Abstract
1. Intracellular recordings were made from bipolar and amacrine cells in the isolated goldfish retina. Cells were identified mainly from their response patterns to a spot and an annulus in reference to the knowledge obtained from the previous work of intracellular Procion Yellow injection. Using white light and monochromatic lights receptive field organization of recorded cells were analysed.
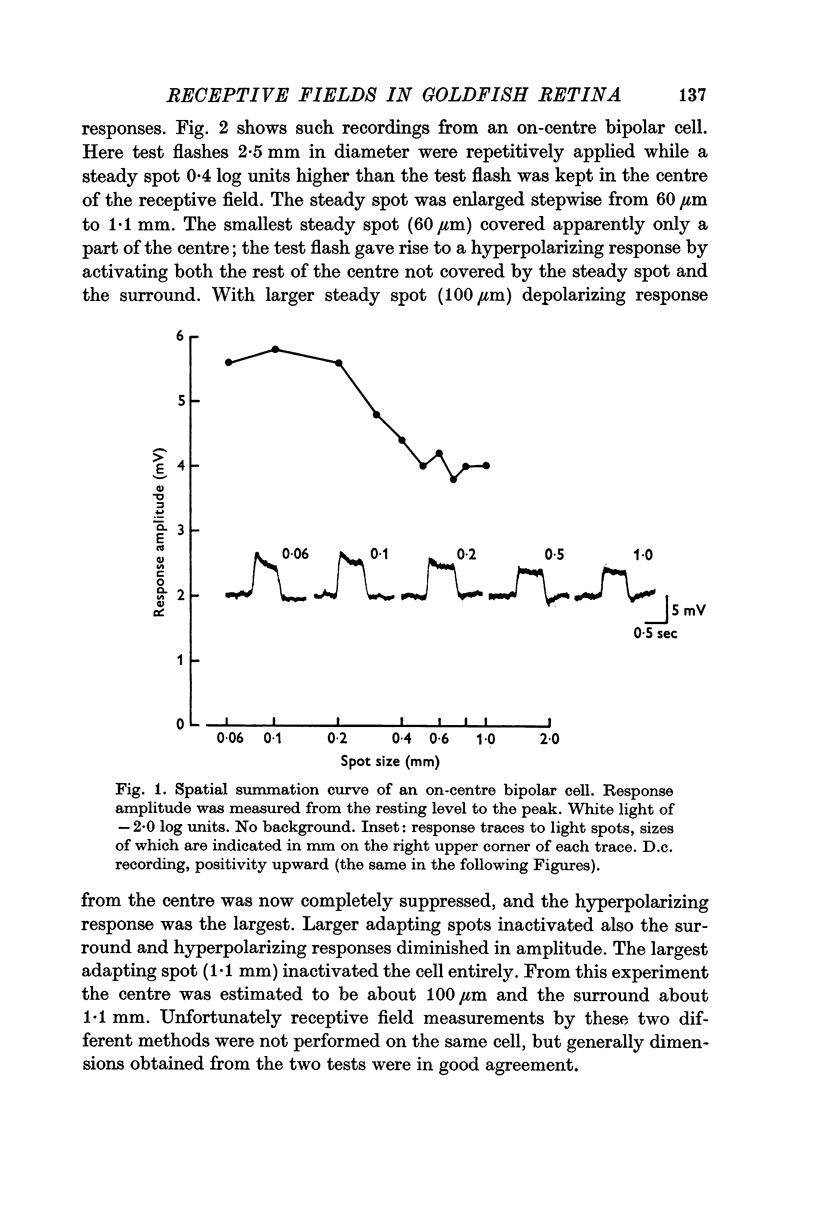
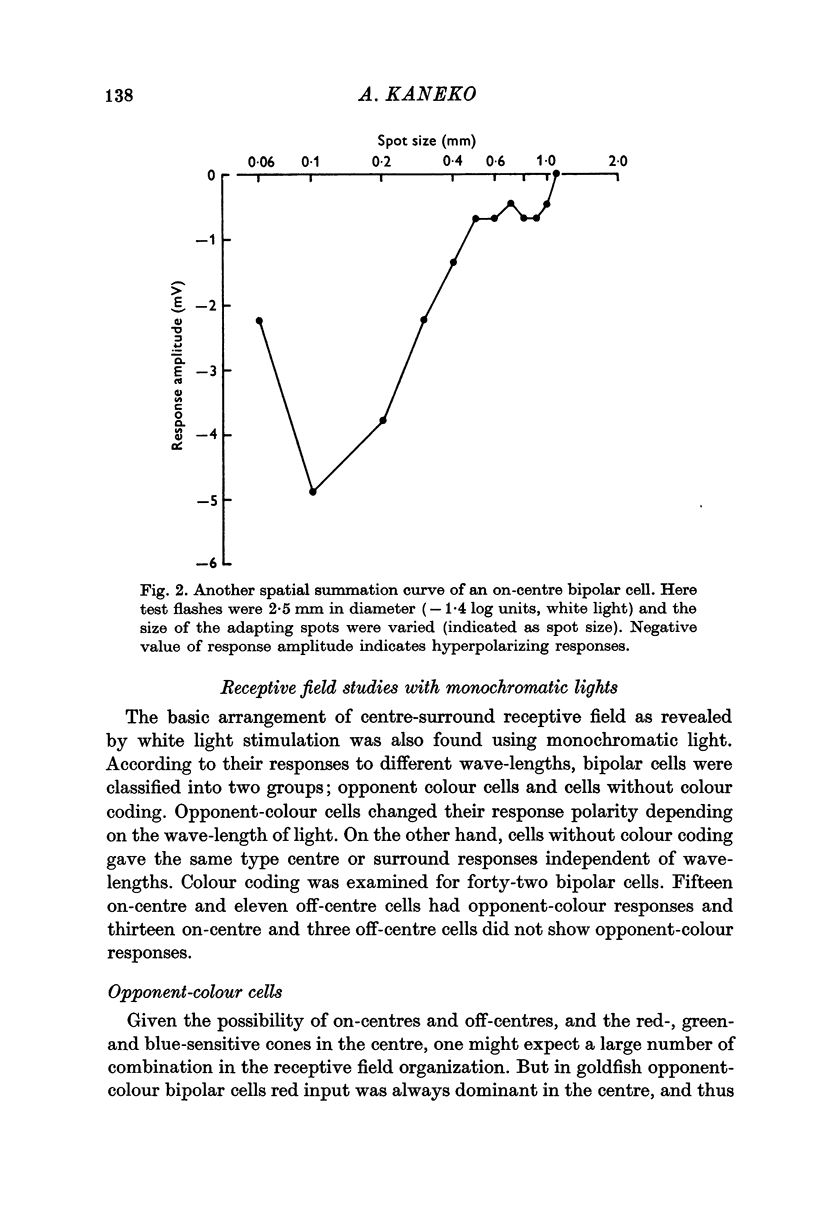
2. All bipolar cells had a centre-surround organization in their receptive fields. The field centre was estimated to be 100-200 μm in diameter, and the surround 1-1·5 mm.
