Abstract
1. Intracellular aequorin was used to monitor changes in Ca entry in response to maintained depolarization either produced electrically or by exposure to K-rich solutions.
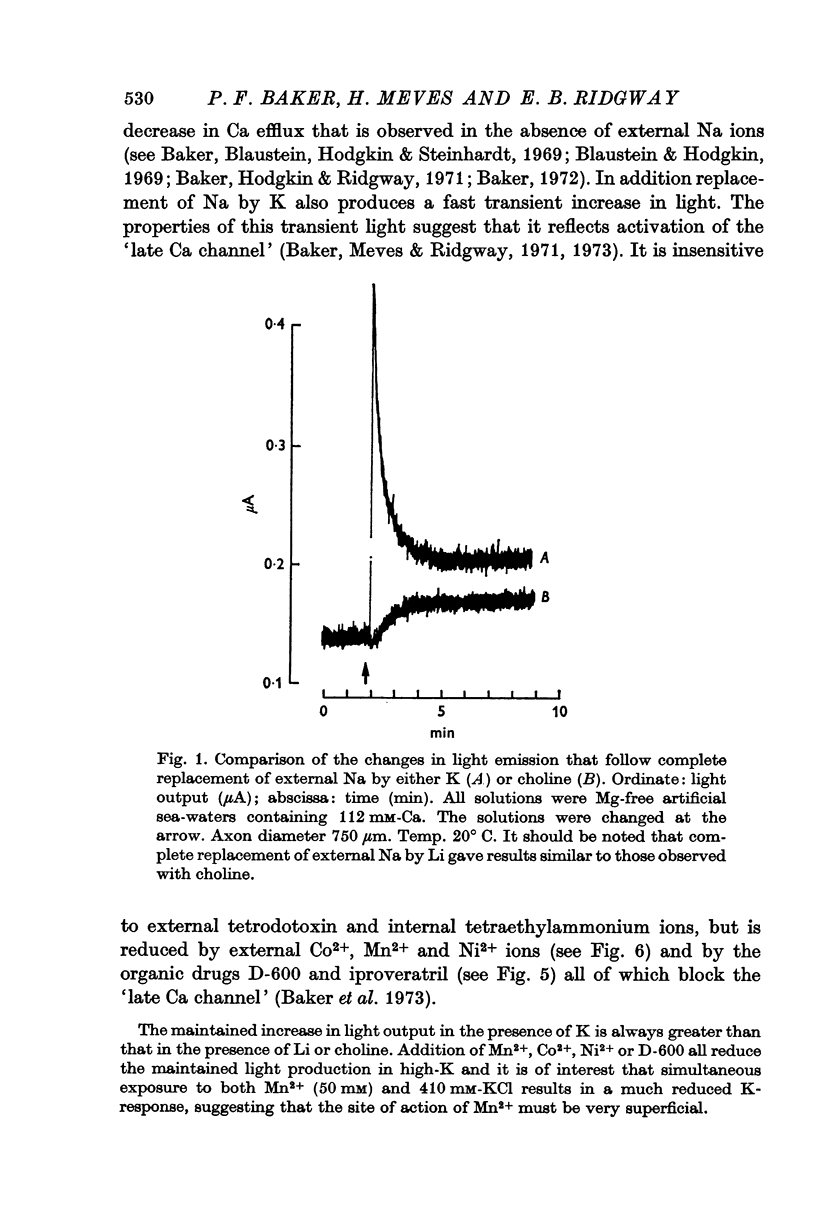
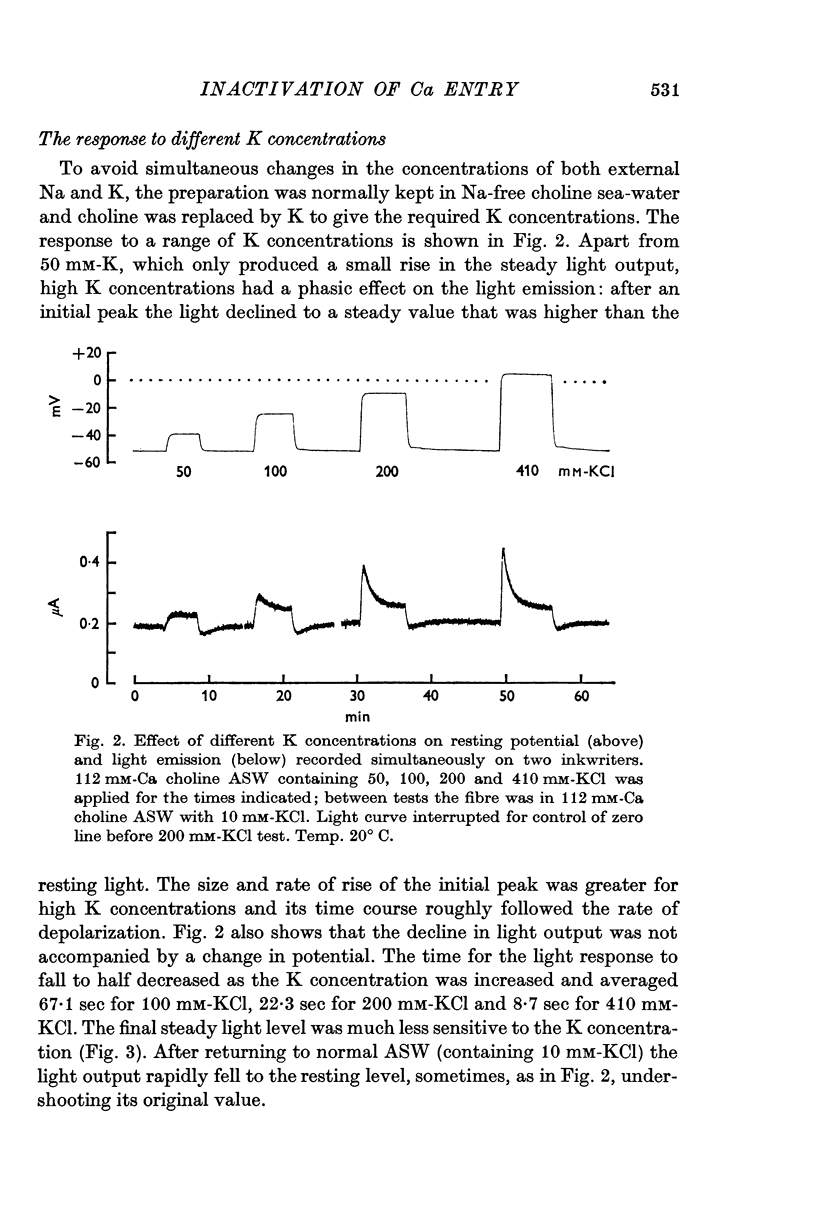
