Abstract
1. The temperature dependence of the sodium and potassium conductances of Myxicola giant axons was studied in the range of 1-18° C.
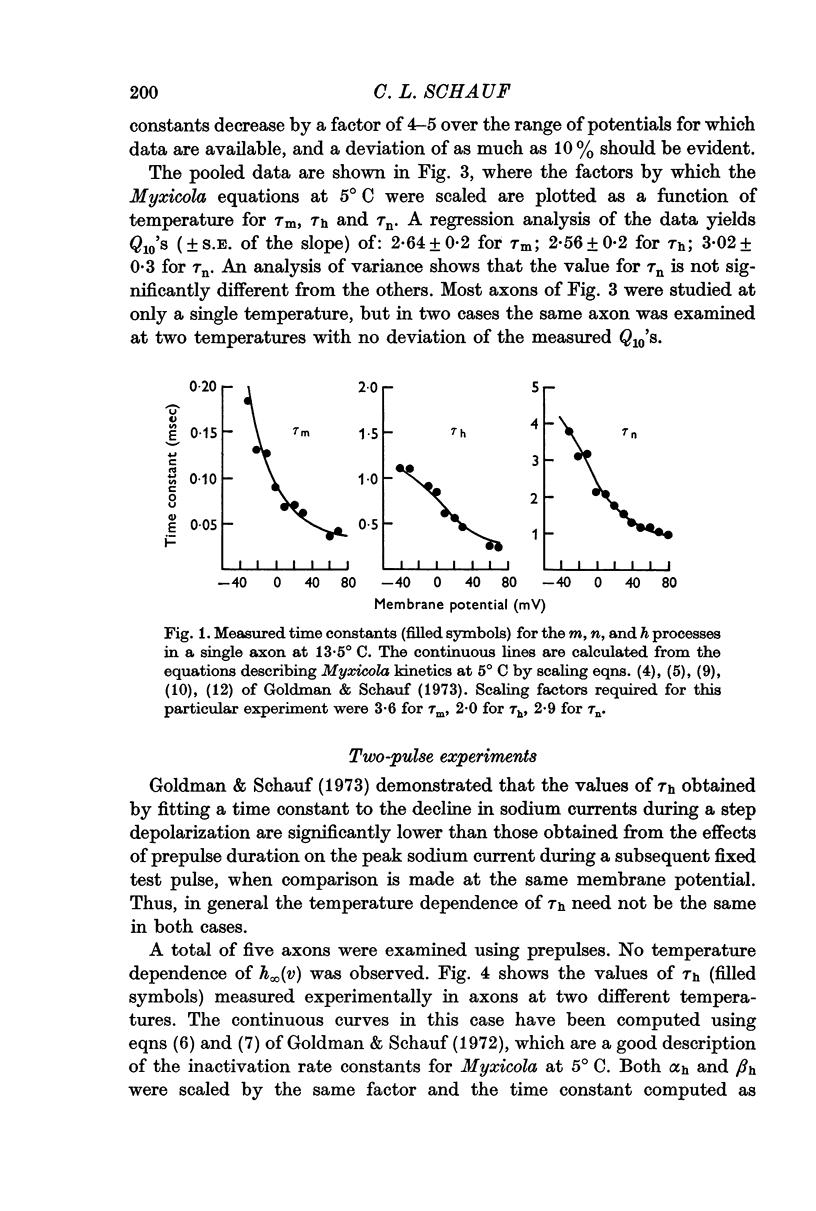
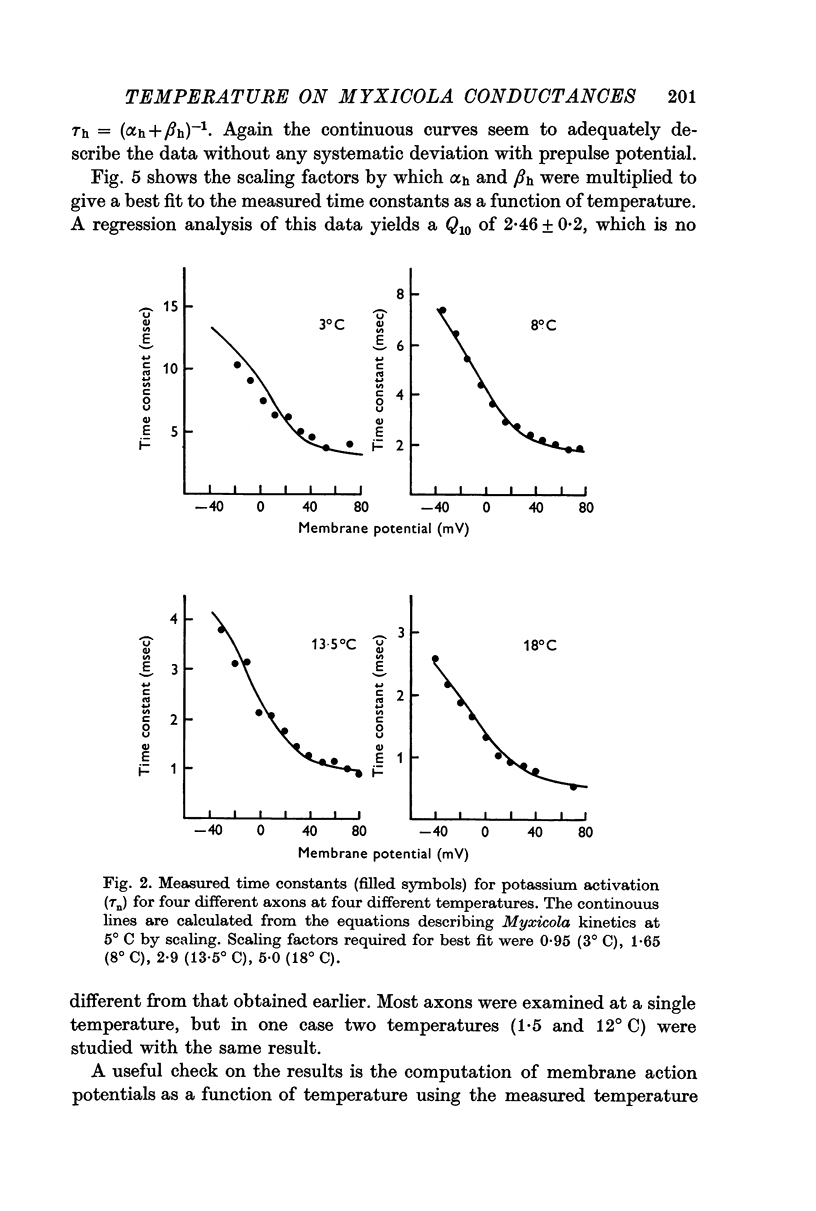
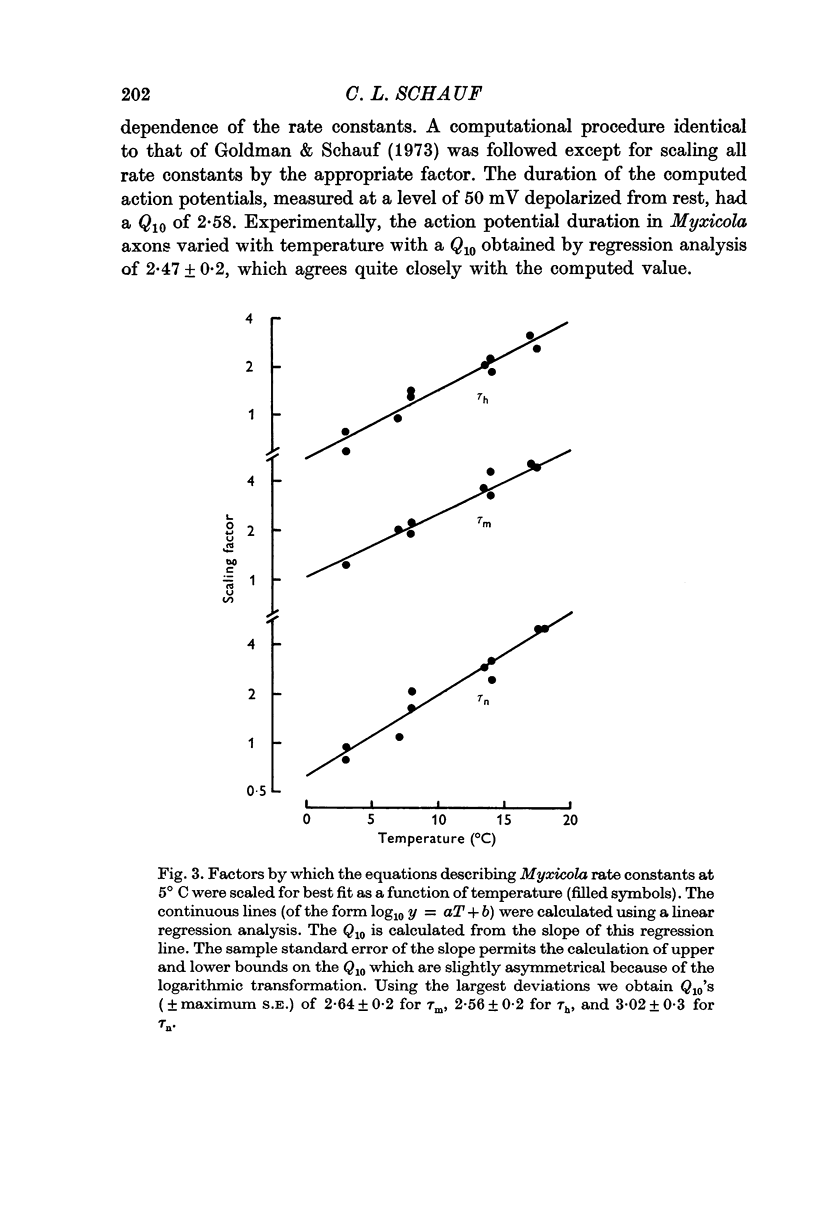
2. Analysis of voltage clamp records for step depolarizations of 20-150 mV yielded Q10's for ḡNa, ḡK, and g1 of 1·3-1·5. The steady-state parameters m∞ (v), n∞ (v) were independent of temperature. The time constants had Q10's of: 2·64 ± 0·2 for τm; 2·56 ± 0·2 for τh; 3·02 ± 0·3 for τn.