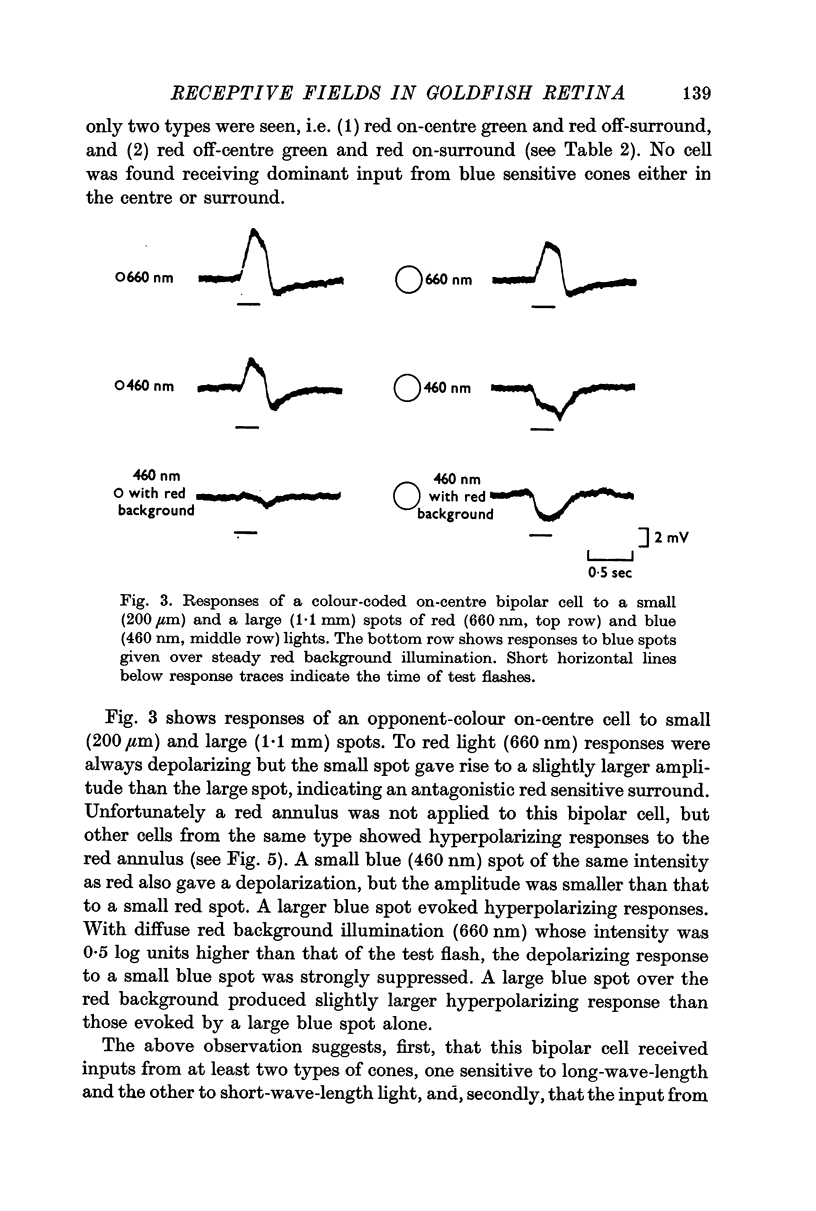
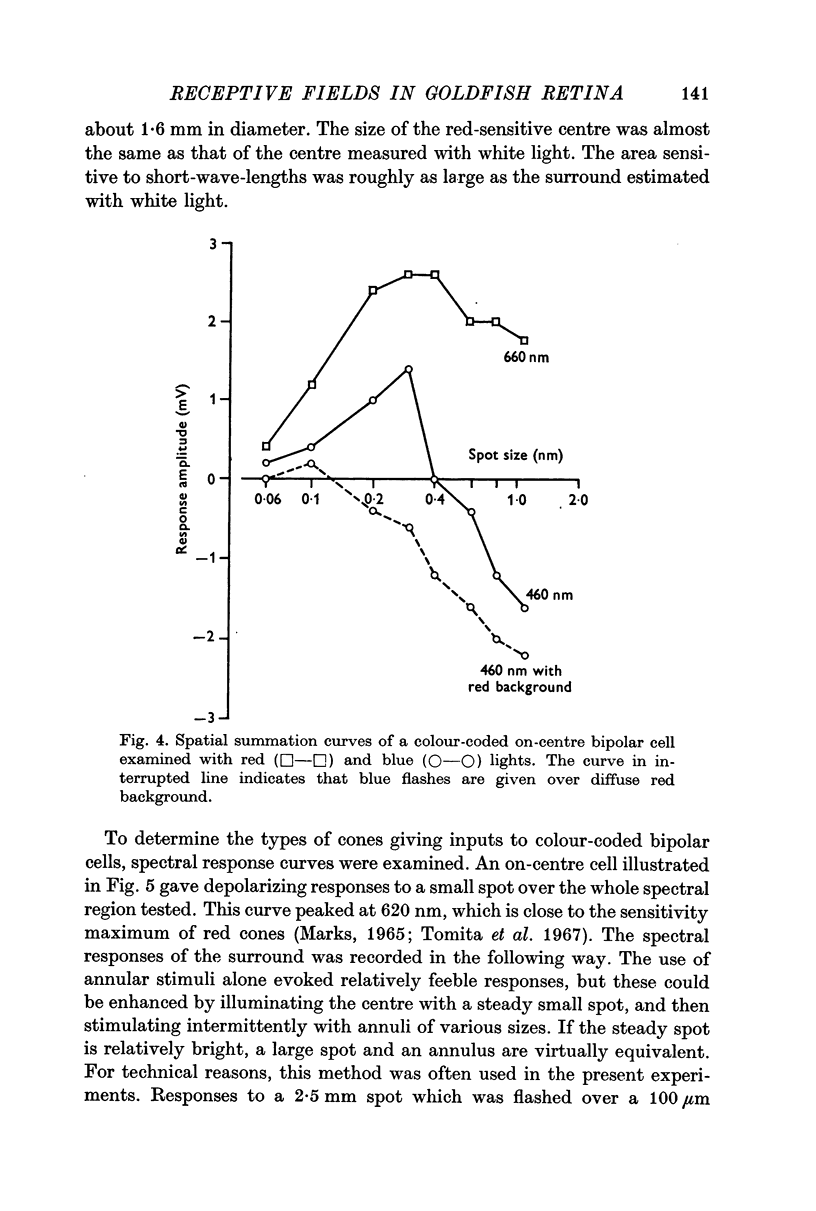
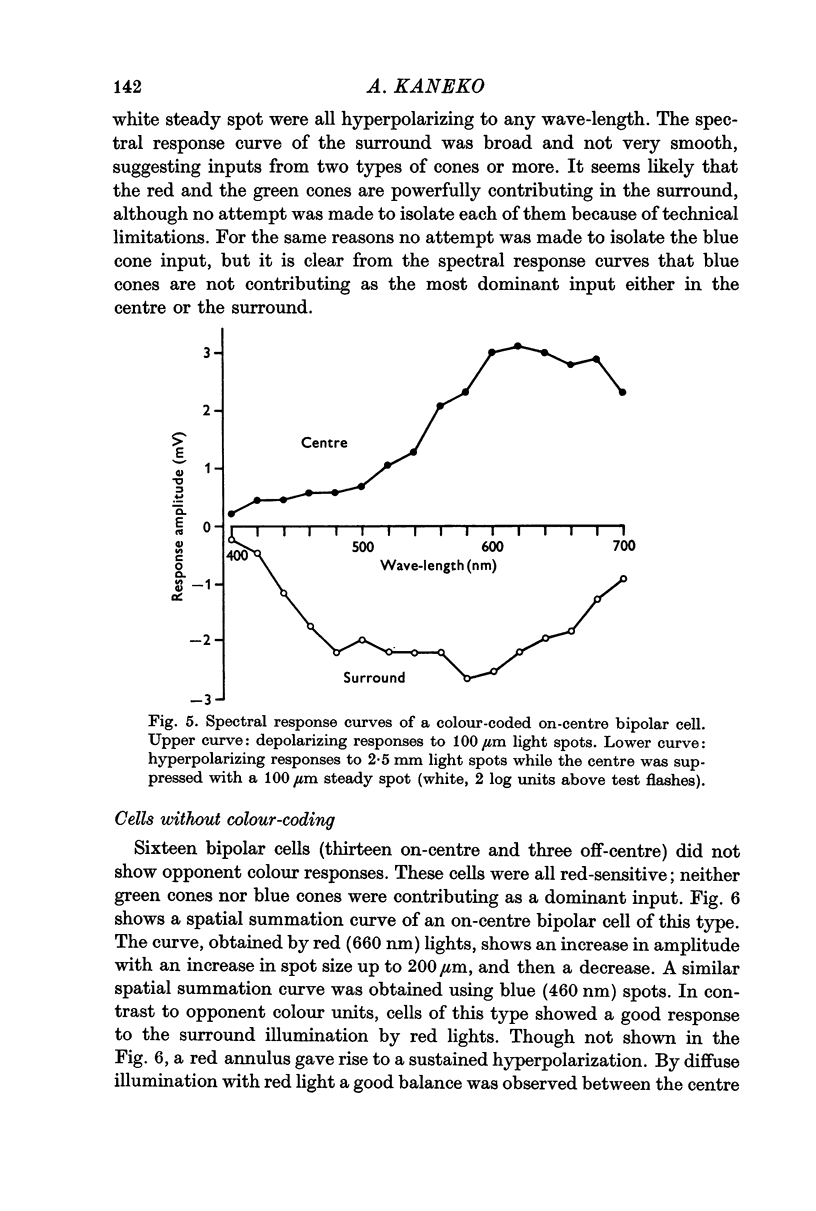
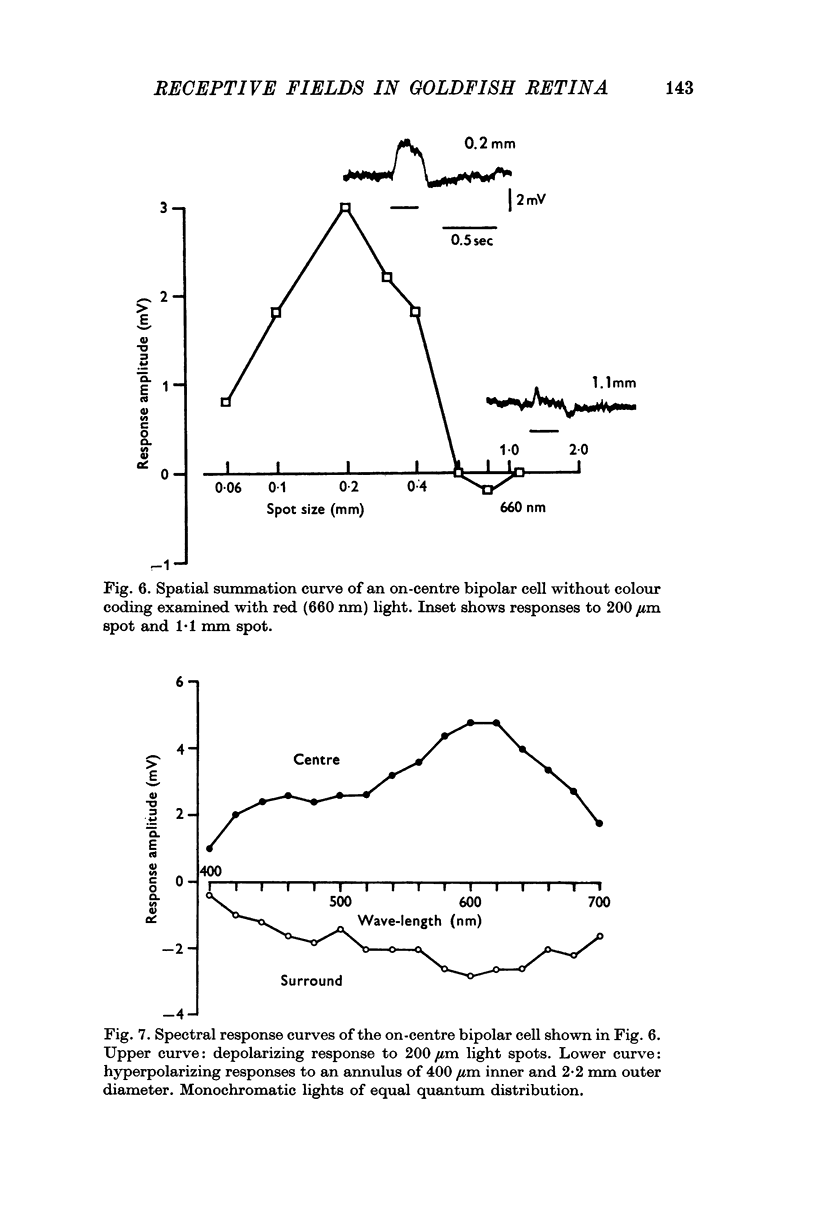
3. Bipolar cells were classified into two types according to the response properties to monochromatic lights. Opponent colour cells received inputs from red and green cones, responding with red on-centre, red and green off-surround or vice versa. Cells without colour coding received input from red cones both in the field centre and the surround. In these cells the centre and the surround were well balanced.
