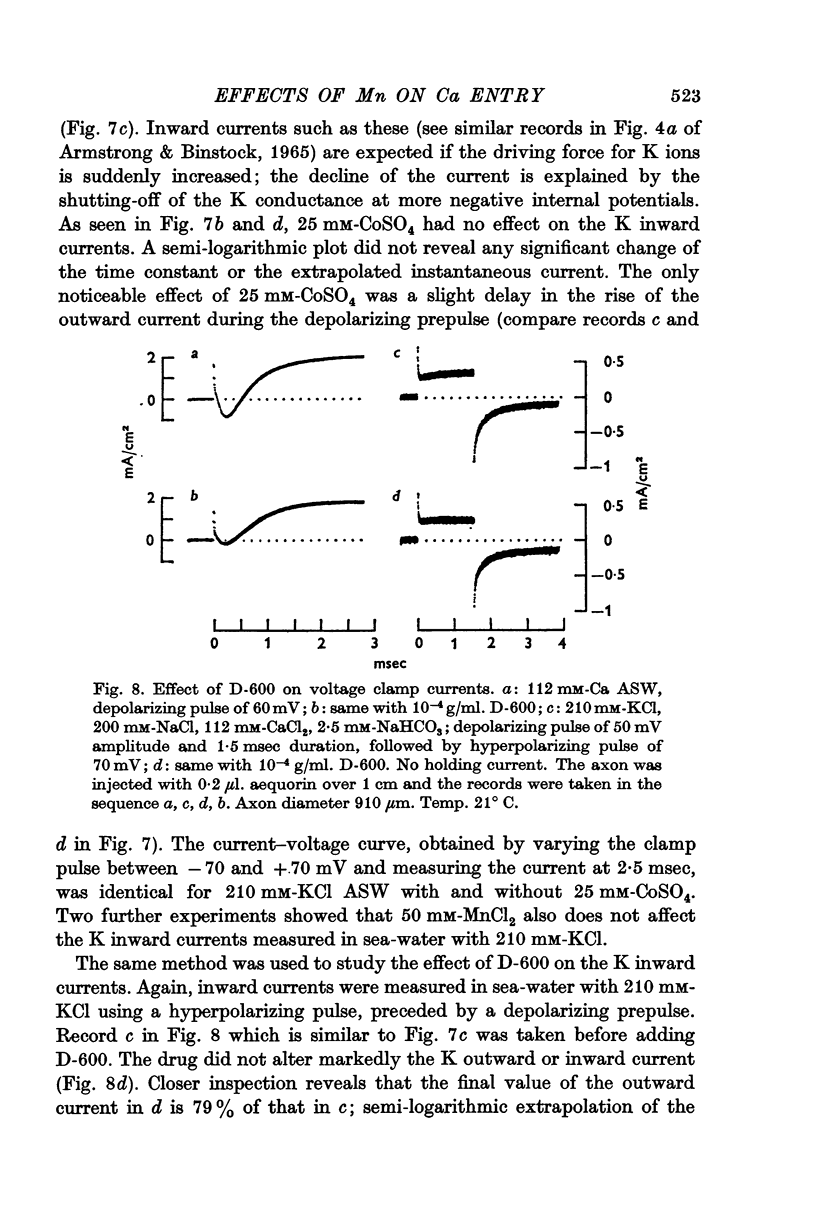
4. Mn2+, Co2+, Ni2+ and the drug D-600 all reduce the Na currents, but have little effect on either outward or inward K currents. Tetraethylammonium blocks the outward K current but has no appreciable effect on the tetrodotoxin-insensitive entry of Ca.