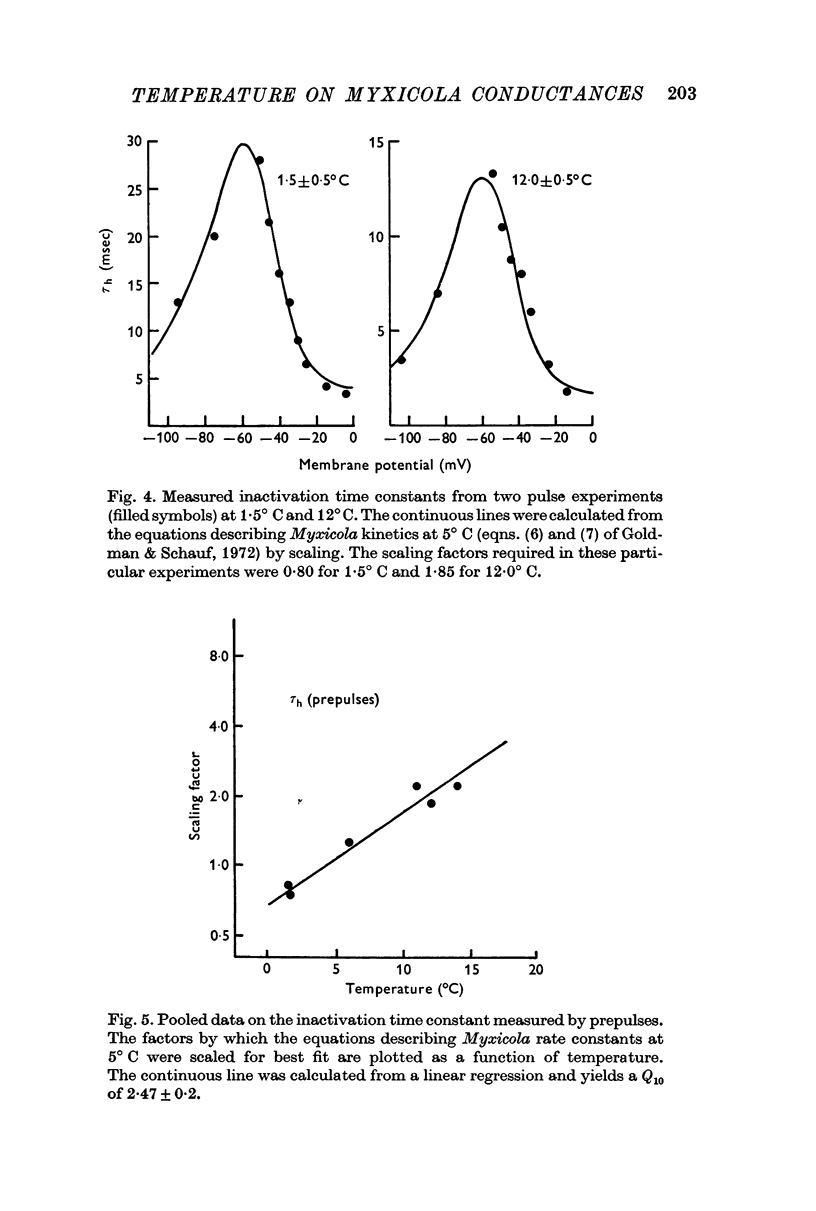
3. τh determined from the effects of prepulse duration rather than the decline in sodium current during a step depolarization had a Q10 of 2·47 ± 0·2.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binstock L., Goldman L. Current- and voltage-clamped studies on Myxicola giant axons. Effect of tetrodotoxin. J Gen Physiol. 1969 Dec;54(6):730–740. doi: 10.1085/jgp.54.6.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Inactivation of the sodium current in Myxicola giant axons. Evidence for coupling to the activation process. J Gen Physiol. 1972 Jun;59(6):659–675. doi: 10.1085/jgp.59.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Quantitative description of sodium and potassium currents and computed action potentials in Myxicola giant axons. J Gen Physiol. 1973 Mar;61(3):361–384. doi: 10.1085/jgp.61.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Ion movements during nerve activity. Ann N Y Acad Sci. 1959 Aug 28;81:221–246. doi: 10.1111/j.1749-6632.1959.tb49311.x. [DOI] [PubMed] [Google Scholar]
- Moore L. E. Effect of temperature and calcium ions on rate constants of myelinated nerve. Am J Physiol. 1971 Jul;221(1):131–137. doi: 10.1152/ajplegacy.1971.221.1.131. [DOI] [PubMed] [Google Scholar]
- Noble D. Conductance mechanisms in excitable cells. Biomembranes. 1972;3:427–447. doi: 10.1007/978-1-4684-0961-1_30. [DOI] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. M., Narahashi T., Scuka M. Mechanism of negative temperature coefficient of nerve blocking action of allethrin. J Pharmacol Exp Ther. 1972 Sep;182(3):442–453. [PubMed] [Google Scholar]