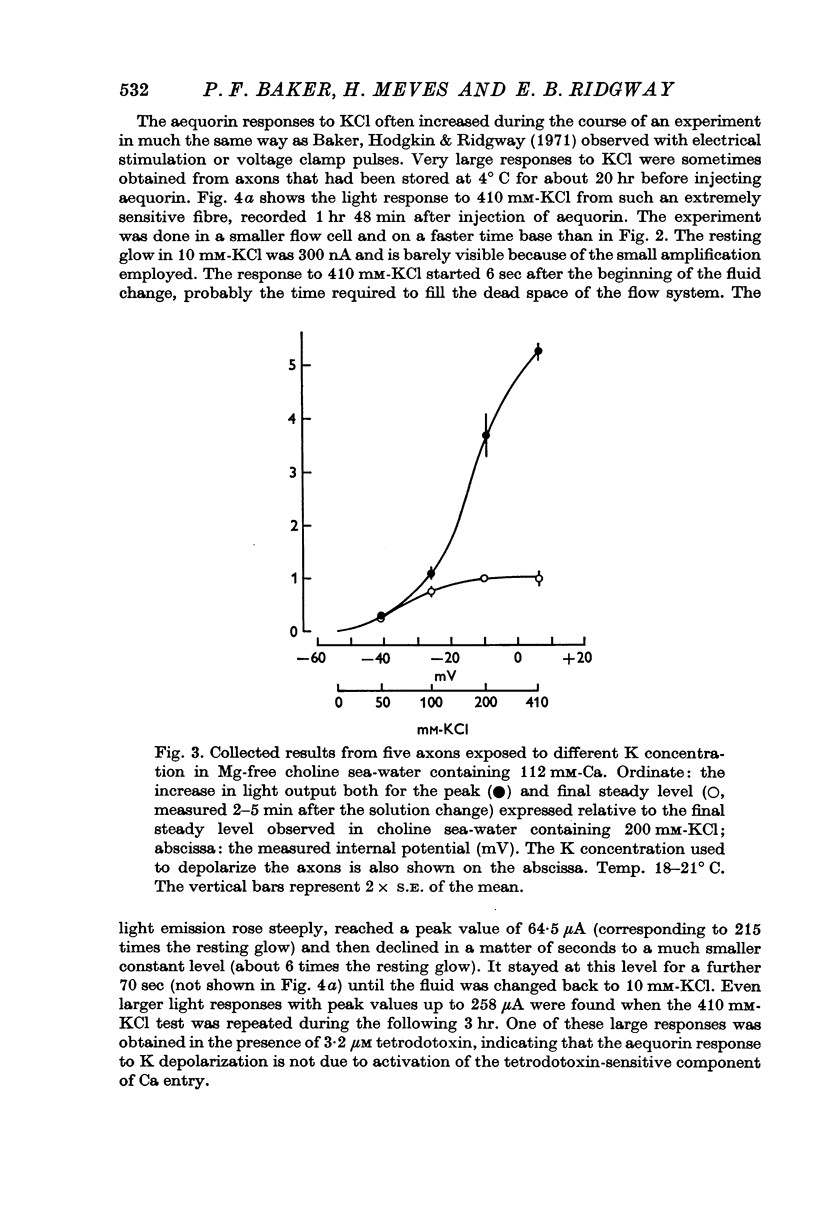
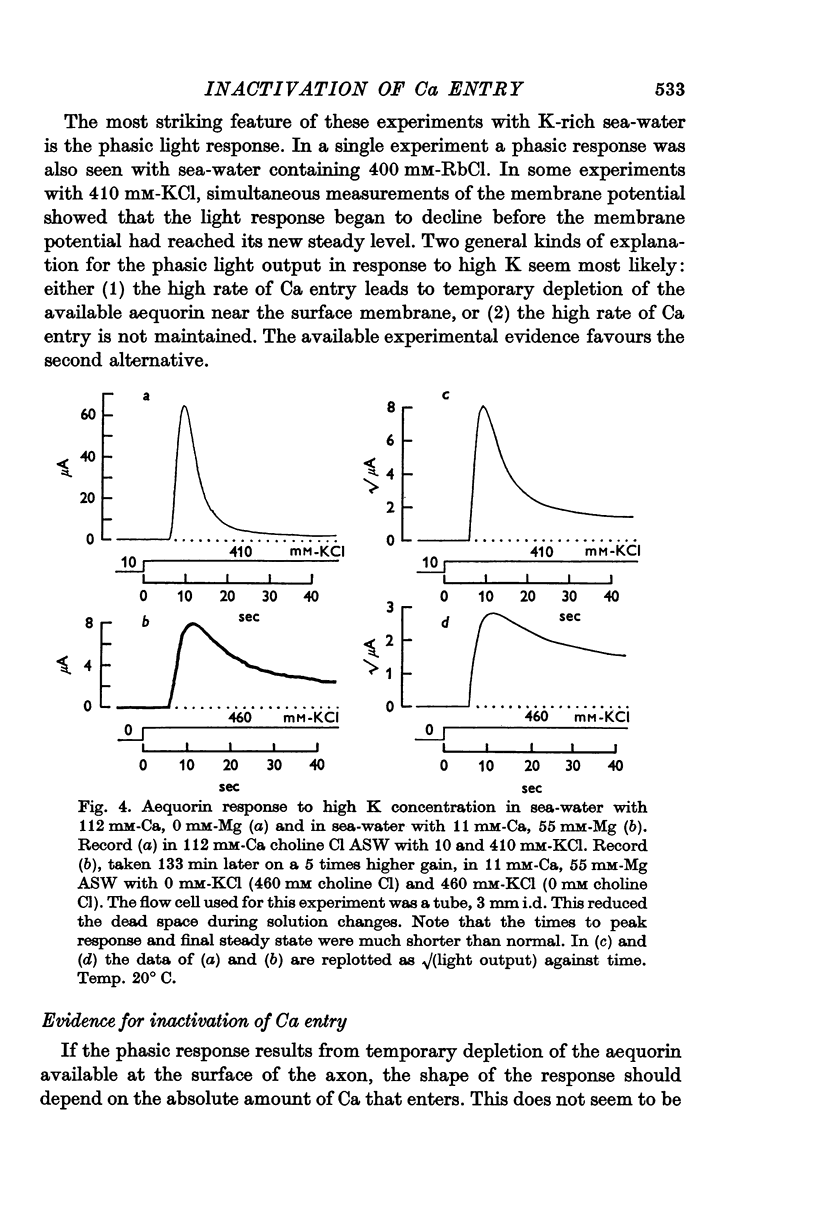
2. External K concentrations greater than 50 mM produce a phasic light response. The light rises to a peak in a few sec and then falls in 0·5-5 min to a new steady level that is always greater than the level in the absence of K.
