Abstract
1. The effects of denervation, reinnervation and pH on the ionic permeability changes mediated by junctional receptors have been studied in muscle fibres of the frog sartorius.
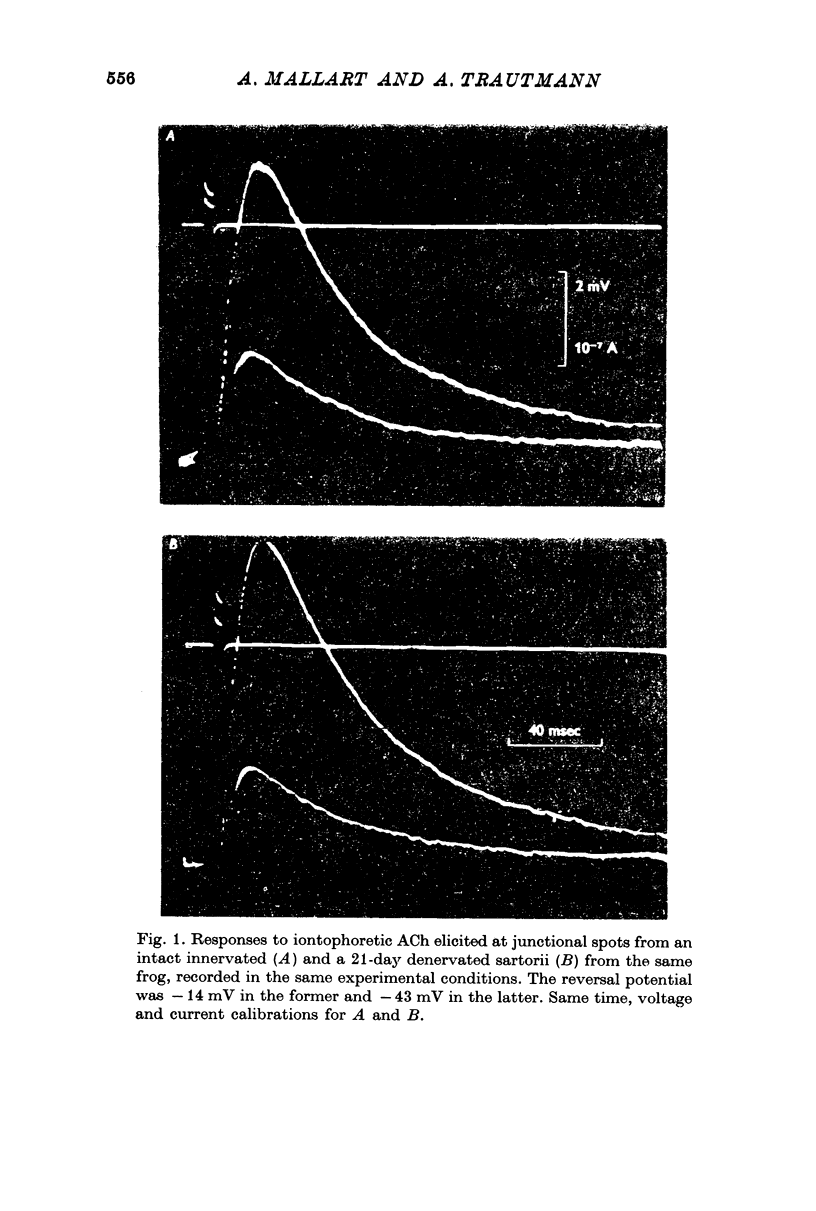
2. The reversal potential of acetylcholine responses in denervated junctions was about 25 mV more negative than in normal junctions.