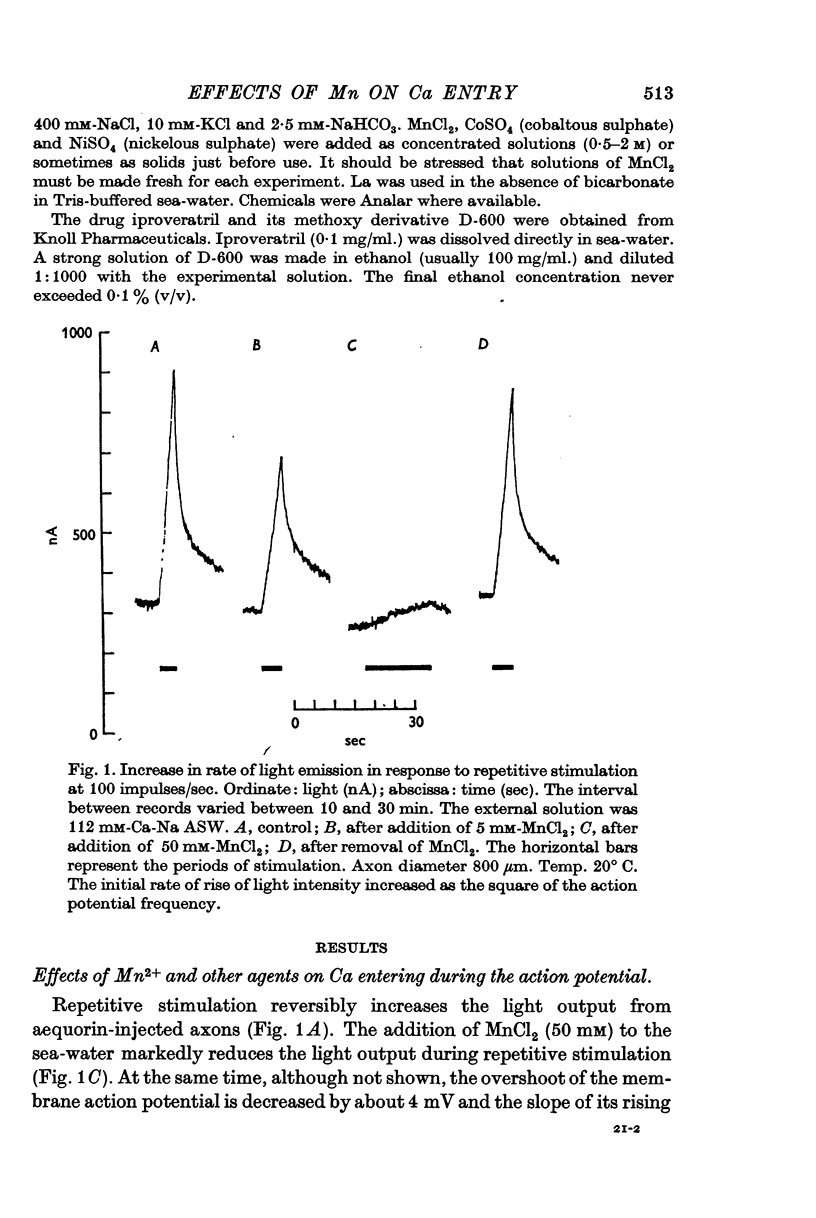
5. Concentrations of Mn between 5 and 50 mM substantially reduce the light output during a train of action potentials; they also slightly reduce the rate of rise of the action potential.
6. On pharmacological grounds it is concluded that the tetrodotoxin-insensitive component of Ca entry does not represent Ca ions passing through the K permeability channels. There must exist a potential-dependent late Ca channel that is distinct from the well known Na and K channels of the action potential. A possible function for this late Ca channel in the coupling of excitation to secretion is discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Calcium entry in response to maintained depolarization of squid axons. J Physiol. 1973 Jun;231(3):527–548. doi: 10.1113/jphysiol.1973.sp010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Phasic entry of calcium in response to depolarization of giant axons of Loligo forbesi. J Physiol. 1971 Jul;216(2):70P–71P. [PubMed] [Google Scholar]
- Blaustein M. P., Goldman D. E. The action of certain polyvalent cations on the voltage-clamped lobster axon. J Gen Physiol. 1968 Mar;51(3):279–291. doi: 10.1085/jgp.51.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. New selective inhibitors of the transmembrane Ca conductivity in mammalian myocardial fibres. Studies with the voltage clamp technique. Experientia. 1972 Mar 15;28(3):288–289. doi: 10.1007/BF01928693. [DOI] [PubMed] [Google Scholar]
- Takata M., Pickard W. F., Lettvin J. Y., Moore J. W. Ionic conductance changes in lobster axon membrane when lanthanum is substituted for calcium. J Gen Physiol. 1966 Nov;50(2):461–471. doi: 10.1085/jgp.50.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]