3. The phasic light response does not result from depletion of available aequorin at the periphery of the axon, but rather seems to reflect a phasic entry of Ca in response to depolarization.
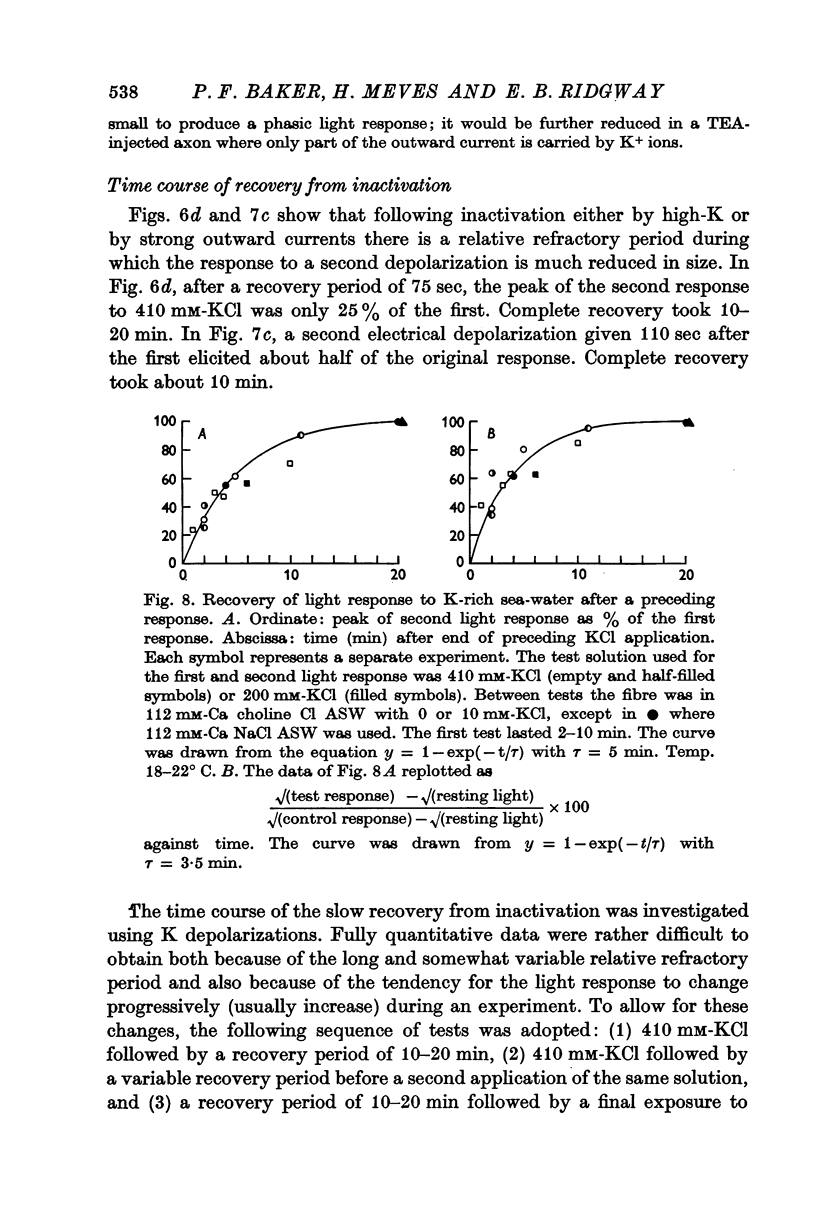