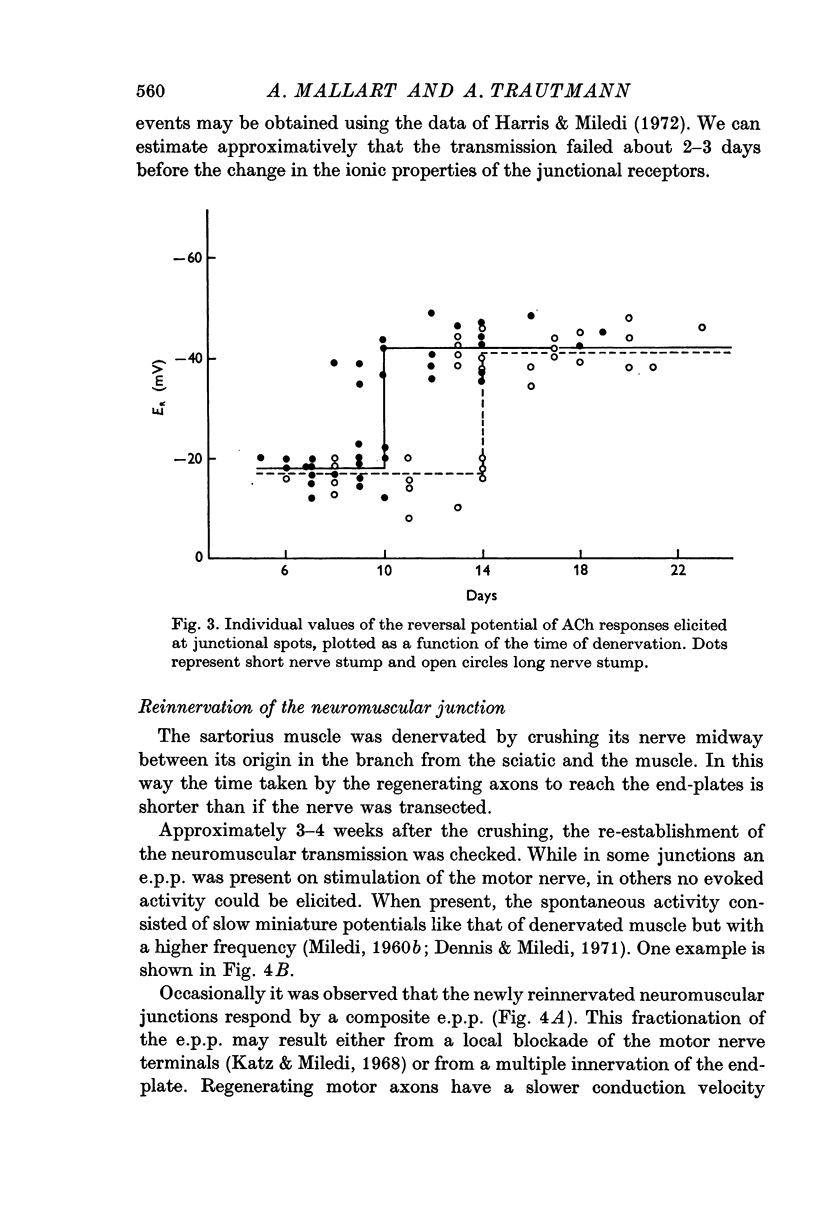
3. The delay of the change in the ionic properties of junctional receptors was proportional to the nerve stump length: 10 and 14 days for lengths of 12 and 33 mm, respectively.
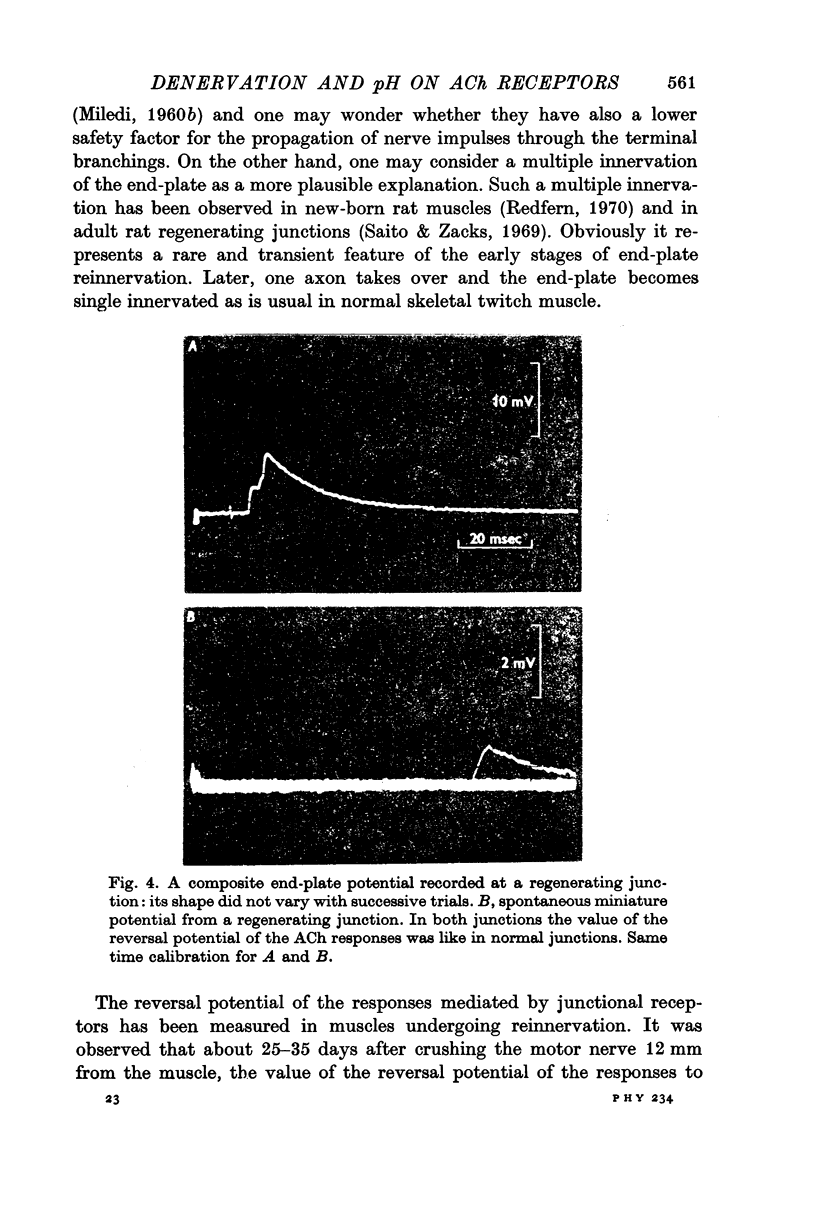
