Abstract
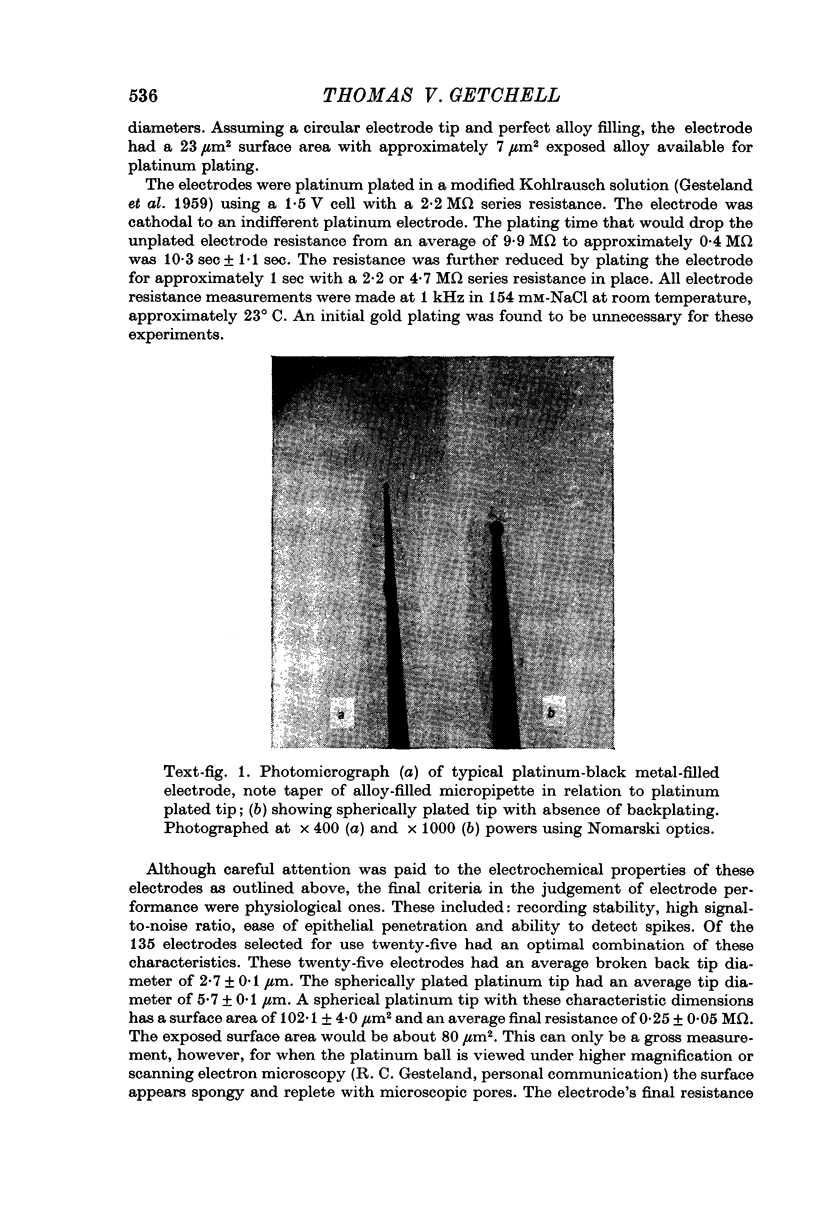
1. Differences in unitary spike conformation were systematically investigated with extracellular recordings at different depths in the frog's olfactory epithelium. Platinum-black metal-filled micro-electrodes were used in the unit recordings. Their properties were carefully investigated and compared with those of micropipettes.