4. Similar phasic responses are produced by prolonged electrical depolarization. These results are consistent with depolarization serving both to activate and also to inactivate Ca entry.
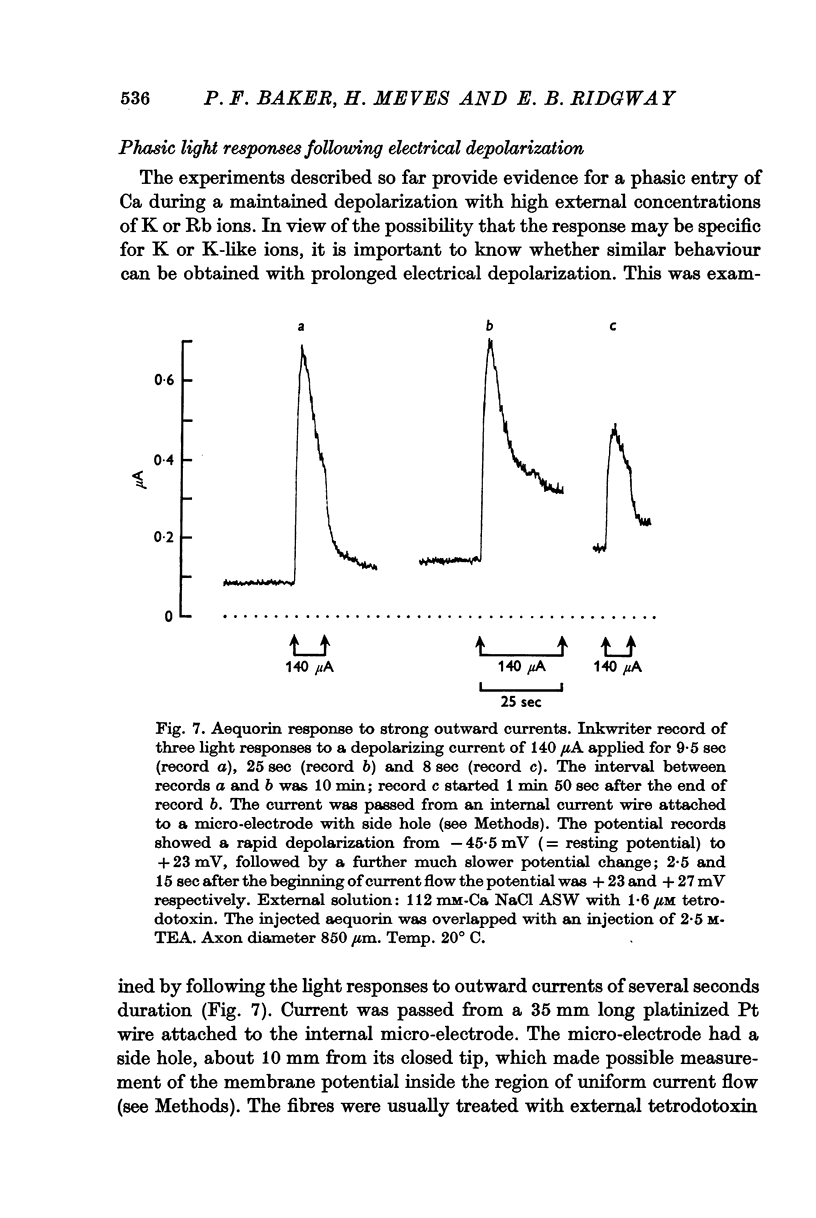
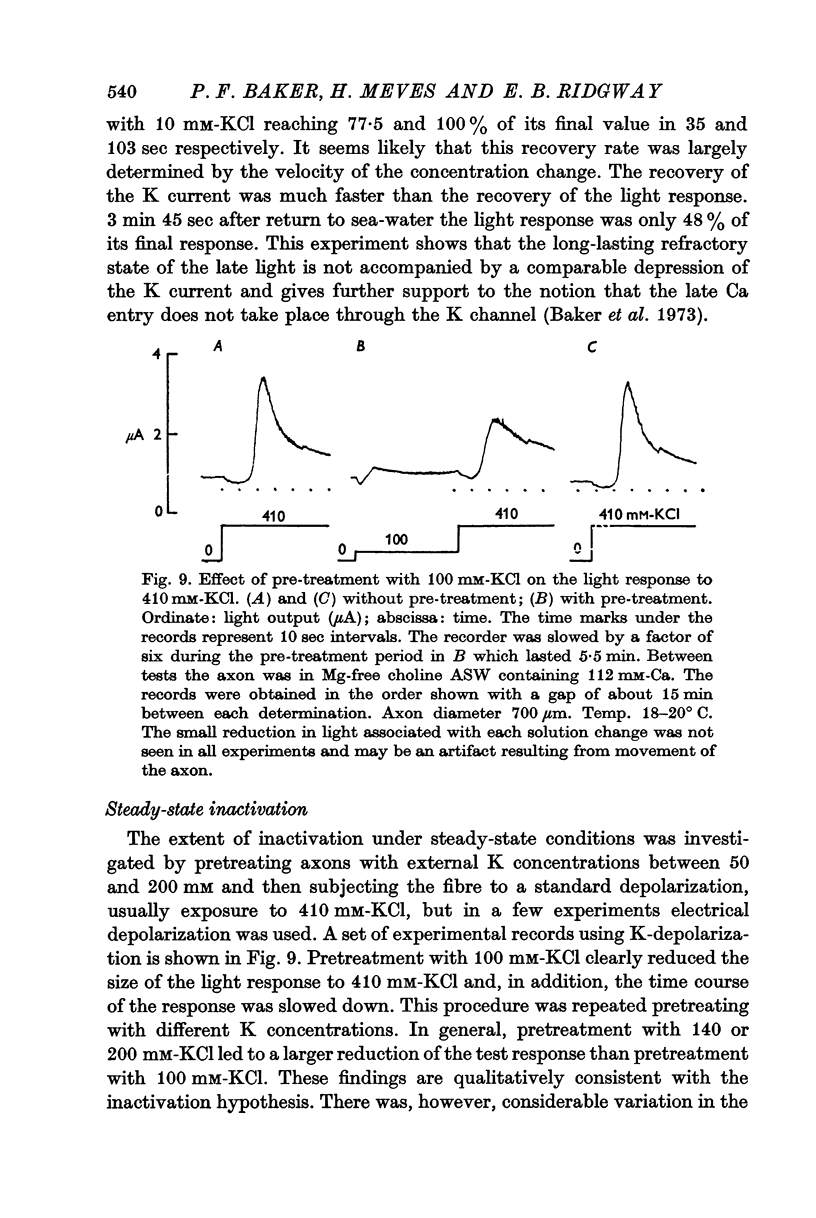