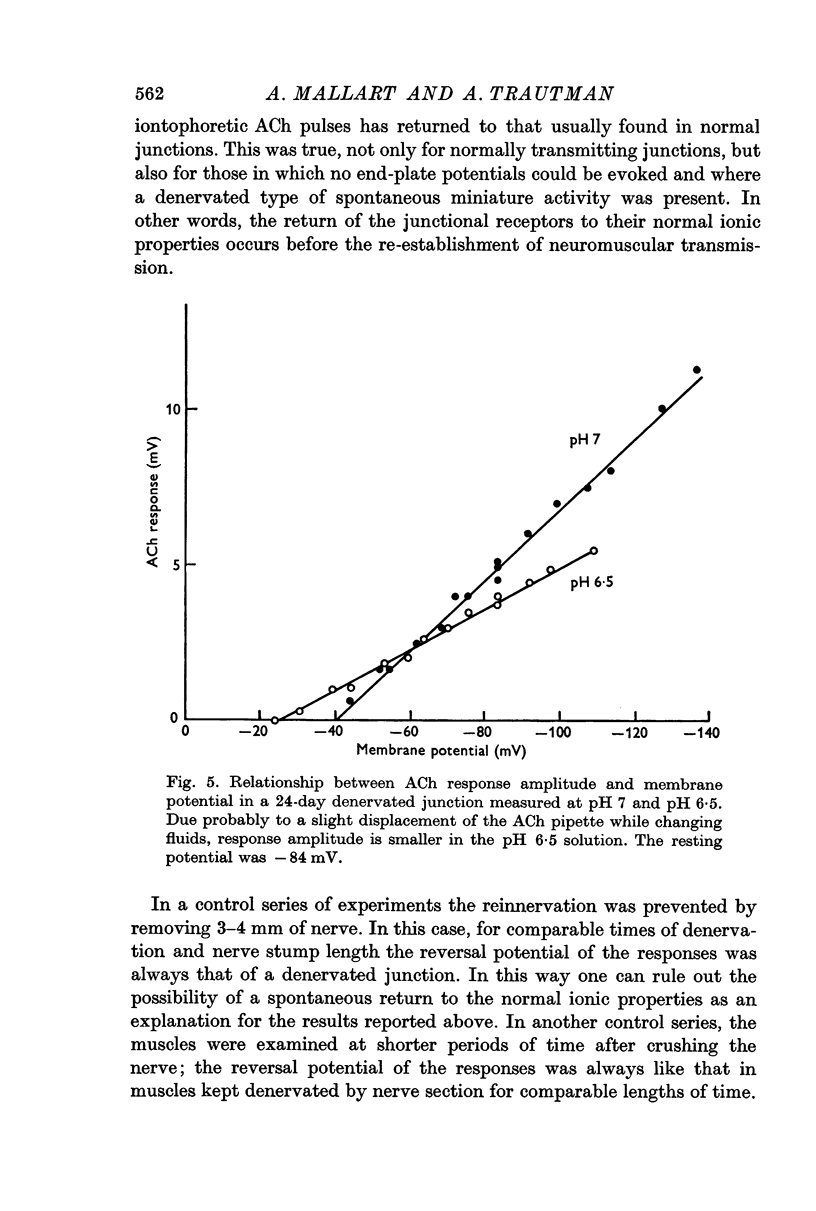
4. When the motor nerve reinnervates the junction, the reversal potential of the acetylcholine responses comes back to the normal value before the neuromuscular transmission is restored.