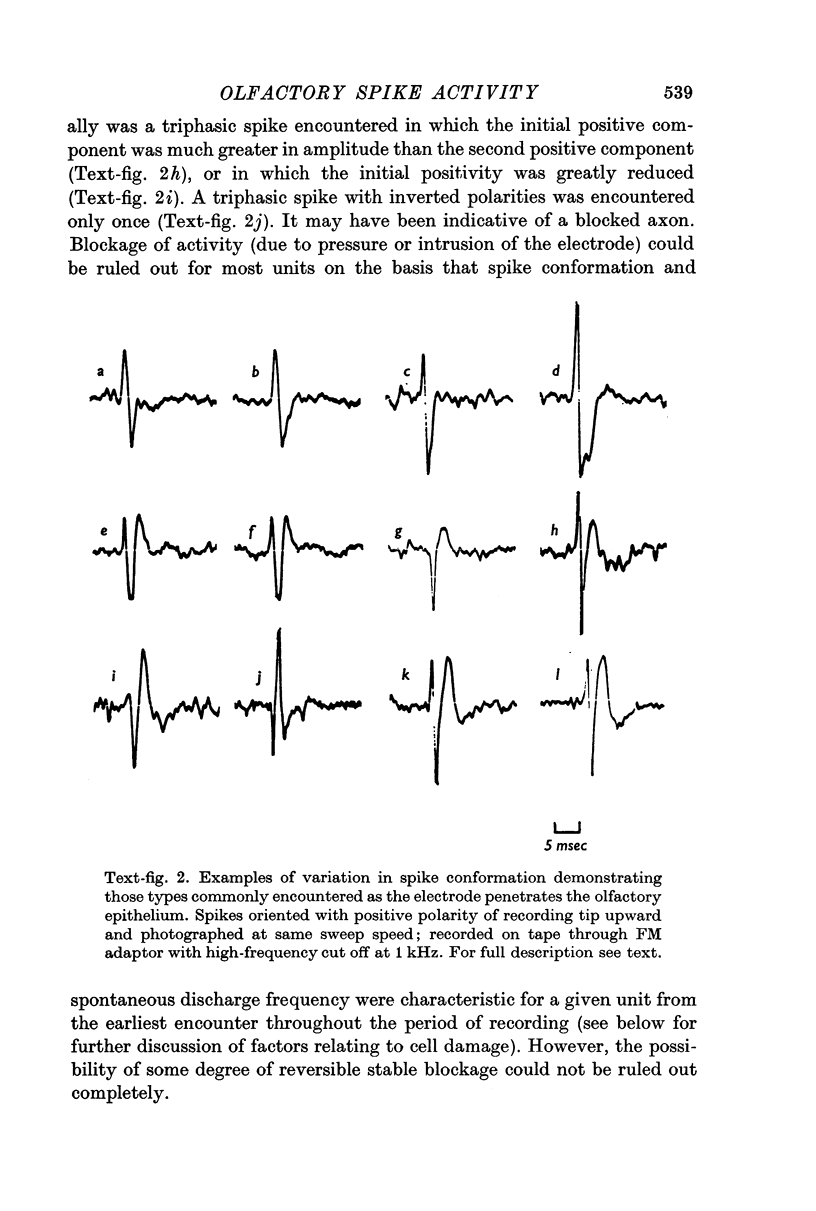
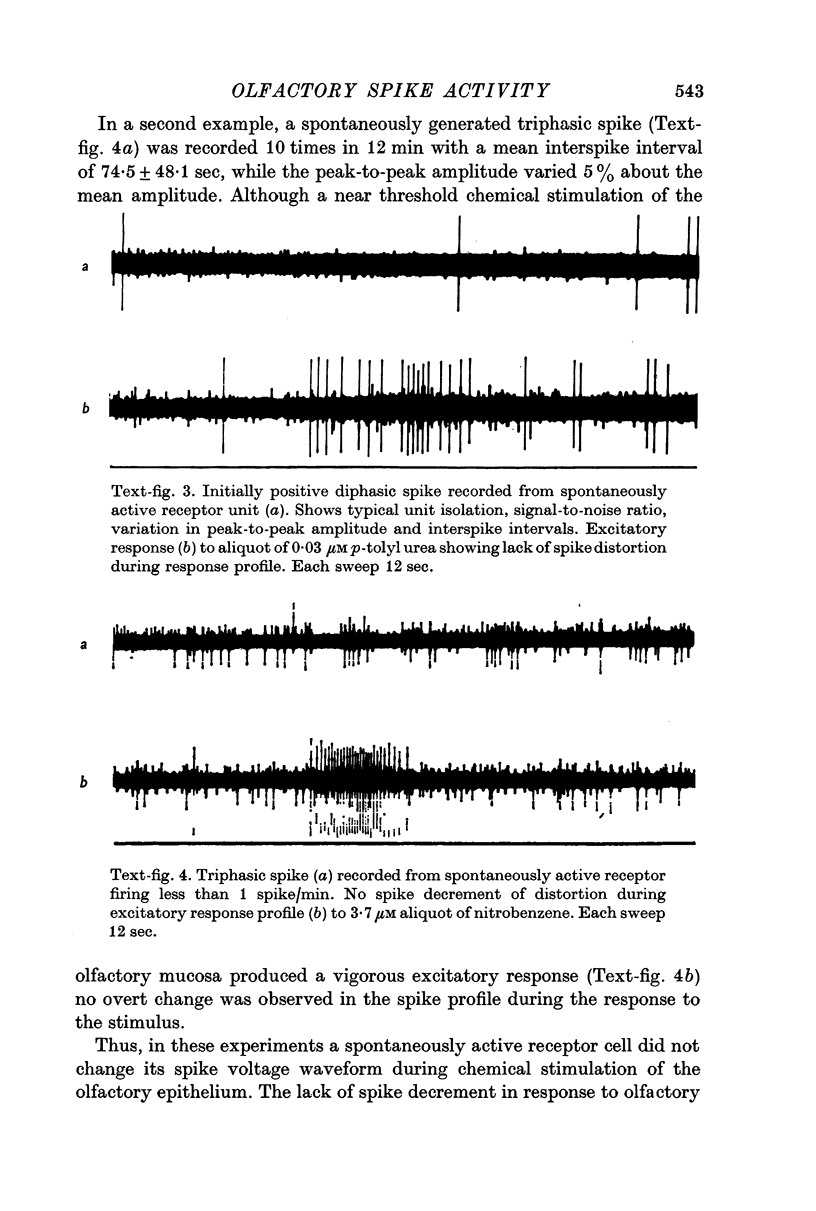
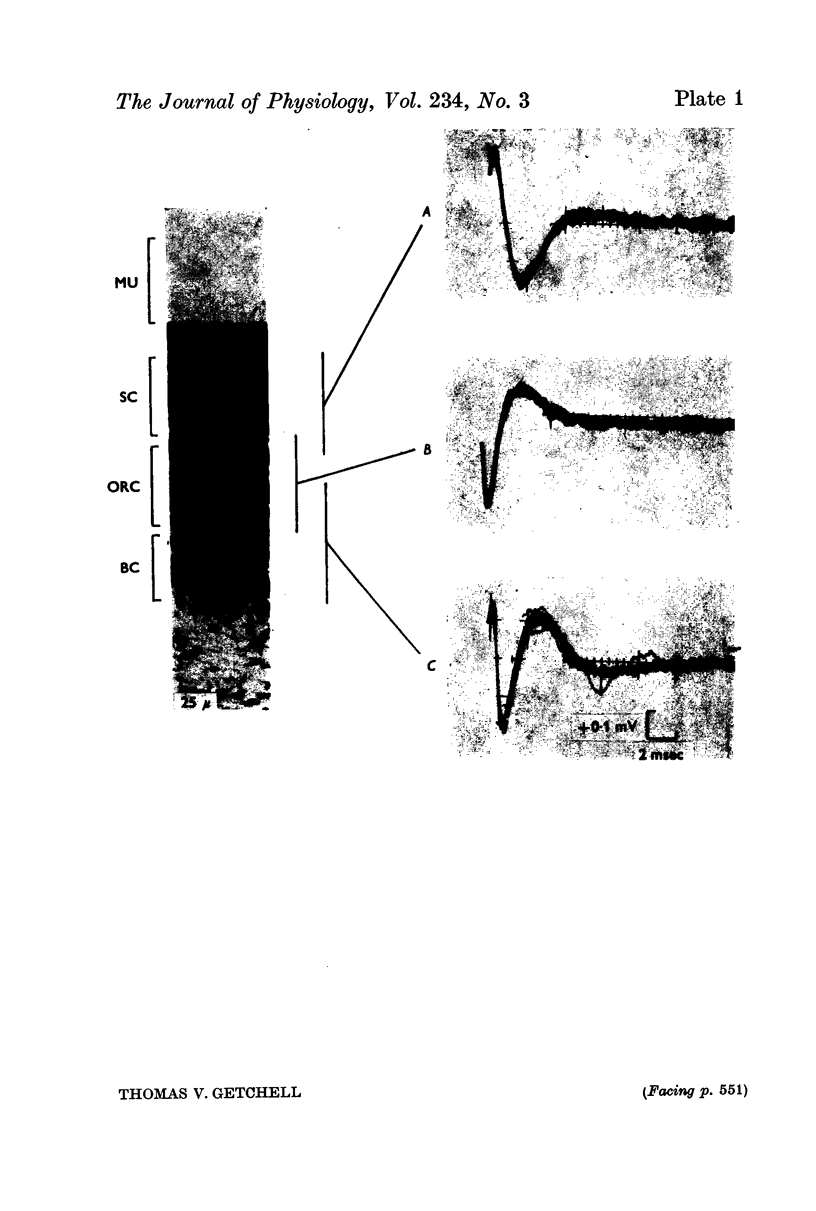
2. Three basic types of spikes were recorded: a diphasic spike with a positive—negative voltage sequence, a diphasic spike with a negative—positive voltage sequence and a triphasic spike with a positive—negative—positive voltage sequence.