5. Following inactivation and after return to normal sea-water, there is an appreciable relative refractory period during which the response both to K-rich sea-water and electrical depolarization is reduced in size. Complete recovery takes 10-15 min.
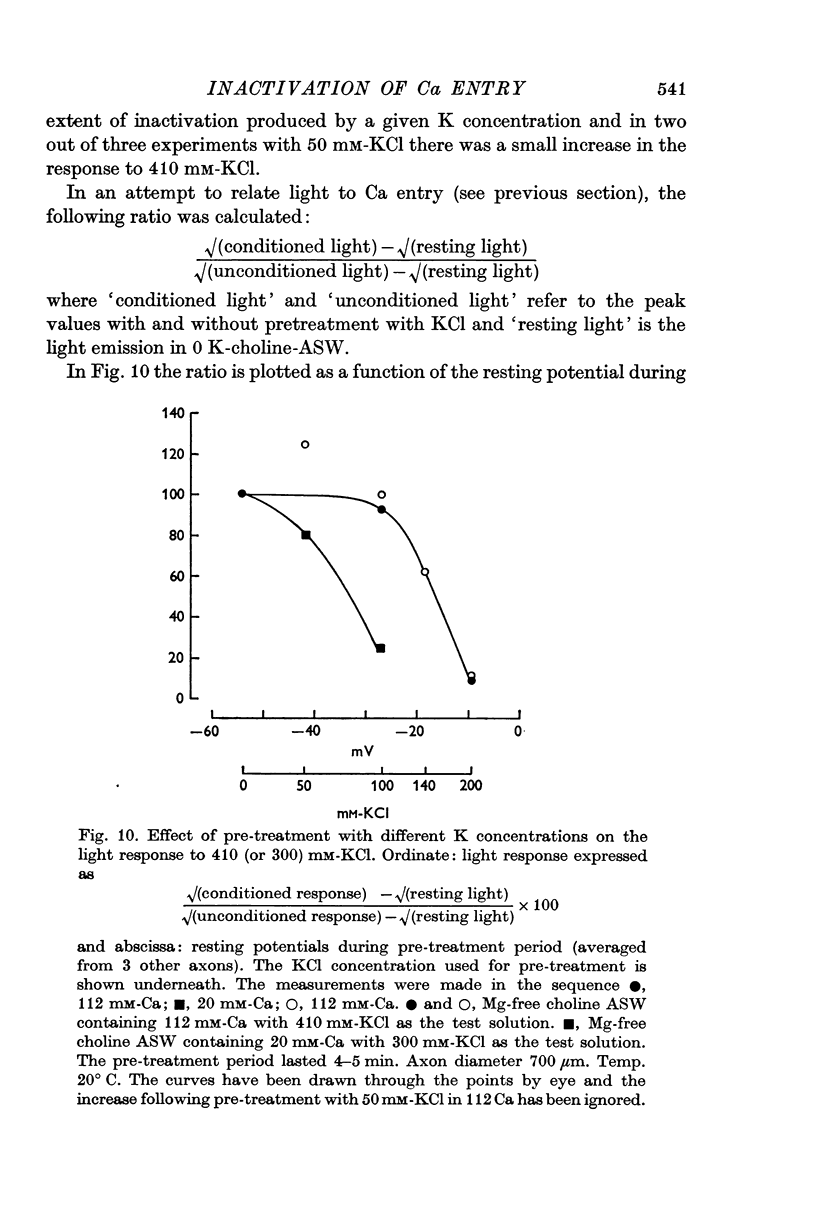