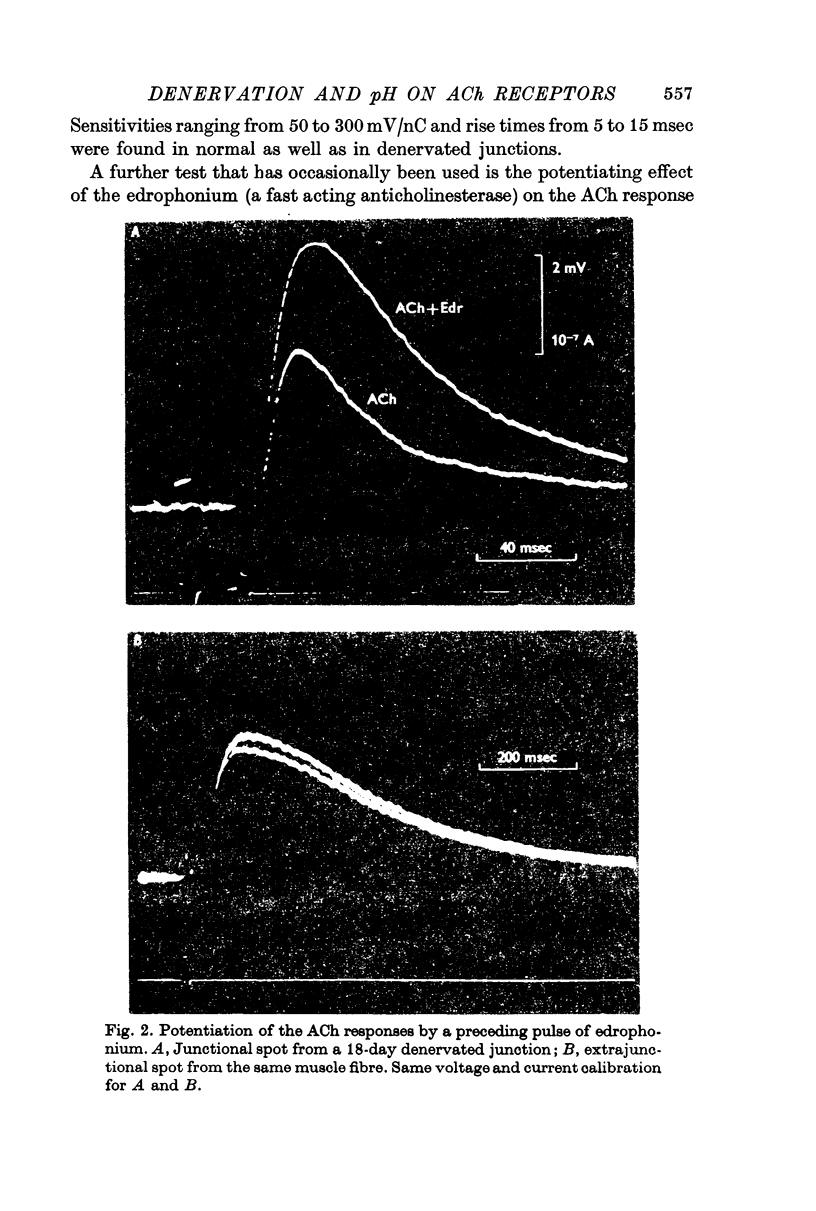
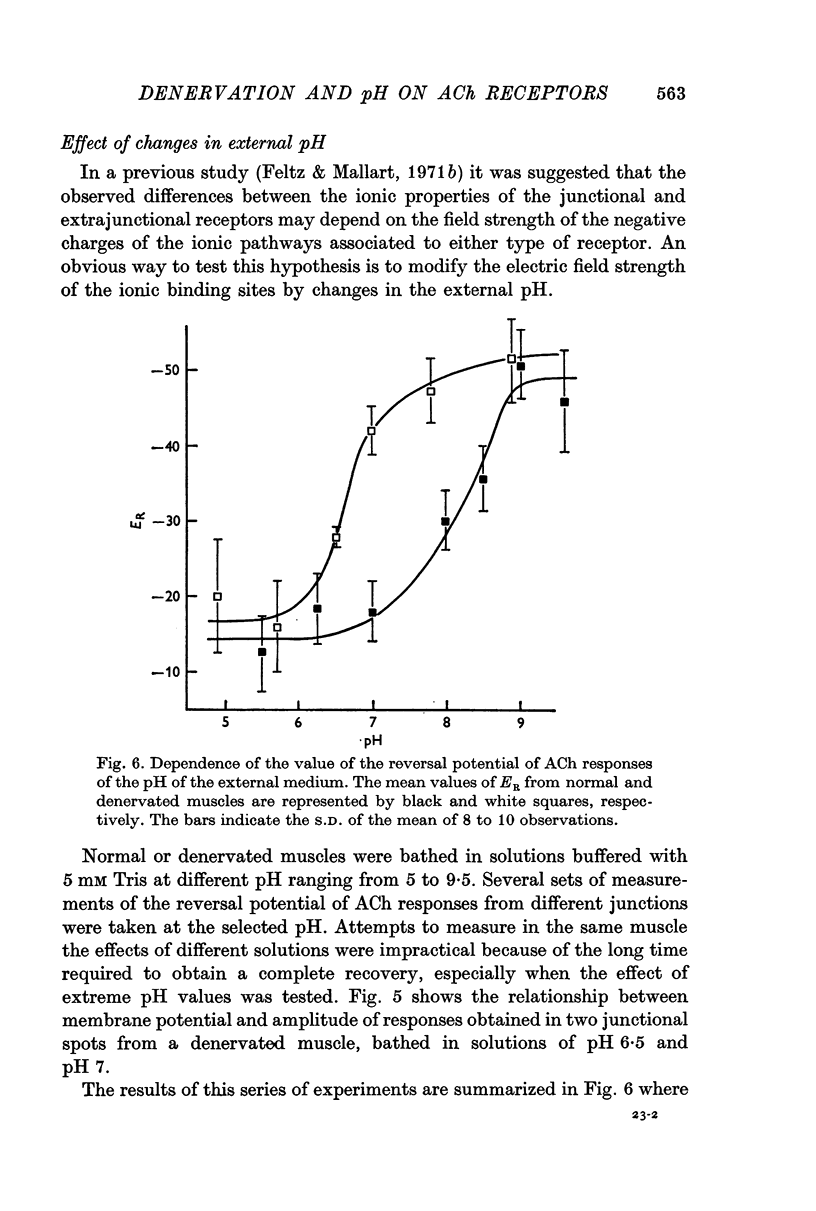
5. The Na/K conductance change decreases in high pH solutions. After denervation, the pH profile of this ratio is shifted to the acid values by about two pH units.