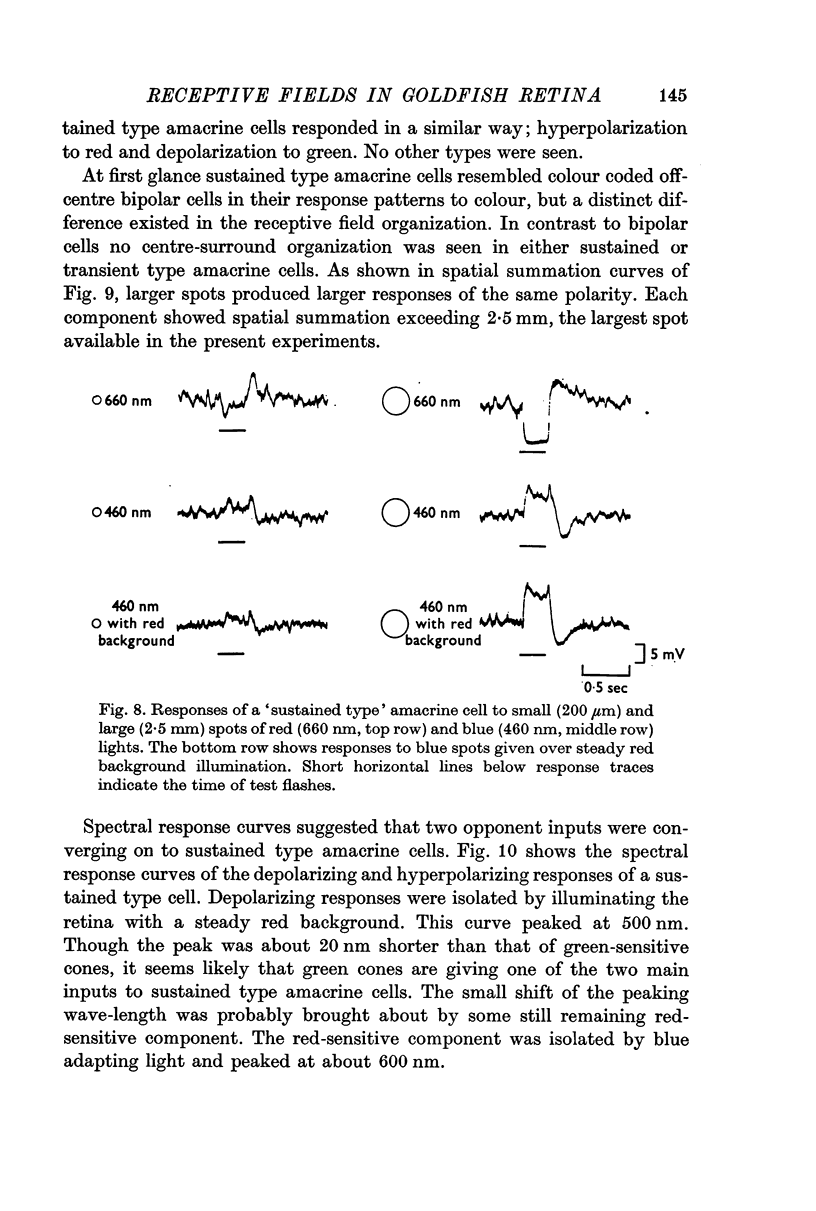
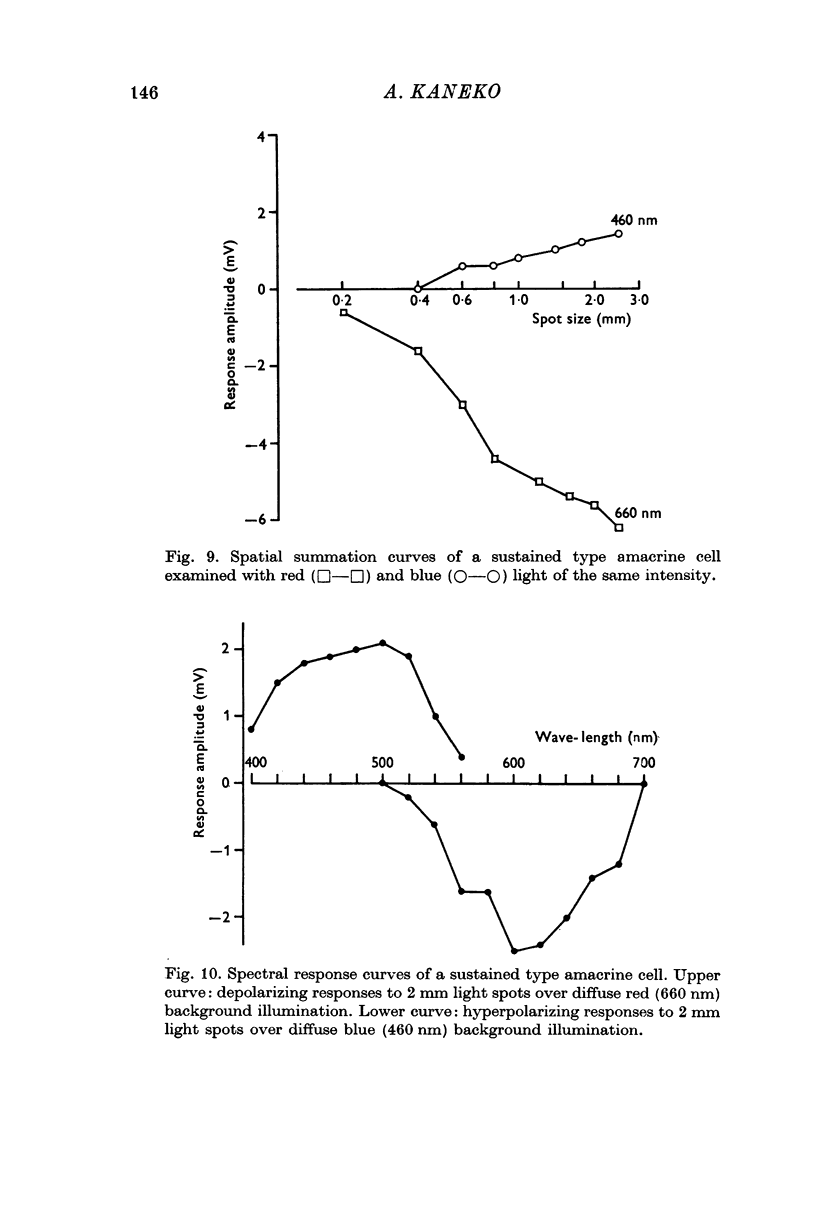
4. Amacrine cells were also classified into two types, a sustained type and a transient type. The sustained type amacrine cells responded with a steady potential change and were colour coded. They were hyperpolarized by red and depolarized by green light. The transient type amacrine cells responded with transient depolarization at on and off of light flashes. They received input chiefly from red cones and were not colour coded. Both types of amacrine cells showed a large spatial summation in an area over 2·5 mm; centre-surround antagonism was not seen.