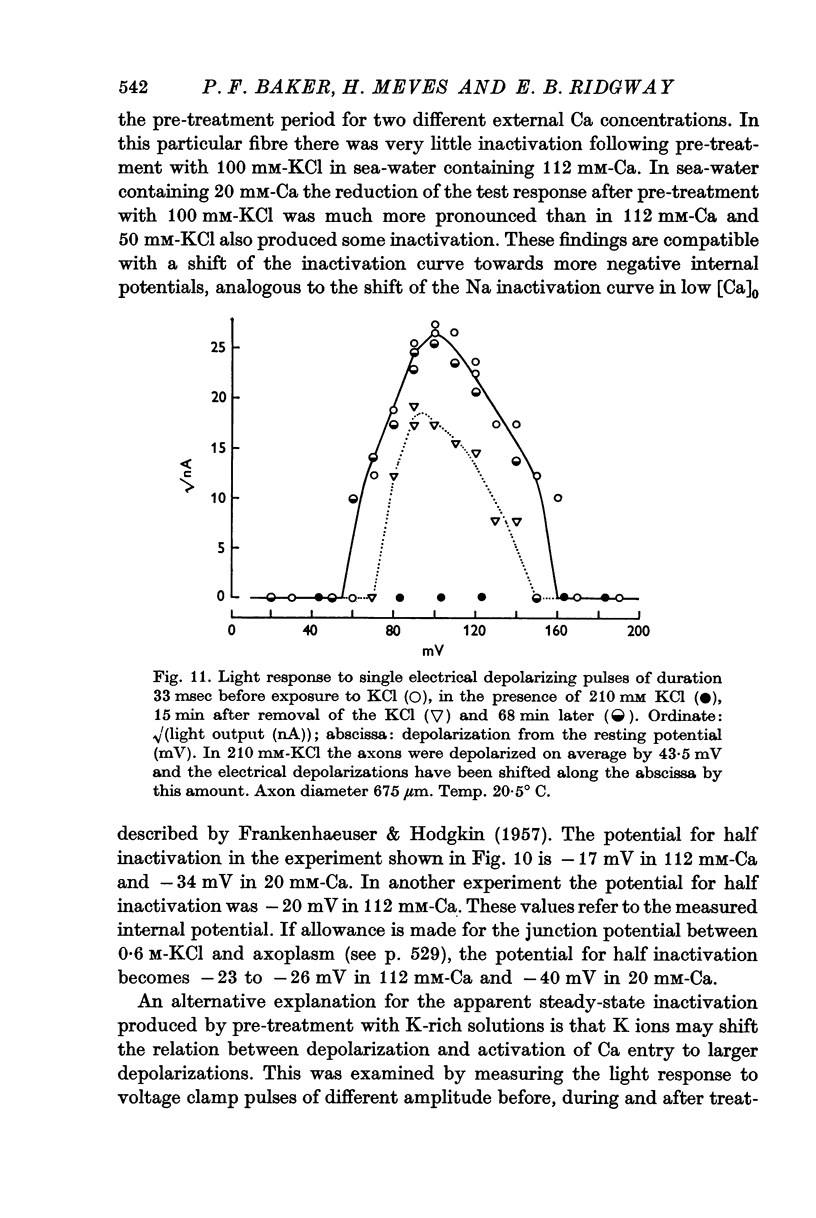
6. The response to 410 mM-KCl is dependent on the previous treatment of the preparation. Pre-treatment with 100 or 200 mM-KCl reduced the response to 410 mM-KCl. The potential for half inactivation was about -25 mV in 112 mM-Ca and -40 mV in 20 mM-Ca.