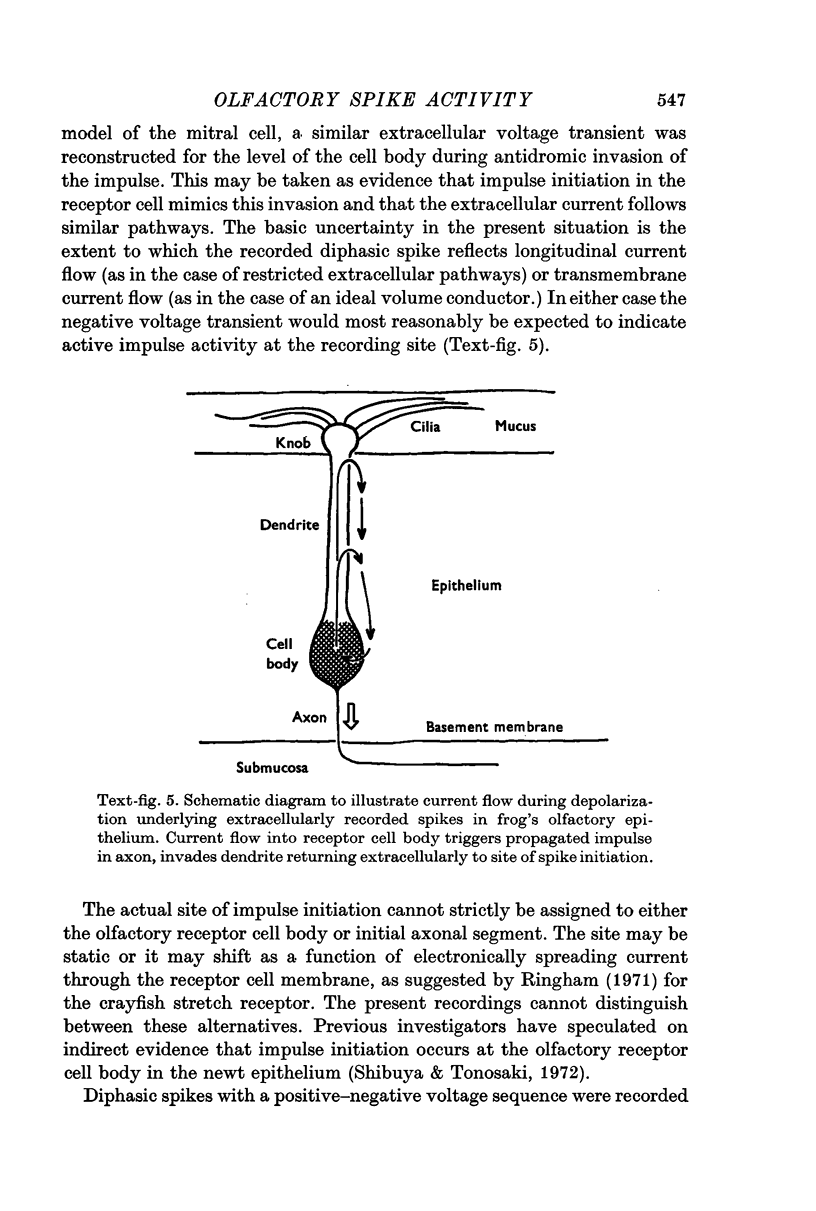
3. The triphasic spike was correlated with the action potential in the axon. The initially negative diphasic spike was correlated with the action potential initiated in the cell body region. The initially positive diphasic spike was correlated with the spread of the impulse into the receptor cell dendrite.