5. Comparing the size of the receptive field with anatomy, especially with the size of dendritic spread, the field centre of bipolar cells agreed in size with their dendritic spread. Bipolar cell surround clearly exceeded its dendritic field. Since the response properties of the bipolar cell surround was mimicked most closely by the receptive field of external horizontal cells, the input to the bipolar cell surround is thought to be mediated by external horizontal cells.
6. By comparing receptive field properties of various retinal cells it is suggested that both the opponent colour bipolar cells and the colour coded amacrine cells converge on to the double opponent ganglion cells.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Major D. Cat retinal ganglion cell dendritic fields. Exp Neurol. 1966 May;15(1):70–78. doi: 10.1016/0014-4886(66)90035-5. [DOI] [PubMed] [Google Scholar]
- Daw N. W. Colour-coded ganglion cells in the goldfish retina: extension of their receptive fields by means of new stimuli. J Physiol. 1968 Aug;197(3):567–592. doi: 10.1113/jphysiol.1968.sp008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of optic nerve fibres in the spider monkey. J Physiol. 1960 Dec;154:572–580. doi: 10.1113/jphysiol.1960.sp006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Hashimoto H. Electrophysiological study of single neurons in the inner nuclear layer of the carp retina. Vision Res. 1969 Jan;9(1):37–55. doi: 10.1016/0042-6989(69)90030-3. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACNICHOL E. J., SVAETICHIN G. Electric responses from the isolated retinas of fishes. Am J Ophthalmol. 1958 Sep;46(3 Pt 2):26–46. doi: 10.1016/0002-9394(58)90053-9. [DOI] [PubMed] [Google Scholar]
- MARKS W. B. VISUAL PIGMENTS OF SINGLE GOLDFISH CONES. J Physiol. 1965 May;178:14–32. doi: 10.1113/jphysiol.1965.sp007611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael C. R. Receptive fields of single optic nerve fibers in a mammal with an all-cone retina. 3. Opponent color units. J Neurophysiol. 1968 Mar;31(2):268–282. doi: 10.1152/jn.1968.31.2.268. [DOI] [PubMed] [Google Scholar]
- Norton A. L., Spekreijse H., Wagner H. G., Wolbarsht M. L. Responses to directional stimuli in retinal preganglionic units. J Physiol. 1970 Jan;206(1):93–107. doi: 10.1113/jphysiol.1970.sp008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell W. K. The structure and relationships of horizontal cells and photoreceptor-bipolar synaptic complexes in goldfish retina. Am J Anat. 1967 Sep;121(2):401–423. doi: 10.1002/aja.1001210213. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrophysiological study of the mechanisms subserving color coding in the fish retina. Cold Spring Harb Symp Quant Biol. 1965;30:559–566. doi: 10.1101/sqb.1965.030.01.054. [DOI] [PubMed] [Google Scholar]
- Tomita T., Kaneko A., Murakami M., Pautler E. L. Spectral response curves of single cones in the carp. Vision Res. 1967 Jul;7(7):519–531. doi: 10.1016/0042-6989(67)90061-2. [DOI] [PubMed] [Google Scholar]
- Wagner H. G., Macnichol E. F., Wolbarsht M. L. The Response Properties of Single Ganglion Cells in the Goldfish Retina. J Gen Physiol. 1960 Jul 1;43(6):45–62. doi: 10.1085/jgp.43.6.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966 Nov;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- Witkovsky P. A comparison of ganglion cell and S-potential response properties in carp retina. J Neurophysiol. 1967 May;30(3):546–561. doi: 10.1152/jn.1967.30.3.546. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Dowling J. E. Synaptic relationships in the plexiform layers of carp retina. Z Zellforsch Mikrosk Anat. 1969;100(1):60–82. doi: 10.1007/BF00343821. [DOI] [PubMed] [Google Scholar]
- Yager D. Behavioral measures and theoretical analysis of spectral sensitivity and spectral saturation in the goldfish, Carassius auratus. Vision Res. 1967 Sep;7(9):707–727. doi: 10.1016/0042-6989(67)90034-x. [DOI] [PubMed] [Google Scholar]