6. These observations can be explained by assuming that the Na and K channels differ by the pK of their anionic groups and that the denervation induces an alteration of the sites that bear the charges.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Schuh F. T., Kauffman F. C. Early membrane depolarization of the fast mammalian muscle after denervation. Pflugers Arch. 1971;328(1):36–50. doi: 10.1007/BF00587359. [DOI] [PubMed] [Google Scholar]
- Deguchi T., Narahashi T. Effects of procaine on ionic conductances of end-plate membranes. J Pharmacol Exp Ther. 1971 Feb;176(2):423–433. [PubMed] [Google Scholar]
- Dennis M., Miledi R. Lack of correspondence between the amplitudes of spontaneous potentials and unit potentials evoked by nerve impulses at regenerating neuromuscular junctions. Nat New Biol. 1971 Jul 28;232(30):126–128. doi: 10.1038/newbio232126a0. [DOI] [PubMed] [Google Scholar]
- Drachman D. B. Neurotrophic regulation of muscle cholinesterase: effects of botulinum toxin and denervation. J Physiol. 1972 Nov;226(3):619–627. doi: 10.1113/jphysiol.1972.sp010000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman D. B., Witzke F. Trophic regulation of acetylcholine sensitivity of muscle: effect of electrical stimulation. Science. 1972 May 5;176(4034):514–516. doi: 10.1126/science.176.4034.514. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Stefani E. Electrophysiological studies of neuromuscular transmission in hereditary 'motor end-plate disease' of the mouse. J Physiol. 1971 Jan;212(2):535–548. doi: 10.1113/jphysiol.1971.sp009340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. S., Howell J. N., Vaughan P. C. The maintenance of resting potentials in glycerol-treated muscle fibres. J Physiol. 1971 May;215(1):95–102. doi: 10.1113/jphysiol.1971.sp009459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Mallart A. An analysis of acetylcholine responses of junctional and extrajunctional receptors of frog muscle fibres. J Physiol. 1971 Oct;218(1):85–100. doi: 10.1113/jphysiol.1971.sp009605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Mallart A. Ionic permeability changes induced by some cholinergic agonists on normal and denervated frog muscles. J Physiol. 1971 Oct;218(1):101–116. doi: 10.1113/jphysiol.1971.sp009606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTH L., ALBERS R. W., BROWN W. C. QUANTITATIVE CHANGES IN CHOLINESTERASE ACTIVITY OF DENERVATED MUSCLE FIBERS AND SOLE PLATES. Exp Neurol. 1964 Sep;10:236–250. doi: 10.1016/0014-4886(64)90065-2. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Capacitance of the surface and transverse tubular membrane of frog sartorius muscle fibers. J Gen Physiol. 1969 Mar;53(3):265–278. doi: 10.1085/jgp.53.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L., Brown W. C., Watson P. K. Studies on the role of nerve impulses and acetylcholine release in the regulation of the cholinesterase activity of muscle. Exp Neurol. 1967 Aug;18(4):443–452. doi: 10.1016/0014-4886(67)90061-1. [DOI] [PubMed] [Google Scholar]
- Harris A. J., Miledi R. A study of frog muscle maintained in organ culture. J Physiol. 1972 Feb;221(1):207–226. doi: 10.1113/jphysiol.1972.sp009749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Thesleff S. Nerve stump length and membrane changes in denervated skeletal muscle. Nat New Biol. 1972 Mar 15;236(63):60–61. doi: 10.1038/newbio236060a0. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. The interaction between edrophonium (tensilon) and acetylcholine at the motor end-plate. Br J Pharmacol Chemother. 1957 Jun;12(2):260–264. doi: 10.1111/j.1476-5381.1957.tb00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of local blockage of motor nerve terminals. J Physiol. 1968 Dec;199(3):729–741. doi: 10.1113/jphysiol.1968.sp008675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCO J. V., EYZAGUIRRE C. Fibrillation and hypersensitivity to ACh in denervated muscle: effect of length of degenerating nerve fibers. J Neurophysiol. 1955 Jan;18(1):65–73. doi: 10.1152/jn.1955.18.1.65. [DOI] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno T. Analysis of sodium and potassium conductances in the procaine end-plate potential. J Physiol. 1966 Apr;183(3):592–606. doi: 10.1113/jphysiol.1966.sp007886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno T., Edwards C., Hashimura S. Difference in effects of end-plate potentials between procaine and lidocaine as revealed by voltage-clamp experiments. J Neurophysiol. 1971 Jan;34(1):32–46. doi: 10.1152/jn.1971.34.1.32. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A., Zacks S. I. Fine structure of neuromuscular junctions after nerve section and implantation of nerve in denervated muscle. Exp Mol Pathol. 1969 Jun;10(3):256–273. doi: 10.1016/0014-4800(69)90056-2. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI N. Effects of calcium on the conductance change of the end-plate membrane during the action of transmitter. J Physiol. 1963 Jun;167:141–155. doi: 10.1113/jphysiol.1963.sp007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THESLEFF S. Supersensitivity of skeletal muscle produced by botulinum toxin. J Physiol. 1960 Jun;151:598–607. doi: 10.1113/jphysiol.1960.sp006463. [DOI] [PMC free article] [PubMed] [Google Scholar]