4. A model is discussed which accounts for the differences in spike conformations and provides a basis for analysing impulse activity in spontaneously active and stimulus-driven olfactory receptor cells.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN K. T., WIESEL T. N. Intraretinal recording with micropipette electrodes in the intact cat eye. J Physiol. 1959 Dec;149:537–562. doi: 10.1113/jphysiol.1959.sp006360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J., Plonsey R. The extracellular potential field of the single active nerve fiber in a volume conductor. Biophys J. 1968 Jul;8(7):842–864. doi: 10.1016/S0006-3495(68)86524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GASSER H. S. Olfactory nerve fibers. J Gen Physiol. 1956 Mar 20;39(4):473–496. doi: 10.1085/jgp.39.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesteland R. C., Lettvin J. Y., Pitts W. H. Chemical transmission in the nose of the frog. J Physiol. 1965 Dec;181(3):525–559. doi: 10.1113/jphysiol.1965.sp007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziadei P. P. Topological relations between olfactory neurons. Z Zellforsch Mikrosk Anat. 1971 Jul;118(4):449–466. doi: 10.1007/BF00324613. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W., FITZHUGH R., BARLOW H. B. Maintained activity in the cat's retina in light and darkness. J Gen Physiol. 1957 May 20;40(5):683–702. doi: 10.1085/jgp.40.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R., Nicholson C. Electrophysiological properties of dendrites and somata in alligator Purkinje cells. J Neurophysiol. 1971 Jul;34(4):532–551. doi: 10.1152/jn.1971.34.4.532. [DOI] [PubMed] [Google Scholar]
- MATURANA H. R., LETTVIN J. Y., MCCULLOCH W. S., PITTS W. H. Anatomy and physiology of vision in the frog (Rana pipiens). J Gen Physiol. 1960 Jul;43(6):129–175. doi: 10.1085/jgp.43.6.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews D. F. Response patterns of single neurons in the tortoise olfactory epithelium and olfactory bulb. J Gen Physiol. 1972 Aug;60(2):166–180. doi: 10.1085/jgp.60.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeranz B., Chung S. H. Dendritic-tree anatomy codes form-vision physiology in tadpole retina. Science. 1970 Nov 27;170(3961):983–984. doi: 10.1126/science.170.3961.983. [DOI] [PubMed] [Google Scholar]
- RODIECK R. W., KIANG N. Y., GERSTEIN G. L. Some quantitative methods for the study of spontaneous activity of single neurons. Biophys J. 1962 Jul;2:351–368. doi: 10.1016/s0006-3495(62)86860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W., Shepherd G. M. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol. 1968 Nov;31(6):884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]
- Ringham G. L. Origin of nerve impulse in slowly adapting stretch receptor of crayfish. J Neurophysiol. 1971 Sep;34(5):773–784. doi: 10.1152/jn.1971.34.5.773. [DOI] [PubMed] [Google Scholar]
- SHIBUYA T., SHIBUYA S. Olfactory epithelium: unitary responses in the tortoise. Science. 1963 May 3;140(3566):495–496. doi: 10.1126/science.140.3566.495. [DOI] [PubMed] [Google Scholar]
- Stein R. B., Pearson K. G. Predicted amplitude and form of action potentials recorded from unmyelinated nerve fibres. J Theor Biol. 1971 Sep;32(3):539–558. doi: 10.1016/0022-5193(71)90155-x. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Tucker D. Amino acids as olfactory stimuli in freshwater catfish, Ictalurus catus (Linn.). Comp Biochem Physiol A Comp Physiol. 1971 Oct;40(2):399–404. doi: 10.1016/0300-9629(71)90030-2. [DOI] [PubMed] [Google Scholar]