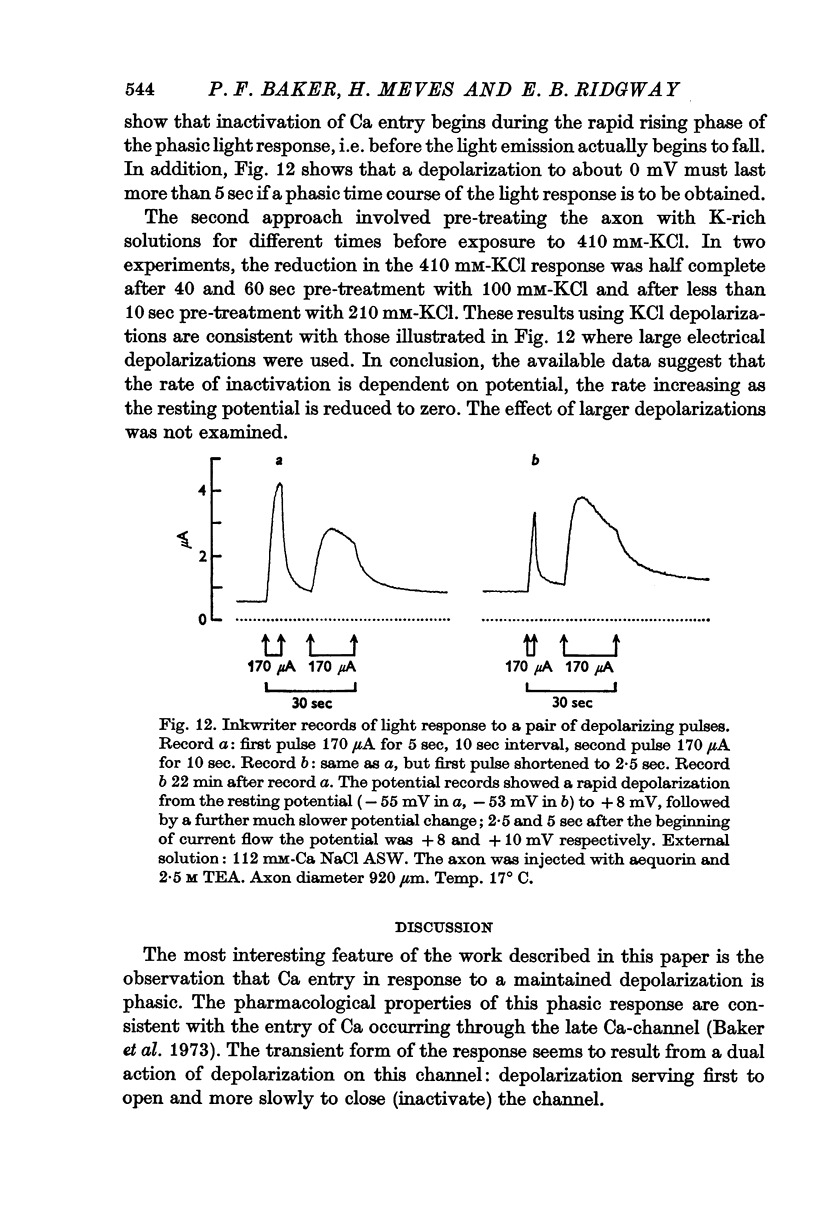
7. The rate of onset of inactivation is potential dependent and is faster for depolarizations to zero potential than for smaller ones.
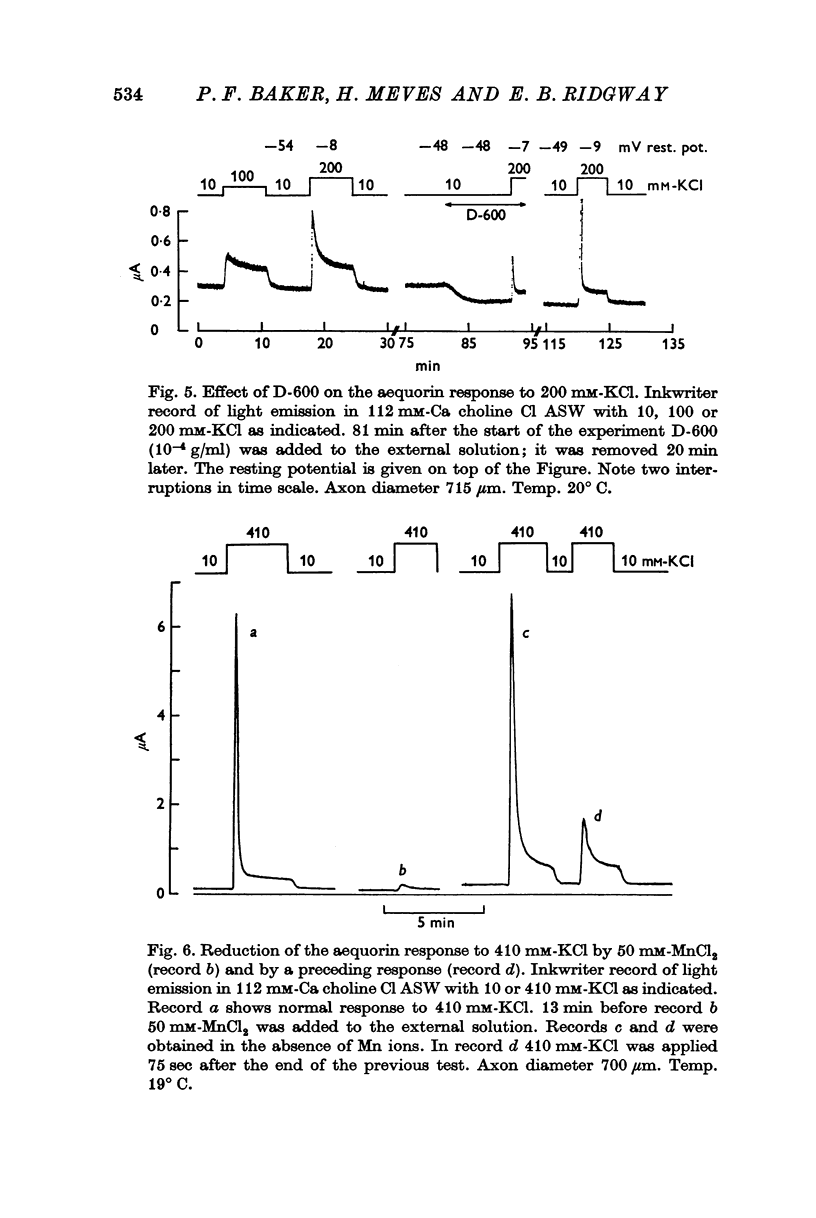
8. The phasic Ca entry produced by K-rich solutions is insensitive to external tetrodotoxin and internal tetraethylammonium ions, but is blocked by external Mn2+, Co2+ and Ni2+ ions and by the drugs D-600 and iproveratril. This suggests that the phasic Ca entry involves the late Ca channel.
9. Recovery of the outward K current after a long depolarization is much faster than recovery of the late Ca entry system. This provides further support for the view that the late Ca channel and the K channel are distinct.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C. An estimate of calcium concentration changes during the contraction of single muscle fibres. J Physiol. 1970 Sep;210(2):133P–134P. [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Phasic entry of calcium in response to depolarization of giant axons of Loligo forbesi. J Physiol. 1971 Jul;216(2):70P–71P. [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Membrane calcium current in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):191–209. doi: 10.1113/jphysiol.1970.sp009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloedel J. R., Gage P. W., Llinás R., Quastel D. M. Transmission across the squid giant synapse in the presence of tetrodotoxin. J Physiol. 1967 Jan;188(2):52P–53P. [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein G., Gilbert D. L. Slow changes of potassium permeability in the squid giant axon. Biophys J. 1966 Sep;6(5):553–566. doi: 10.1016/S0006-3495(66)86677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhaeuser B., Lännergren J. The effect of calcium on the mechanical response of single twitch muscle fibres of Xenopus laevis. Acta Physiol Scand. 1967 Mar;69(3):242–254. doi: 10.1111/j.1748-1716.1967.tb03518.x. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., TASAKI I. A study on the mechanism of impulse transmission across the giant synapse of the squid. J Physiol. 1958 Aug 29;143(1):114–137. doi: 10.1113/jphysiol.1958.sp006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistracher P., Hunt C. C. The relation of membrane changes ot contraction in twitch muscle fibres. J Physiol. 1969 May;201(3):589–611. doi: 10.1113/jphysiol.1969.sp008774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I. Mechanism of transmitter release. Prog Biophys Mol Biol. 1970;21:33–124. [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of prolonged depolarization on synaptic transfer in the stellate ganglion of the squid. J Physiol. 1971 Jul;216(2):503–512. doi: 10.1113/jphysiol.1971.sp009537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUETTGAU H. C. THE ACTION OF CALCIUM IONS ON POTASSIUM CONTRACTURES OF SINGLE MUSCLE FIBRES. J Physiol. 1963 Oct;168:679–697. doi: 10.1113/jphysiol.1963.sp007215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecar H., Ehrenstein G., Binstock L., Taylor R. E. Removal of potassium negative resistance in perfused squid giant axons. J Gen Physiol. 1967 Jul;50(6):1499–1515. doi: 10.1085/jgp.50.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J. Contractures of single slow muscle fibres of Xenopus laevis elicited by potassium, acetylcholine or choline. Acta Physiol Scand. 1967 Apr;69(4):362–372. doi: 10.1111/j.1748-1716.1967.tb03533.x. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. The action of calcium on neuronal synapses in the squid. J Physiol. 1966 May;184(2):473–498. doi: 10.1113/jphysiol.1966.sp007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H., Saiga Y. Microdetermination of Calcium by Aequorin Luminescence. Science. 1963 Jun 21;140(3573):1339–1340. doi: 10.1126/science.140.3573.1339. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Electrical changes in pre- and postsynaptic axons of the giant synapse of Loligo. J Gen Physiol. 1962 Jul;45:1181–1193. doi: 10.1085/jgp.45.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]


